Abstract
Background
Long-distance road transportation is a common practice in the beef industry, frequently resulting in bovine respiratory disease (BRD) and compromised growth performance. However, a comprehensive investigation integrating clinical performance, physiological conditions, and nasopharyngeal microflora remains lacking.Methods
This study aimed to evaluate the respiratory health and immunometabolic status of 54 beef calves subjected to a 3000-km journey. The respiratory health of calves was monitored over 60 days post-arrival using a modified clinical scoring system. Nasopharyngeal microflora and venous blood samples were collected at 3 time points: before transportation (A), 30 days post-arrival (B), and 60 days post-arrival (C), for 16S rRNA microbiomics, whole-blood transcriptomics, serum metabolomics, and laboratory assays.Result
Within the first week post-arrival, the appetite and mental scores of calves dropped to zero, while other respiratory-related scores progressively declined over the 60 days. The α-diversity of nasopharyngeal microflora in calves was similar at time points A and B, both significantly higher than at time point C. The structure of these microbial communities varied significantly across different time points, with a notably higher relative abundance of BRD-related genera, such as Pasteurella and Mannheimia, detected at time point A compared to B and C. The composition and gene expression profiles of circulating blood cells at time point A were significantly different from those at B and C. Specifically, higher expression levels of oxidative- and inflammatory-related genes, cytokines, and enzymes were observed at time point A compared to B and C. Higher levels of catabolism-related metabolites and enzymes were detected at time point A, while higher levels of anabolism-related metabolites and enzymes were observed at time points B and C. Additionally, significant correlations were found among microorganisms, genes, and metabolites with differing abundances, expression levels, and concentrations across time points. Stronger correlations were observed between calves' performance and nasopharyngeal microflora and immunometabolic status at time point A compared to B or C.Conclusions
Collectively, these results confirm that 3000 km of road transportation significantly alters the composition and gene expression profiles of circulating white blood cells in calves, affects their metabolic processes, disrupts the balance of the respiratory microbial community, and leads to pronounced respiratory symptoms that persist for at least 60 days. During this period, the influenced composition and gene expression of circulating blood cells, metabolic processes, and nasopharyngeal microbial community gradually return to equilibrium, and the respiratory symptoms gradually diminish. This observational research indicates that transportation induces BRD in calves by disrupting the homeostasis of their immune function, metabolic processes, and nasopharyngeal microbial community. However, these results and their underlying molecular mechanisms warrant further validation through well-designed in vivo and in vitro confirmatory experiments with larger sample size. Video Abstract.Free full text

Multi-omics investigation into long-distance road transportation effects on respiratory health and immunometabolic responses in calves
Abstract
Background
Long-distance road transportation is a common practice in the beef industry, frequently resulting in bovine respiratory disease (BRD) and compromised growth performance. However, a comprehensive investigation integrating clinical performance, physiological conditions, and nasopharyngeal microflora remains lacking.
Methods
This study aimed to evaluate the respiratory health and immunometabolic status of 54 beef calves subjected to a 3000-km journey. The respiratory health of calves was monitored over 60 days post-arrival using a modified clinical scoring system. Nasopharyngeal microflora and venous blood samples were collected at 3 time points: before transportation (A), 30 days post-arrival (B), and 60 days post-arrival (C), for 16S rRNA microbiomics, whole-blood transcriptomics, serum metabolomics, and laboratory assays.
Result
Within the first week post-arrival, the appetite and mental scores of calves dropped to zero, while other respiratory-related scores progressively declined over the 60 days. The α-diversity of nasopharyngeal microflora in calves was similar at time points A and B, both significantly higher than at time point C. The structure of these microbial communities varied significantly across different time points, with a notably higher relative abundance of BRD-related genera, such as Pasteurella and Mannheimia, detected at time point A compared to B and C. The composition and gene expression profiles of circulating blood cells at time point A were significantly different from those at B and C. Specifically, higher expression levels of oxidative- and inflammatory-related genes, cytokines, and enzymes were observed at time point A compared to B and C. Higher levels of catabolism-related metabolites and enzymes were detected at time point A, while higher levels of anabolism-related metabolites and enzymes were observed at time points B and C. Additionally, significant correlations were found among microorganisms, genes, and metabolites with differing abundances, expression levels, and concentrations across time points. Stronger correlations were observed between calves’ performance and nasopharyngeal microflora and immunometabolic status at time point A compared to B or C.
