Abstract
Background
Long-COVID symptoms remain incompletely defined due to a large heterogeneity in the populations studied, case definitions, and settings of care. The aim of this study was to assess, in patients accessing care for Long-COVID, the profile of symptoms reported, the possible clustering of symptoms and cases, the functional status compared to pre-infection, and the impact on working activity.Methods
Multicentre cohort study with a collection of both retrospective and prospective data. Demographics, comorbidities, severity and timing of acute COVID, subjective functional status, working activity and presence of 30 different symptoms were collected using a shortened version of the WHO Post COVID-19 Case Report Form. The impact on working activity was assessed in multivariable logistic regression models. Clustering of symptoms was analysed by hierarchical clustering and the clustering of cases by two-step automatic clustering.Results
The study evaluated 1297 individuals (51.5% women) from 30 clinical centres. Men and women had different profiles in terms of comorbidities, vaccination status, severity and timing of acute SARS-CoV-2 infection. Fatigue (55.9%) and dyspnea (47.2%) were the most frequent symptoms. Women reported more symptoms (3.6 vs. 3.1, p < 0.001), with a significantly higher prevalence of memory loss, difficult concentration, cough, palpitation or tachycardia, dermatological abnormalities, brain fog, headache and visual disturbances. Dyspnea was more common in men. In the cluster analysis of the 19 more common symptoms, five aggregations were found: four two-symptom clusters (smell and taste reduction; anxiety and depressed mood; joint pain or swelling and muscle pain; difficult concentration and memory loss) and one six-symptom cluster (brain fog, equilibrium/gait disturbances, headache, paresthesia, thoracic pain, and palpitations/tachycardia). In a multivariable analysis, headache, dyspnea, difficult concentration, disturbances of equilibrium or gait, visual disturbances and muscular pain were associated with reduced or interrupted working activity. Clustering of cases defined two clusters, with distinct characteristics in terms of phase and severity of acute infection, age, sex, number of comorbidities and symptom profile.Conclusions
The findings provide further evidence that Long-COVID is a heterogeneous disease with manifestations that differ by sex, phase of the pandemic and severity of acute disease, and support the possibility that multiple pathways lead to different clinical manifestations.Free full text

Symptom profile, case and symptom clustering, clinical and demographic characteristics of a multicentre cohort of 1297 patients evaluated for Long-COVID
Abstract
Background
Long-COVID symptoms remain incompletely defined due to a large heterogeneity in the populations studied, case definitions, and settings of care. The aim of this study was to assess, in patients accessing care for Long-COVID, the profile of symptoms reported, the possible clustering of symptoms and cases, the functional status compared to pre-infection, and the impact on working activity.
Methods
Multicentre cohort study with a collection of both retrospective and prospective data. Demographics, comorbidities, severity and timing of acute COVID, subjective functional status, working activity and presence of 30 different symptoms were collected using a shortened version of the WHO Post COVID-19 Case Report Form. The impact on working activity was assessed in multivariable logistic regression models. Clustering of symptoms was analysed by hierarchical clustering and the clustering of cases by two-step automatic clustering.
Results
The study evaluated 1297 individuals (51.5% women) from 30 clinical centres. Men and women had different profiles in terms of comorbidities, vaccination status, severity and timing of acute SARS-CoV-2 infection. Fatigue (55.9%) and dyspnea (47.2%) were the most frequent symptoms. Women reported more symptoms (3.6 vs. 3.1, p <
< 0.001), with a significantly higher prevalence of memory loss, difficult concentration, cough, palpitation or tachycardia, dermatological abnormalities, brain fog, headache and visual disturbances. Dyspnea was more common in men. In the cluster analysis of the 19 more common symptoms, five aggregations were found: four two-symptom clusters (smell and taste reduction; anxiety and depressed mood; joint pain or swelling and muscle pain; difficult concentration and memory loss) and one six-symptom cluster (brain fog, equilibrium/gait disturbances, headache, paresthesia, thoracic pain, and palpitations/tachycardia). In a multivariable analysis, headache, dyspnea, difficult concentration, disturbances of equilibrium or gait, visual disturbances and muscular pain were associated with reduced or interrupted working activity. Clustering of cases defined two clusters, with distinct characteristics in terms of phase and severity of acute infection, age, sex, number of comorbidities and symptom profile.
0.001), with a significantly higher prevalence of memory loss, difficult concentration, cough, palpitation or tachycardia, dermatological abnormalities, brain fog, headache and visual disturbances. Dyspnea was more common in men. In the cluster analysis of the 19 more common symptoms, five aggregations were found: four two-symptom clusters (smell and taste reduction; anxiety and depressed mood; joint pain or swelling and muscle pain; difficult concentration and memory loss) and one six-symptom cluster (brain fog, equilibrium/gait disturbances, headache, paresthesia, thoracic pain, and palpitations/tachycardia). In a multivariable analysis, headache, dyspnea, difficult concentration, disturbances of equilibrium or gait, visual disturbances and muscular pain were associated with reduced or interrupted working activity. Clustering of cases defined two clusters, with distinct characteristics in terms of phase and severity of acute infection, age, sex, number of comorbidities and symptom profile.
Conclusions
The findings provide further evidence that Long-COVID is a heterogeneous disease with manifestations that differ by sex, phase of the pandemic and severity of acute disease, and support the possibility that multiple pathways lead to different clinical manifestations.
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has affected since its beginning a vast number of individuals worldwide, with over 775 million confirmed cases and more than seven million deaths as of April 2024 [1]. Although the severity of the acute disease has reduced, with a currently decreasing number of deaths and of hospitalisations in the first few weeks from infection [1], post-acute sequelae have gained increased public health importance because of their significant prevalence, long-term persistence, and negative impact on quality of life, daily activities, working capacity, and employment status [2].
National and international institutions have soon recognised post-acute sequelae of SARS-CoV-2 infection as a distinct clinical entity, although with slightly different definitions. The National Institute for Health and Care Excellence (NICE) defines as ‘ongoing symptomatic Coronavirus Disease 19 (COVID-19)’ the persistence of symptoms between 4 and 12 weeks, as ‘post COVID-19 syndrome’ the persistence for 12 weeks or longer and as ‘Long-COVID’ any persistence beyond 4 weeks [3]. The United States (US) Department of Health and Human Services (DHHS) and Centers for Disease Control and Prevention (CDC) also define Long-COVID as signs, symptoms, and conditions that continue or develop four weeks or more after the initial phase of infection [4]. Finally, the World Health Organization (WHO) defines as Post COVID-19 condition, commonly known as Long-COVID, the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months [5]. In all the above definitions, Long-COVID is characterised by a wide spectrum of health problems that have no other explanation. This premise, together with the extremely high number of signs, symptoms and conditions attributed to Long-COVID, and the absence of a specific marker for the condition, has led in published studies to a large range of prevalences for both the general Long-COVID condition and the individual symptoms [2, 6–8]. Overall, the spectrum of Long-COVID symptoms remains incompletely defined. With the aim to contribute information on this issue, we implemented a multicentre national study of patients accessing care for Long-COVID in specialised centres, evaluating their clinical characteristics, the profile of symptoms reported, the possible clustering of symptoms and cases, the subjective functional status compared to pre-infection, and the changes occurred in working activity.