Conclusions
Collectively, these results confirm that 3000 km of road transportation significantly alters the composition and gene expression profiles of circulating white blood cells in calves, affects their metabolic processes, disrupts the balance of the respiratory microbial community, and leads to pronounced respiratory symptoms that persist for at least 60 days. During this period, the influenced composition and gene expression of circulating blood cells, metabolic processes, and nasopharyngeal microbial community gradually return to equilibrium, and the respiratory symptoms gradually diminish. This observational research indicates that transportation induces BRD in calves by disrupting the homeostasis of their immune function, metabolic processes, and nasopharyngeal microbial community. However, these results and their underlying molecular mechanisms warrant further validation through well-designed in vivo and in vitro confirmatory experiments with larger sample size.
Video Abstract(145M, mp4)
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-024-01962-2.
Introduction
Road transportation is a ubiquitous practice in the beef and dairy cattle industry worldwide, with both short- and long-term effects on calf health, welfare, and productivity [1–3]. In China, for example, beef calves from the north often need to be transported thousands of kilometers across provinces to the south for fattening, usually called long-distance transportation [4–6]. Stressors encountered during transport, such as varied handling techniques, exposure to new environments [7], commingling with unfamiliar calves [8], and food and water deprivation [9], can compromise the immune system [10, 11]. This immunosuppression often leads to an increased incidence of morbidity and mortality and can stunt the growth of calves [12]. For example, Scott et al. observed that up to 90% of calves experienced diarrhea or bovine respiratory disease (BRD) in the weeks following their arrival [13], Renaud et al. documented a 42% mortality rate within the first 3 weeks post-arrival [14], and Cole et al. observed inhibited growth performance in feeder steers for 28 days after transport [15]. Transportation is widely recognized as a key factor that contributes to the development and progression of BRD, which is a major concern when managing young calves [11, 16].
A variety of indicators are employed to gauge the impact of transportation on calves, including but not limited to behavioral signs, hematological variables, and physiological responses. Clinical scoring systems have been developed to objectively evaluate the respiratory health of calves by monitoring performance such as rectal temperature, mental status, respiration rates, nasopharyngeal discharge, and food intake [17, 18]. For example, Devon et al. used a clinical scoring system from the University of Wisconsin-Madison to assess the respiratory health of 640 transported calves [19]. Numerous blood parameters like albumin (ALB), aspartate aminotransferase (AST), bilirubin (BIL), and C-reactive protein (CRP) have been measured to assess the effects of transportation on calves [20]. Chibisa et al. noted an increase in haptoglobin concentration and changes in the amino acid profile in Jersey calves post-transport [21].
The rapid advancement in high-throughput sequencing technology and the associated decrease in costs have made multi-omics analyses increasingly popular for investigating physiological and pathological alterations in cattle. For instance, Li et al. investigated the genetic background of BRD infection in feedlot crossbred cattle using a multi-omics analysis involving genomics, transcriptomics, and metabolomics [22, 23]. These methods have recently been applied to study the impact of transportation on calves. Li et al., for instance, examined the development of the rumen microbiome in transported calves during the first 30 days post-arrival [24]. Alterations in the nasopharyngeal microbiome, commonly associated with BRD [25], are frequently considered indicators of respiratory health status in transported calves [6, 26]. Blood transcriptome and serum metabolome analyses have also been sporadically employed in studies on calf transportation [5, 27].
However, there remains a noticeable gap in research regarding a comprehensive assessment of behavioral, physiological, hematological, and multi-omics changes in calves following transportation. Moreover, existing studies have primarily focused on short-term effects within the first-month post-transport on calves by examining a limited set of indicators. The long-term effects of transportation beyond this period are still unclear. Therefore, our investigation aims to evaluate the multifaceted effect of long-distance road transportation on calves during the first 60 days post-arrival. This includes clinical assessments, routine blood tests (RBT), hematological responses, nasopharyngeal microbiome variations, whole blood transcriptome alterations, and serum small molecular metabolome changes. Our results will provide insights into the extended and comprehensive consequences of road transportation on calf respiratory health and contribute to enhancing post-transport care and management practices.