Methods
The present clinical study is part of the project “Analysis and strategies for responding to the long-term effects of the COVID-19 infection (Long-COVID)”, funded by the National Centre for Disease Prevention and Control (CCM) of the Italian Ministry of Health, and aimed at monitoring the long-term effects of SARS-CoV-2 infection, increasing knowledge about this condition, and providing recommendations to standardise the approach nationwide [9].
Participation to the study was offered to all the 124 centres for assistance to Long-COVID previously identified in a national survey [10]. The study started in January 2023 and was closed in March 2024, with data extracted on April 2, 2024. The study included patients attending for the first time the clinics during the study and patients already followed, who returned after the study started for a planned follow-up visit after acute infection or for occurrence/persistence of potential Long-COVID symptoms. Consents were collected at the time of visits, after the start of the study. The study design was partly retrospective and partly prospective: symptom collection was retrospective, based on clinical records and patient interview, for patients who had previous visits before January 2023 and taken at the time of visits for the patients without previous visits before the start of the study. Data on demographics, acute infection and comorbidities were taken from clinical records. At the time of study closure, the cohort was 80% retrospective and 20% prospective.
Study data were entered by medical staff at the participating centres using an online dedicated platform. For data collection, a shortened version of the Post COVID-19 Case Report Form (CRF) from the WHO Global Clinical Platform for COVID-19 was used, which included patient demographics, comorbidities, severity and timing of acute COVID, subjective functional status after infection, impact of SARS-CoV-2 infection on working activity, plus 30 different symptoms [11].
Inclusion criteria were age at least 18, a recorded date of acute SARS-CoV-2 infection, and at least one symptom persisting at a clinical evaluation performed at least four weeks after acute infection. The severity of acute SARS-CoV-2 disease was defined as mild, moderate, severe or critical according to the WHO grading [11]. Respiratory assistance was categorised as none, low or high flow oxygen, continuous positive airway pressure (CPAP), mechanical ventilation (MV) and extracorporeal membrane oxygenation (ECMO). The phase of the pandemic was categorised as Pre-Omicron or Omicron according to the occurrence of the date of acute infection before or after December 23, 2021 [12]. The comorbidities considered were neoplastic disease, ischaemic heart disease, heart failure, renal failure, anxiety or depressive disorders, chronic liver disease, respiratory failure, chronic obstructive pulmonary disease, diabetes, hypertension, obesity, autoimmune diseases, asthma, plus a general category of other major conditions, reviewed by two of the authors.
Data were summarised as proportions for categorical variables and as means with standard deviations for quantitative variables. Mean values were compared by Student's T test and proportions by the chi-square test in contingency tables. The impact of multiple covariates on changes in working activity was assessed in a multivariable logistic regression model that adjusted for gender, number of comorbidities and WHO grade of severity of acute SARS-CoV-2 infection (severe/critical vs. mild/moderate). The clustering of symptoms was analysed by hierarchical clustering of variables, considering as variables all 19 symptoms with an observed prevalence of at least 5%, using the nearest neighbour method, binary measures, and simple correspondence algorithm. Several solutions between 5 and 12 clusters were considered, with a final 10-cluster solution selected. Clustering of cases was performed with the two-step automatic clustering method, which considered all symptoms with an observed frequency of at least 5% plus the following clinical and demographic variables: gender, age, number of comorbidities, smoking, phase of the pandemic (Omicron vs. pre-Omicron), WHO grade of severity of acute disease (severe/critical vs. mild/moderate) and level of respiratory assistance during acute SARS-CoV-2 infection (none/low or high-flow oxygen vs. continuous positive airway pressure/mechanical ventilation/extracorporeal membrane oxygenation), with the final number of clusters selected according to the quality of the model provided by the Schwarz Bayesian information criterion. No input was used to substitute missing data. All analyses were performed using the SPSS software, version 27.0 (IBM Corp, 2017, Armonk, NY, US).
Results
Study sample
As of April 2, 2024, data for 1910 patients from 30 clinical centres were entered into the platform. According to the eligibility criteria of the study, the following patients were excluded: unknown date of acute infection (n: 65); evaluated within 4 weeks from acute infection (n: 5); no symptoms reported at clinical evaluation (n: 179); symptoms solved or only sporadically present at clinical evaluation (n: 267) and age <
< 18 (n: 97), for a final sample of 1297 adult patients evaluated at least 4 weeks after acute infection. For most of the patients (78.9%), symptom data were collected retrospectively, based on clinical records and patient interviews. Symptoms were evaluated after a mean interval from acute infection of 224.1 days (women 224.6 days, men 223.6, p
18 (n: 97), for a final sample of 1297 adult patients evaluated at least 4 weeks after acute infection. For most of the patients (78.9%), symptom data were collected retrospectively, based on clinical records and patient interviews. Symptoms were evaluated after a mean interval from acute infection of 224.1 days (women 224.6 days, men 223.6, p =
= 0.935). The specialties of the coordinators of the 30 centres were infectious diseases (n: 10), internal medicine (7), respiratory diseases (5), cardiology (2), paediatrics (2), geriatrics (1), dermatology (1); rehabilitation (1) and hepatology (1).
0.935). The specialties of the coordinators of the 30 centres were infectious diseases (n: 10), internal medicine (7), respiratory diseases (5), cardiology (2), paediatrics (2), geriatrics (1), dermatology (1); rehabilitation (1) and hepatology (1).
The overall characteristics of the population studied and their distribution by gender is reported in Table 1. Women were significantly younger and leaner, and although the number of comorbidities was similar in men and women, their distribution was different, with ischaemic heart disease, respiratory failure and hypertension more common in men, and anxiety, depression and autoimmune diseases more common in women. Women were also more frequently vaccinated, infected during the Omicron phase, and had less severe acute SARS-CoV-2 infection, as expressed by hospitalisations, admissions to intensive care unit, WHO grade of severity of acute SARS-CoV-2 infection, level of respiratory assistance and some support treatments administered during acute infection (Table 1).
Table 1
Population descriptives and differences between women and men in demographics, comorbidities, vaccination status, and characteristics of acute infection
| All | Women | Men | p | |
|---|---|---|---|---|
| Gender: (n, %) | 1297 (100%) | 668 (51.5%) | 629 (48.5%) | |
| Age (years, mean, SD) | 59.6 (13.9) | 58.4 (14.3) | 60.9 (27.3) | 0.001 |
| Body mass index (kg/m2, mean, SD) | 27.0 (5.1) | 26.8 (5.7) | 27.3 (4.3) | 0.045 |
| Current smoking (n, %) (n: 1206) | 122 (10.1) | 65 (10.5) | 57 (9.7) | 0.649 |
| Comorbidities (mean number, SD) | 1.3 (1.4) | 1.3 (1.3) | 1.4 (1.4) | 0.431 |
| Neoplastic disease | 87 (6.7%) | 47 (7.0%) | 40 (6.4%) | 0.626 |
| Ischaemic heart disease | 86 (6.6%) | 35 (5.2%) | 5 (8.1%) | 0.038 |
| Heart failure | 45 (3.5%) | 18 (2.7%) | 27 (4.3%) | 0.116 |
| Renal failure | 57 (4.4%) | 29 (4.3%) | 28 (4.5%) | 0.923 |
| Anxiety | 93 (7.2%) | 60 (9.0%) | 33 (5.2%) | 0.009 |
| Depression | 73 (5.6%) | 48 (7.2%) | 25 (4.0%) | 0.012 |
| Chronic liver disease | 20 (1.5%) | 11 (1.6%) | 9 (1.4%) | 0.753 |
| Respiratory failure or COPD | 85 (6.6%) | 32 (4.8%) | 53 (8.4%) | 0.008 |
| Diabetes | 147 (11.3%) | 70 (10.5%) | 77 (12.2%) | 0.317 |
| Hypertension | 552 (42.6%) | 255 (38.2%) | 297 (47.2%) | < 0.001 0.001 |
| Obesity | 214 (16.5%) | 109 (16.3%) | 105 (16.7%) | 0.855 |
| Autoimmune diseases | 109 (8.4%) | 86 (12.9%) | 23 (3.7%) | < 0.001 0.001 |
| Asthma | 62 (4.8%) | 39 (5.8%) | 33 (3.7%) | 0.066 |
| Other major conditions | 74 (5.7%) | 31 (4.6%) | 43 (6.8%) | 0.088 |
| Vaccinateda(n: 748): | 0.029 | |||
 Before infection Before infection | 213 (28.5%) | 128 (32.7%) | 85 (23.9%) | |
 After infection After infection | 124 (16.6%) | 60 (15.3%) | 64 (18.0%) | |
 Not vaccinated Not vaccinated | 411 (54.9%) | 204 (52.0%) | 207 (58.1%) | |
| Acute infection pandemic phase: | < 0.001 0.001 | |||
 Pre-omicron Pre-omicron | 916 (70.6%) | 438 (65.6%) | 478 (76.0%) | |
 Omicron Omicron | 381 (29.4%) | 230 (34.4%) | 151 (24.0%) | |
 Hospitalised during acute phase: Hospitalised during acute phase: | 787 (63.5%) | 355 (55.8%) | 432 (71.6%) | < 0.001 0.001 |
 Admitted to intensive care unit: Admitted to intensive care unit: | 120 (9.4%) | 38 (5.8%) | 82 (13.2%) | < 0.001 0.001 |
| WHO COVID severity grade: | < 0.001 0.001 | |||
 Mild Mild | 433 (33.4%) | 266 (39.8%) | 167 (26.6%) | |
 Moderate Moderate | 241 (18.6%) | 128 (19.2%) | 113 (18.0%) | |
 Severe Severe | 410 (31.6%) | 187 (28.0%) | 223 (35.5%) | |
 Critical Critical | 165 (12.7%) | 56 (8.4%) | 109 (17.3%) | |
 Unknown Unknown | 48 (3.7%) | 31 (4.6%) | 17 (2.7%) | |
| Respiratory assistance: | < 0.001 0.001 | |||
 None None | 514 (39.6%) | 314 (47.0%) | 200 (31.8%) | |
 Low-flow O2 Low-flow O2 | 266 (20.5%) | 138 (20.7%) | 128 (20.3%) | |
 High-flow O2 High-flow O2 | 117 (9.0%) | 46 (6.9%) | 71 (11.3%) | |
 CPAP CPAP | 226 (17.4%) | 93 (13.9%) | 133 (21.1%) | |
 Mechanical ventilation Mechanical ventilation | 90 (6.9%) | 34 (5.1%) | 56 (8.9%) | |
 ECMO ECMO | 8 (0.6%) | 2 (0.3%) | 6 (1.0%) | |
 Unknown Unknown | 76 (5.9%) | 41 (6.1%) | 35 (5.6%) | |
| Treatments during acute phase: | ||||
 Remdesivir Remdesivir | 236 (19.2%) | 109 (17.2%) | 127 (21.3%) | 0.067 |
 Oral or IV steroids Oral or IV steroids | 654 (53.2%) | 295 (46.9%) | 359 (59.8%) | < 0.001 0.001 |
 Anticoagulants Anticoagulants | 617 (50.1) | 268 (42.5%) | 349 (58.2%) | < 0.001 0.001 |
 Monoclonal antibodies Monoclonal antibodies | 55 (4.5%) | 30 (4.8%) | 25 (4.2%) | 0.627 |
 IL or TK inhibitors IL or TK inhibitors | 102 (8.3%) | 39 (6.2%) | 63 (10.6%) | 0.006 |
SD standard deviation, COPD chronic obstructive pulmonary disease, O2 oxygen, CPAP continuous positive airway pressure, ECMO extracorporeal membrane oxygenation, IV intravenous, IL interleukins, TK tyrosine kinase
aAny dose
Long-COVID symptoms
The mean number of symptoms observed in the entire population was 3.4, significantly higher in women compared to men (3.6 vs. 3.1, p <
< 0.001), but with no differences by pandemic phase (3.4 for Omicron vs. 3.3 for pre-Omicron, p
0.001), but with no differences by pandemic phase (3.4 for Omicron vs. 3.3 for pre-Omicron, p =
= 0.754), WHO grade of severity of acute disease (3.5 for severe or critical vs. 3.4 for mild or moderate, p
0.754), WHO grade of severity of acute disease (3.5 for severe or critical vs. 3.4 for mild or moderate, p =
= 0.723), level of respiratory assistance (3.4 for CPAP, mechanical ventilation or ECMO vs. 3.5 for no assistance or only low/high flow oxygen, p
0.723), level of respiratory assistance (3.4 for CPAP, mechanical ventilation or ECMO vs. 3.5 for no assistance or only low/high flow oxygen, p =
= 0.382), with a trend for a higher number of symptoms in patients admitted to intensive care unit (3.8 vs. 3.3 in patients without admittance, p
0.382), with a trend for a higher number of symptoms in patients admitted to intensive care unit (3.8 vs. 3.3 in patients without admittance, p =
= 0.069). The mean number of symptoms was also higher in individuals below 65 of age compared to those 65 or older (3.6 vs. 3.0, p
0.069). The mean number of symptoms was also higher in individuals below 65 of age compared to those 65 or older (3.6 vs. 3.0, p <
< 0.001).