Materials and methods
Management of animals
On October 1, 2022, a total of 54 male Simmental crossbred beef calves, approximately 8 months old and in excellent health, vaccinated against foot-and-mouth disease and nodular skin disease, were purchased from individual farmers in Yitong County, Siping City, Jilin Province, China. For the initial 3 days (October 2–4), these calves were housed together in an 80 ×
× 10 m2 pen. A veterinarian employed by the facility regularly checked their health during these 3 days, and no calf exhibited any visible signs of illness. Twice daily, at 8:30 and 16:30, the calves received adequate hay, concentrated feed, and water. During this commingling process, all these calves were labeled with ear tags numbered from 1 to 54, and 10 of them were randomly selected for subsequent sample collection procedures. During this period, all calves were orally given an immune booster prepared by the facility.
10 m2 pen. A veterinarian employed by the facility regularly checked their health during these 3 days, and no calf exhibited any visible signs of illness. Twice daily, at 8:30 and 16:30, the calves received adequate hay, concentrated feed, and water. During this commingling process, all these calves were labeled with ear tags numbered from 1 to 54, and 10 of them were randomly selected for subsequent sample collection procedures. During this period, all calves were orally given an immune booster prepared by the facility.
On the morning of October 5, after being fed, the calves were loaded onto a spacious truck with a hay-covered floor and an enclosed roof. The truck then departed for the facility, a journey of about 3000 km that took roughly 40 h, concluding on the morning of October 7. Throughout the trip, the calves had no access to food or water. Upon arrival, they were unloaded into another 80 ×
× 10 m enclosure and tethered at 2-m intervals. Each calf was then given 3 L of water boiled with 0.15 kg of brown sugar and 0.09 kg of ginger (BSG water), but no food was offered for the following 6 h. In the following 6 days, known as the adaptive feeding period, the calves were fed a total mixed ration (TMR) including forage, silage, and hay (Supplementary Table 1), which exceeded the nutrient requirements set by the National Research Council [28]. The calves were provided with normal water and TMR (leaving 5–10% leftovers) twice daily following the 3 L of BSG water at 8:30 and 16:30. After this adaptive feeding period, the calves had free access to ample regular water and TMR twice daily at the same times. The enclosure was disinfected and sterilized weekly, and all calves were dewormed on the 15th day post-arrival. It is important to note that the researchers did not participate in deciding or administering any of these procedures, which were carried out by the facility’s staff according to their standard regulations. The experimental design of this study is illustrated in Fig. 1. Considering the limitations in budget and workload, we chose to sample 10 calves for further omics and laboratory analysis without conducting a power analysis.
10 m enclosure and tethered at 2-m intervals. Each calf was then given 3 L of water boiled with 0.15 kg of brown sugar and 0.09 kg of ginger (BSG water), but no food was offered for the following 6 h. In the following 6 days, known as the adaptive feeding period, the calves were fed a total mixed ration (TMR) including forage, silage, and hay (Supplementary Table 1), which exceeded the nutrient requirements set by the National Research Council [28]. The calves were provided with normal water and TMR (leaving 5–10% leftovers) twice daily following the 3 L of BSG water at 8:30 and 16:30. After this adaptive feeding period, the calves had free access to ample regular water and TMR twice daily at the same times. The enclosure was disinfected and sterilized weekly, and all calves were dewormed on the 15th day post-arrival. It is important to note that the researchers did not participate in deciding or administering any of these procedures, which were carried out by the facility’s staff according to their standard regulations. The experimental design of this study is illustrated in Fig. 1. Considering the limitations in budget and workload, we chose to sample 10 calves for further omics and laboratory analysis without conducting a power analysis.