0.001).
The cumulative higher occurrence of symptoms among women compared to men was confirmed by assessing the prevalence of individual symptoms (Table 2). Many symptoms were significantly more frequent in women: memory loss (23.5% vs. 15.6%, p <
< 0.001), difficult concentration (18.0% vs. 13.4%, p
0.001), difficult concentration (18.0% vs. 13.4%, p =
= 0.023), cough (14.4% vs. 10.3%, p
0.023), cough (14.4% vs. 10.3%, p =
= 0.028), palpitation or tachycardia (13.2% vs. 9.2%, p
0.028), palpitation or tachycardia (13.2% vs. 9.2%, p =
= 0.025), skin disorders or alopecia (13.0% vs. 6.4%, p
0.025), skin disorders or alopecia (13.0% vs. 6.4%, p <
< 0.001), brain fog (9.0% vs. 5.6%, p
0.001), brain fog (9.0% vs. 5.6%, p =
= 0.019), headache (8.5% vs. 4.1%, p
0.019), headache (8.5% vs. 4.1%, p =
= 0.002) and visual disturbances (5.8% vs. 3.3%, p
0.002) and visual disturbances (5.8% vs. 3.3%, p =
= 0.034), with anxiety, pharyngodynia, sleep disturbances and fatigue showing a similar trend but with an association slightly below the level of statistical significance. The only symptom significantly more frequent in men was weight loss (5.2% vs. 3.0% in women, odds ratio (OR) for men: 1.794, 95% confidence interval (CI) 1.018–3.161, p
0.034), with anxiety, pharyngodynia, sleep disturbances and fatigue showing a similar trend but with an association slightly below the level of statistical significance. The only symptom significantly more frequent in men was weight loss (5.2% vs. 3.0% in women, odds ratio (OR) for men: 1.794, 95% confidence interval (CI) 1.018–3.161, p =
= 0.043). The other symptoms showed no major differences between men and women (Table 2).
0.043). The other symptoms showed no major differences between men and women (Table 2).
Table 2
Prevalence of the 30 symptoms studied in the entire population and in the two subgroups of women and men
| All | Women | Men | Odds ratio (95% CI) | p | |
|---|---|---|---|---|---|
| Fatigue | 725 (55.9%) | 389 (58.2%) | 336 (53.4%) | 1.216 (0.976–1.514) | 0.081 |
| Dyspnea | 612 (47.2%) | 309 (46.3%) | 303 (48.2%) | 0.926 (0.745–1.152) | 0.490 |
| Sleep disturbances | 277 (21.4%) | 156 (23.4%) | 121 (19.2%) | 1.279 (0.979–1.671) | 0.071 |
| Memory loss | 255 (19.7%) | 157 (23.5%) | 98 (15.6%) | 1.665 (1.258–2.203) | < 0.001 0.001 |
| Joint pain or swelling | 212 (16.3%) | 120 (18.0%) | 92 (14.6%) | 1.278 (0.950–1.719) | 0.105 |
| Muscle pain | 209 (16.1%) | 118 (17.7%) | 91 (14.5%) | 1.268 (0.941–1.709) | 0.118 |
| Difficult concentration | 204 (15.7%) | 120 (18.0%) | 84 (13.4%) | 1.421 (1.049–1.924) | 0.023 |
| Cough | 161 (12.4%) | 96 (14.4%) | 65 (10.3%) | 1.456 (1.041–2.037) | 0.028 |
| Anxiety | 160 (12.3%) | 94 (14.1%) | 66 (10.5%) | 1.397 (0.999–1.954) | 0.051 |
| Taste reduction | 155 (12.0%) | 78 (11.7%) | 77 (12.2%) | 0.948 (0.678–1.326) | 0.754 |
| Smell reduction | 151 (11.6%) | 75 (11.2%) | 76 (12.1%) | 0.920 (0.655–1.292) | 0.631 |
| Palpitations, tachycardia | 146 (11.3%) | 88 (13.2%) | 58 (9.2%) | 1.494 (1.051–2.122) | 0.025 |
| Depressed mood | 142 (10.9%) | 76 (11.4%) | 66 (10.5%) | 1.095 (0.772–1.553) | 0.610 |
| Skin disorders, alopecia | 127 (9.8%) | 87 (13.0%) | 40 (6.4%) | 2.205 (1.491–3.262) | < 0.001 0.001 |
| Thoracic pain | 122 (9.4%) | 69 (10.3%) | 53 (8.4%) | 1.252 (0.860–1.823) | 0.241 |
| Paresthesia | 113 (8.7%) | 50 (7.5%) | 63 (10.0%) | 0.727 (0.493–1.072) | 0.107 |
| Brain fog | 95 (7.3%) | 60 (9.0%) | 35 (5.6%) | 1.675 (1.087–2.580) | 0.019 |
| Headache | 83 (6.4%) | 57 (8.5%) | 26 (4.1%) | 2.164 (1.342–3.487) | 0.002 |
| Disorders of equilibrium or gait | 71 (5.5%) | 37 (5.5%) | 34 (5.4%) | 1.026 (0.636–1.657) | 0.916 |
| Visual disturbances | 60 (4.6%) | 39 (5.8%) | 21 (3.3%) | 1.795 (1.044–3.087) | 0.034 |
| Weight loss | 53 (4.1%) | 20 (3.0%) | 33 (5.2%) | 0.557 (0.316–0.982) | 0.043 |
| Diarrhoea | 45 (3.5%) | 19 (2.8%) | 26 (4.1%) | 0.679 (0.372–1.239) | 0.207 |
| Hearing disturbances | 41 (3.2%) | 24 (3.6%) | 17 (2.7%) | 1.342 (0.714–2.522) | 0.361 |
| Pharygodynia | 41 (3.2%) | 27 (4.0) | 14 (2.2%) | 1.850 (0.961–3.562) | 0.062 |
| Loss of appetite | 39 (3.0%) | 22 (3.3) | 17 (2.7%) | 1.226 (0.645–2.331) | 0.534 |
| Nausea or vomiting | 27 (2.1%) | 17 (2.5%) | 10 (1.6%) | 1.616 (0.734–3.557) | 0.233 |
| Fever | 19 (1.5%) | 12 (1.8%) | 7 (1.1%) | 1.625 (0.636–4.155) | 0.310 |
| Menstrual disorders | 9 (0.7%) | 9 (1.3%) | |||
| Chilblains | 8 (0.6%) | 2 (0.3%) | 6 (1.0%) | 0.312 (0.063–1.551) | 0.154 |
| Delirium, hallucinations | 3 (0.2%) | 1 (0.1%) | 2 (0.3%) | 0.470 (0.043–5.196) | 0.538 |
Symptoms clusters
Given the observed common occurrence of multiple symptoms in the population studied, we analysed the possible aggregation of symptoms in a clustering analysis, which considered as variables all 19 symptoms with an observed prevalence of at least 5%. The results of the final 10-cluster solution are reported in Fig. 1. Five symptoms did not aggregate with others, representing single-unit clusters (skin disorders/alopecia, cough, sleep disturbances, dyspnea, fatigue). The other fourteen symptoms aggregated in four clusters of two symptoms (smell and taste reduction; anxiety and depressed mood; joint pain or swelling and muscle pain; difficult concentration and memory loss) and in one six-symptom cluster, that included brain fog, equilibrium or gait disturbances, headache, paresthesia, thoracic pain, and palpitations/tachycardia.
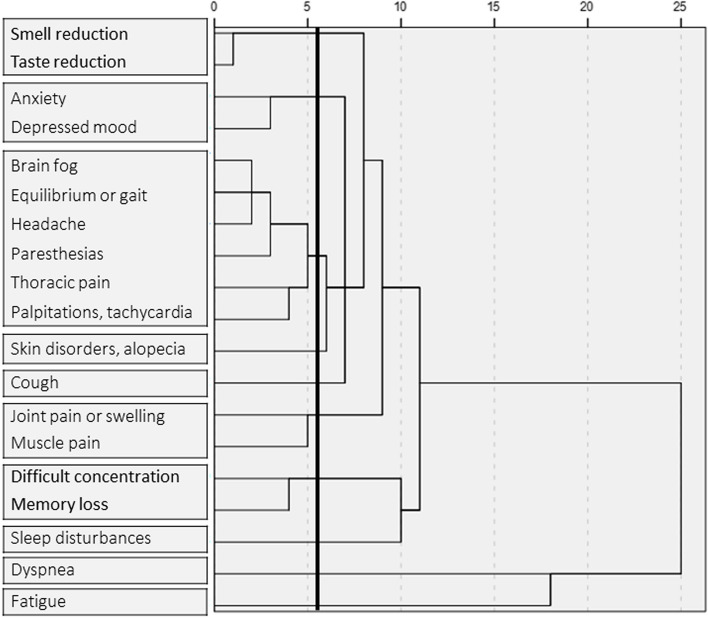
Dendrogram of symptom clustering. In the dendrogram, the horizontal direction (x-axis) represents a measure of the distance between clusters, with longer lines indicating greater distances. Points of clustering are defined by vertical connections between horizontal lines. The bold vertical line indicates the cut-off for the definition of clusters in the model. The branches present at the left of this line represent individual clusters, and lines that do not aggregate before the bold line represent individual variables not clustering with others
Impact on working activity.
Among 579 patients with age under 60, 138 (23.8%) reported reduced or interrupted working activity following COVID-19, with no significant differences by gender (24.3% in females vs. 23.3% in males, p =
= 0.777). In a multivariable regression model that included as independent variables all the 30 symptoms evaluated plus gender, number of comorbidities and severity of acute SARS-CoV-2 infection, six symptoms (headache, dyspnea, difficult concentration, disturbances of equilibrium or gait, visual disturbances and muscular pain) were significantly associated with this occurrence (Table 3).
0.777). In a multivariable regression model that included as independent variables all the 30 symptoms evaluated plus gender, number of comorbidities and severity of acute SARS-CoV-2 infection, six symptoms (headache, dyspnea, difficult concentration, disturbances of equilibrium or gait, visual disturbances and muscular pain) were significantly associated with this occurrence (Table 3).
Table 3
Symptoms significantly associated with reduced or interrupted working activity in multivariable analysis
| Adjusted odds ratio | 95% CI | p | |
|---|---|---|---|
| Headache | 3.295 | 1.57–6.91 | 0.002 |
| Dyspnea | 1.735 | 1.09–2.76 | 0.020 |
| Difficult concentration | 2.103 | 1.06–4.16 | 0.033 |
| Disturbances of equilibrium or gait | 2.887 | 1.06–7.84 | 0.037 |
| Visual disturbances | 2.943 | 1.06–8.16 | 0.038 |
| Muscular pain | 1.814 | 1.02–3.21 | 0.041 |
Multivariable logistic regression model based on 555 cases with age <
< 60 years (86.6%). Independent variables were all the 30 study symptoms, gender, number of comorbidities and severity of acute SARS-CoV-2 disease
60 years (86.6%). Independent variables were all the 30 study symptoms, gender, number of comorbidities and severity of acute SARS-CoV-2 disease
CI confidence interval
Case clustering
Clustering of cases was performed with the aim to define possible subpopulations with different characteristics and distinct symptom profiles. The analysis considered all symptoms with an observed frequency of at least 5% plus ten additional clinical and demographic variables. Two clusters were defined (Table 4). Individuals in cluster 2 were older, more frequently males, had a higher number of comorbidities, were more commonly infected prior to the Omicron phase, and had a more severe acute SARS-CoV-2 infection, as shown by WHO severity grade, hospitalisations, admissions to intensive care unit, and level of respiratory assistance. The symptoms profile of the two clusters also showed some differences, with dyspnea and paresthesia more common in cluster 2 and headache, difficult concentration and tachycardia/palpitations more common in cluster 1 (Table 4).
Table 4
Case clustering in two groups according to demographics, clinical variables and symptoms reported
| Cluster 1 | Cluster 2 | p | |
|---|---|---|---|
| N (all: 1154) | 840 | 314 | |
| Age (years, mean, SD) | 57.5 (14.2) | 64.6 (11.1) | < 0.001 0.001 |
| Women (n, %) | 474 (56.5%) | 123 (39.2%) | < 0.001 0.001 |
| Omicron phase of the pandemic (n, %) | 301 (35.8%) | 30 (9.6%) | < 0.001 0.001 |
| Current smoking (n, %) | 97 (11.5%) | 20 (6.4%) | 0.009 |
| Comorbidities (mean number, SD) | 1.2 (1.2) | 1.9 (1.6) | < 0.001 0.001 |
| Acute infection severe or critical (n, %) | 228 (27.1%) | 313 (99.7%) | < 0.001 0.001 |
| CPAP, mechanical ventilation or ECMO (n, %) | 0 (0%) | 311 (99.0%) | < 0.001 0.001 |
| Hospitalised during acute phase (n, %) | 414 (49.3%) | 314 (100%) | < 0.001 0.001 |
| Admitted to intensive care unit (n, %) | 6 (0.7%) | 110 (35.0%) | < 0.001 0.001 |
| Fatigue | 496 (59.0%) | 169 (53.8%) | 0.110 |
| Dyspnea | 383 (45.6%) | 187 (59.6%) | < 0.001 0.001 |
| Sleep disturbances | 193 (23.0%) | 66 (21.0%) | 0.478 |
| Memory loss | 170 (20.2%) | 66 (21.0%) | 0.770 |
| Joint pain or swelling | 145 (17.3%) | 55 (17.5%) | 0.919 |
| Muscle pain | 148 (17.6%) | 47 (15.0%) | 0.285 |
| Difficult concentration | 149 (17.7%) | 40 (12.7%) | 0.041 |
| Cough | 120 (14.3%) | 36 (11.5%) | 0.212 |
| Anxiety | 114 (13.6%) | 35 (11.1%) | 0.274 |
| Taste reduction | 107 (12.7%) | 31 (9.9%) | 0.182 |
| Smell reduction | 104 (12.4%) | 32 (10.2%) | 0.305 |
| Palpitations, tachycardia | 106 (12.6%) | 27 (8.6%) | 0.057 |
| Depressed mood | 90 (10.7%) | 43 (13.7%) | 0.158 |
| Skin disorders, alopecia | 57 (6.8%) | 29 (9.2%) | 0.158 |
| Thoracic pain | 90 (10.7%) | 25 (8.0%) | 0.165 |
| Paresthesia | 64 (7.6%) | 45 (14.3%) | < 0.001 0.001 |
| Brain fog | 72 (8.6%) | 19 (6.1%) | 0.157 |
| Headache | 68 (8.1%) | 12 (3.8%) | 0.011 |
| Disorders of equilibrium or gait | 52 (6.2%) | 16 (5.1%) | 0.482 |
CPAP continuous positive airway pressure, ECMO extracorporeal membrane oxygenation
No significant differences were observed between the two clusters in terms of reduction/interruption of working activity (20.8% in both clusters, p =
= 0.982), and of mean number of symptoms reported (3.5 in cluster 1 vs. 3.3 in cluster 2, p
0.982), and of mean number of symptoms reported (3.5 in cluster 1 vs. 3.3 in cluster 2, p =
= 0.130). However, the proportion of individuals that reported a worsened subjective functional status compared to pre-infection was significantly higher in cluster 1 (60.7% vs. 47.1%, p
0.130). However, the proportion of individuals that reported a worsened subjective functional status compared to pre-infection was significantly higher in cluster 1 (60.7% vs. 47.1%, p <
< 0.001).
0.001).
Discussion
The present study characterised a multicentre cohort of more than one thousand patients evaluated for Long-COVID symptoms in specialised centres. Such characterisation defined demographic and clinical characteristics, timing and severity of acute infection, a detailed profile of persisting symptoms, and their impact on working capacity.
A first finding of the study was represented by significant differences between male and female patients. Women were usually younger, more commonly infected during the Omicron phase, more frequently vaccinated, and had, compared to men, a similar number of comorbidities but with a different clinical profile, characterised by the more common presence of anxiety, depression and autoimmune disorders, while men had a higher prevalence of cardiovascular and respiratory comorbidities. Women also had a significantly milder acute SARS-CoV-2 infection, as consistently expressed by hospitalisations and admissions to the intensive care unit, WHO grade of severity of acute SARS-CoV-2 infection, level of respiratory assistance and support treatments. Despite such better clinical characteristics and history, and despite being more commonly infected by the recent Omicron variants, which are usually considered clinically less virulent [13], women showed a higher cumulative number of symptoms and a higher prevalence of several individual symptoms, that included memory loss, difficult concentration, cough, heart palpitations or tachycardia, dermatological disorders, brain fog, headache and visual disturbances, with a higher, although not significantly, occurrence also of anxiety, pharyngodynia, sleep disturbances and fatigue.
A higher number of symptoms in women compared to men had already been described in COVID-19 survivors with previous hospitalisation [14]. Our observation extends these findings to a larger population that includes roughly one third of previously non-hospitalised patients. This indirectly confirms the heavier clinical burden of Long-COVID in women already described by several authors [15–17], and adds information on the symptom profiles, highlighting important gender differences.
An interesting finding of our study is the lack of correlation between the number of symptoms reported and the number of comorbidities, the severity of disease, advanced age and early phase of the pandemic, which were commonly reported as risk factors for Long-COVID insurgence or persistence [15, 17, 18]. The cumulative number of symptoms was actually higher in younger individuals, and among the markers of severity of acute SARS-CoV-2 disease, only admission to the intensive care unit showed an association of borderline statistical significance with higher symptom burden. Among published studies that have addressed this issue, some reported more symptoms in patients without previous hospitalisation compared to those who were hospitalised [19], and others, consistent with our findings, found that gender, time since acute COVID infection, and its severity did not affect subjective status or symptoms [20]. These findings suggest that Long-COVID is a heterogeneous disease, with variable manifestations that do not necessarily rely on worse baseline conditions or worse severity of acute disease [21–23].
Fatigue and dyspnea were the most commonly reported symptoms, affecting roughly half of the cases, with no major differences between men and women. Other studies have reported fatigue [18, 24, 25] or dyspnea/respiratory symptoms [26, 27] as the most common and relevant symptoms in patients with Long-COVID. In patients with Long-COVID their presence was associated with a significantly lower quality of life [28], and in our study dyspnea was associated with a reduced or interrupted working activity. Dyspnea may be particularly common in previously hospitalised patients [19, 26], a finding which is consistent with our cluster analysis, which showed dyspnea to be more frequent in the cluster with more severe acute SARS-CoV-2 disease. Fatigue, in its most severe form, is the main symptom of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME), a debilitating condition which may preclude working and even common daily activities and which is increasingly considered as a possible severe manifestation triggered by SARS-CoV-2, as already described for other viral infections [7]. In our study, fatigue was not associated in a multivariable analysis with reduction or interruption of working activity, but we did not collect the severity grade of symptoms, and the fatigue reported included both mild and severe forms that may have a very different impact on functional status.
In our study, 23.8% of patients with age below 60 reported a reduction or interruption of working activity, matching the reported proportion of 23% of patients unable to return to work after hospitalisation needing intensive care [29]. The symptoms independently associated with reduction or interruption of working activity were headache (also a significant cause of absence from work in the study by Kenny et al. [19]), dyspnea, difficult concentration, disturbances of equilibrium or gait, visual disturbances and muscular pain. This combination of neurological, muscular, respiratory and ocular symptoms suggests that a large spectrum of symptoms affects working capacity.
The clustering of symptoms showed some associations that could be further analysed in order to explore possible distinct pathogenetic pathways. The final associations that we observed were actually well separated in different clinical compartments that included sensorial loss (taste, smell), multidistrict pain (muscular, articular), cognitive dysfunction (memory, concentration), mood changes (anxiety, depression), and a complex of symptoms that might involve sensorial, neurological and autonomic disturbances (brain fog, equilibrium and gait, headache, paresthesia, thoracic pain, palpitations/tachycardia).
The results of the second cluster analysis, based on aggregation of cases according to clinical characteristics and symptomatology, are also potentially relevant for further clinical research. Two distinct subpopulations were identified. The first was characterised by younger age, prevalence of females, more recent infection (Omicron phase), lower number of comorbidities and less severe acute SARS-CoV-2 infection, with almost no admissions in intensive care and no need for intensive respiratory assistance (CPAP, mechanical ventilation or ECMO). The second had older age, prevalence of males, more baseline comorbidities, and a significantly more severe course of acute SARS-CoV-2 infection, which more often occurred during the early (pre-Omicron) phases of the pandemic. Such differences were accompanied by some important differences in symptoms reported, with dyspnea dominating the symptom profile in the subpopulation with severe acute disease, headache, difficult concentration and palpitations/tachycardia more frequent in the population with less severe acute disease. The similar mean number of symptoms and the similar rate of reduction or interruption of working activity in the two clusters indirectly suggest similar severity of Long-COVID in the two groups. Actually, the proportion of individuals reporting worsened subjective functional status compared to pre-infection was significantly higher in the cluster with milder acute disease and less pre-existing comorbidities. The above findings, in our opinion, do not rule out the reported link between greater severity of acute disease, particularly respiratory, and subsequent negative Long-COVID outcomes, because the risk of dyspnea was actually higher in patients with more severe acute disease. This might indicate that an earlier subpopulation was progressively sided by a more recent one, predominantly represented by women, younger and in better health when contracting COVID. Our findings indicate that this subpopulation, despite a milder acute disease, is nonetheless affected by multiple symptoms, that less commonly include dyspnea, but are equally accompanied by worsened subjective functional status and frequent impact on working activity.
Other studies have analysed cluster distribution in Long-COVID, with heterogeneous results. In some studies, clusters were based on both severity and quality of symptoms, in others on the systems affected, and often correlated with patient demographics, pre-existing conditions, severity of acute disease, or impact on functional and working status [19, 30, 31]. Gentilotti et al. identified four clinical phenotypes with a clinically meaningful and comprehensive distribution of symptoms, also showing that these distinct clinical phenotypes have different impacts on quality of life [25]. Although a direct comparison of these studies is difficult given the different populations studied, the heterogeneous techniques used for clustering, and the variable number and composition of the clusters, all the above indicate consistently that there are different phenotypes of Long-COVID, that are likely to be caused by distinct mechanisms. Our findings also suggest that the strains responsible for acute COVID-19 might play a role, as also suggested by others [17, 24].
In terms of study limitations, the selected population of symptomatic patients did not allow any estimate of the prevalence of Long-COVID. This remains a problematic issue in the absence of disease markers. Our study did not contribute to this issue because did not include assessment of biomarkers. We also did not evaluate the possible impact of social determinants of health, such as economic, occupational and psychosocial factors, that are recognised as important cofactors affecting the risk of Long-COVID [22] and that might also have affected disease severity, vaccination attitudes and access to care. We also, focusing on patients evaluated in specialised centres, may have selected more severe cases. Our data also do not allow any conclusion about the possible role of vaccination in preventing Long-COVID, analysed by some authors, with variable results [18, 32]. In our study, the proportion of vaccinated individuals, particularly before infection, was low. Although this might suggest indirectly a protective effect of vaccination in preventing Long-COVID, it has also to be considered that the study included a subgroup of patients who developed COVID-19 between February and December 2020, when no vaccine for SARS-CoV-2 was available. Finally, as already discussed above for fatigue and CFS/ME, we did not assess the severity of symptoms, and we were only able to evaluate it indirectly through self-reported functional status and impact on working activity. As for other studies based on large samples, some small but statistically significant differences may not necessarily have clinical relevance, and a clinical interpretation of data is always necessary. Most of the symptom data were collected retrospectively, and the potential for information or recall bias should also be considered.
The strengths of the present study were the evaluation of a relatively large number of symptoms, accompanied by a comprehensive evaluation of demographic and clinical characteristics, which allowed to adjust some analysis for baseline factors and to better define distinct subpopulations. We also used for symptom collection a form specifically designed and developed by WHO. Our study also assessed both clustering of symptoms and clustering of cases, providing hints for future research. The first analysis allowed to identify different clusters of symptoms possibly associated with distinct pathogenetic pathways, and the second indicated two possible distinct subpopulations characterized not only by different symptom profiles, but also by different demographics, baseline characteristics, and timing and severity of acute infection. Finally, our study was conducted in multiple centres in different regions, with a variety of specialty clinics and settings of care, potentially reducing the selection bias that commonly affects single-centre, locally circumscribed studies [8].
Conclusions
The findings provide further evidence that Long-COVID is a heterogeneous disease with manifestations that differ by sex, phase of the pandemic and severity of acute disease. Future research should explore whether multiple clinical pathways ultimately lead to the different manifestations observed.
Acknowledgements
We thank Fabio Galati, IT services, Istituto Superiore di Sanità, Rome, Italy, for implementing the online platform used for data collection and for data management and extraction; Tiziana Grisetti and Katia Salomone, Istituto Superiore di Sanità, Rome, Italy, for administrative support; Cosimo Polizzi, Istituto Superiore di Sanità, Rome, Italy, for data management during all the study.
The ISS Long-Covid Study Group:
Graziano Onder, Marco Floridia, Marina Giuliano, Tiziana Grisetti, Flavia Pricci, Tiziana Grassi, Dorina Tiple, Marika Villa, Liliana Elena Weimer, Cosimo Polizzi, Fabio Galati (Istituto Superiore di Sanità, Rome, Italy); Maria Rosa Ciardi, Patrizia Pasculli (Dipartimento di Sanità Pubblica e Malattie Infettive, Università La Sapienza, Roma); Piergiuseppe Agostoni, Francesca Colazzo, Irene Mattavelli, Elisabetta Salvioni (Centro Cardiologico Fondazione Monzino, Milano e Dipartimento di Scienze Cliniche e di Comunità, Università degli Studi di Milano); Paolo Palange, Daniela Pellegrino, Marco Bezzio, Federica Olmati, Arianna Sanna, Arianna Schifano, Dario Angelone, Antonio Fabozzi (Dipartimento di Sanità Pubblica e Malattie Infettive, Università La Sapienza, Roma); Patrizia Rovere Querini, Simona Santoro, Anna Fumagalli, Aurora Merolla, Valentina Canti, Maria Pia Ruggiero, Marco Messina, Marina Biganzoli (U.O. Medicina Generale ad Indirizzo Specialistico e della Continuità Assistenziale, IRCCS Ospedale S. Raffaele, Milano); Danilo Buonsenso (Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma); Silvia Zucco, Alice Ianniello (ASL Città di Torino, Ospedale Amedeo di Savoia, Torino); Matteo Tosato, Vincenzo Galluzzo, Laura Macculi (Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma); Aldo Lo Forte, Valeria Maria Bottaro (USL Toscana Centro, Ospedale S. Giovanni di Dio Torregalli, Firenze); Paolo Bonfanti, Luca Bonaffini, Anna Spolti, Nicola Squillace (Unità di Malattie Infettive, Fondazione IRCCS San Gerardo dei Tintori, Monza); Donato Lacedonia, Terence Campanino (AOU OORR Foggia Pneumologia, Dipartimento di Scienze Mediche e Chirurgiche, Università di Foggia); Emanuela Barisione, Teresita Aloè, Elena Tagliabue (IRCCS Ospedale Policlinico San Martino, Genova); Stefano Figliozzi, Federica Testerini (Ospedale di Ricerca IRCCS Humanitas, Rozzano); Paola Andreozzi, Marzia Miglionico, Antonia Barbitta, Chiara Cenciarelli (Unità di Medicina Predittiva, Azienda Ospedaliero Universitaria Policlinico Umberto I and Dipartimento di Medicina Interna, Scienze Endocrino-Metaboliche e Malattie Infettive Università La Sapienza, Roma); Gianluca Pagnanelli, Giuseppe Piccinni (Istituto Dermopatico dell'Immacolata—IDI IRCCS—Roma); Paola Gnerre, Lionello Parodi, Eugenia Monaco, Sandra Buscaglia, Antonella Visconti (ASL Liguria 2, Medicina Interna, Ospedale S. Paolo, Savona); Kwelusukila Loso (Ospedale di Manduria, ASL TA); Giuseppe Pio Martino, Giuseppina Bitti, Laura Postacchini, Antonella Cognigni (U.O.C. Medicina Interna, Ospedale “A. Murri”, Fermo); Maria Antonietta di Rosolini, Sergio Mavilla (UOSD di Malattie Infettive, PO Giovanni Paolo II, Ragusa); Domenico Maurizio Toraldo (Polo Riabilitativo, Plesso S. Cesario, Ospedale di Lecce); Guido Vagheggini, Giulio Bardi, Giuseppa Levantino (Azienda USL Toscana Nord Ovest); Cristina Stefan (IRCCS Eugenio Medea, Polo Scientifico Veneteo, Pieve di Soligo); Gianfranco Parati, Elisa Perger, Enrico Gianfranceschi, Francesca Pozzoli (IRCCS Istituto Auxologico Italiano, Università di Milano Bicocca, Milano); Pasqualina De Leo, Sara Grignolo (ASL Liguria 2, Struttura Complessa Malattie Infettive, Ospedale S. Paolo, Savona); Caterina Monari (AOU Unicam Vanvitelli, Napoli); Leila Bianchi, Luisa Galli (Azienda Ospedaliero-Universitaria Meyer, Firenze); Lorenzo Surace, Elisabetta Falbo (Centro Medicina del Viaggiatore e delle Migrazioni, Dipartimento di Prevenzione PO Lamezia Terme, ASP Catanzaro); Silvia Boni (S.C. Malattie Infettive, EO Ospedali Galliera, Genova); Claudia Battello (Azienda Sanitaria Universitaria Friuli Centrale, Medicina Latisana, PO Palmanova,); Caterina Baghiris (SC Pneumologia ASL Friuli Occidentale, Pordenone); Gaetano Serviddio (UOC di Epatologia, AOU OO RR, Foggia).
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 19 |
| US | United States |
| DHHS | Department of Health and Human Services |
| CDC | Centers for Disease Control and Prevention |
| WHO | World Health Organization |
| CCM | National Centre for Disease Prevention and Control |
| CRF | Case Report Form |
| CPAP | Continuous positive airway pressure |
| MV | Mechanical ventilation |
| ECMO | Extracorporeal membrane oxygenation |
| OR | Odds ratio |
| CI | Confidence interval |
| CFS/ME | Chronic fatigue syndrome/myalgic encephalomyelitis |
| SD | Standard deviation |
| COPD | Chronic obstructive pulmonary disease |
| O2 | Oxygen |
| IV | Intravenous |
| IL | Interleukins |
| TK | Tyrosine kinase |
Authors’ contributions
MF was responsible for data acquisition, directly accessed and verified with MG the underlying data reported in the manuscript, and performed the analyses. MF drafted the manuscript. Full data were available to all authors. MF, MG and GO contributed to the conception and design of the study. CD contributed to the cluster analyses. LEW, MRC, PA1, PP, PRQ, SZ, MT, ALF, PB, DL, EB, SF, PA2, CD and FP contributed to the data acquisition. All authors contributed to interpretation of results and review of the manuscript for intellectual content. All authors read and approved the final manuscript and accepted responsibility to submit for publication.
Funding
The present study is part of the project “Analysis and strategies of response to the long-term effects of COVID-19 infection (Long-COVID)” funded by the National Center for Disease Prevention and Control of the Italian Ministry of Health in 2021 (Grant I85F21003410005) and approved by the Ethics Committee of the ISS (ref. PRE BIO CE 01.00 0015066, 2022). The study sponsor had no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Data availability
The study data can be made available upon reasonable request. Ethics Committee consultation may be necessary in order to obtain permission to share. Requests to access the datasets should be directed to [email protected].
Declarations
The Italian National Ethics Committee approved the project (AOO-ISS—19/04/2022–0015066 Class: PRE BIO CE 01.00). Written informed consent was required for patient inclusion, using a patient information and consent form also approved by the Italian National Ethics Committee.
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
the I. S. S. Long-COVID Study Group:
Marco Floridia, Email: [email protected].
References
Articles from BMC Medicine are provided here courtesy of BMC
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/170424547

 1
1 