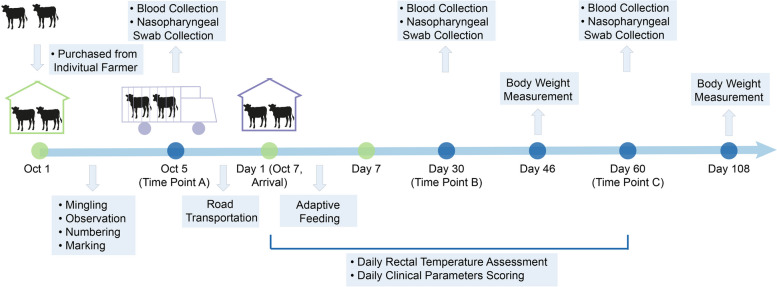
Overview of experimental design. This study involved 54 Simmental crossbred beef calves purchased from individual farmers. Initially, the calves were co-housed for 3 days to allow mingling, during which 10 calves were randomly selected and marked. Subsequently, they underwent a 40-h highway transit, followed by a 6-day acclimation period in the facility. Over the 60 days after their arrival, their rectal temperatures and respiratory-related scores were evaluated twice daily. Body weights were assessed on days 46 and 108 post-arrival. Venous blood and nasopharyngeal swab samples were collected from the 10 marked calves at designated time points A, B, and C for high-throughput sequencing and subsequent bioinformatic analyses. The green dot represents the procedure conducted by staff and the blue dot represents the procedure conducted by researchers
Environmental factors measurement
To assess the potential influence of ambient temperature, air relative humidity, and temperature and humidity index (THI), known to have significant direct or indirect effects on calves [29], we monitored these variables within the enclosure. Over 60 days post-arrival, ambient temperatures (T, °C) and air relative humidities (RH, %) were recorded twice daily at 8:00 and 16:00 using a Deli 9010 in-outdoor thermo-hygrometer from Deli Group (Beijing, China). Moreover, we calculated the THI at each time point using the formula: THI =
= (1.8
(1.8 ×
× T
T +
+ 32)
32) −
− (0.55
(0.55 −
− 0.0055
0.0055 ×
× RH)
RH) ×
× (1.8
(1.8 ×
× T
T −
− 26) [30], which provides a composite measure of environmental stress levels experienced by the calves.
26) [30], which provides a composite measure of environmental stress levels experienced by the calves.
Rectal temperature and body weight measurements
Rectal temperatures (°C), a widely recognized metric for assessing the health status of calves [31, 32], were measured in all calves twice daily at 7:00 and 15:00. This monitoring was carried out for 30 days following their arrival, using a BT-A21G soft-head electronic thermometer from Fudakang Industry (Guangdong, China). Additionally, we measured the body weight of each calf on the 46th and 108th days post-arrival using an A12E-1 T weighbridge from Shanghai Henggang Instrument (Shanghai, China).
Respiratory health assessment
To evaluate and quantify the respiratory health status of calves, we established a clinical scoring system tailored for local beef cattle. We adhered to specific criteria outlined in Supplementary Table 2 to evaluate mental, respiration, appetite, ocular and ear, and nasal discharge status in all calves over the 60th days following their arrival. A single, qualified veterinarian conducted these evaluations to ensure consistency in scoring.
Nasopharyngeal microflora 16S rRNA gene sequencing and analysis
Microflora from the nasopharyngeal mucosa of the 10 marked calves was collected using 20-cm sterile deep nasopharyngeal swabs (Merlin Technology; Tianjin, China) at 3 time points: at 7:00 on the day of departure (A), the 30th day post-arrival (B), and the 60th day post-arrival (C). The swabs were immediately stored in dry ice and sent to Novogene Biotech (Beijing, China) for 16S rRNA microbiome sequencing within 3 days. Genomic DNA was extracted from the nasopharyngeal swabs using a T3010S Monarch® Genomic DNA Purification Kit (New England Biolabs; Ipswich, MA, USA), according to the provided manual. The integrity and concentration of the extracted DNA were verified via electrophoresis on a 1% agarose gel and measured with a NanoDrop ND-2000 spectrophotometer (Thermo Fisher; Waltham, MA, USA), respectively. Thirty complementary DNA libraries were then constructed and sequenced on an Illumina NovaSeq 6000 platform. Polymerase chain reaction (PCR) was employed to amplify the V3-V4 hypervariable regions of the 16S rRNA gene from each sample, employing primers 341F: 5′-CCTAYGGGRBGCASCAG-3′ and 806R: 5′-GGACTACNNGGGTATCTAAT-3′. The sequences were processed using FLASH software (v1.2.7; http://ccb.jhu.edu/software/FLASH/index.shtml) [33] and QIIME software (v1.9.1; http://qiime.org/scripts/split_libraries_fastq.html) [34] to remove unwanted barcode and primer sequences, merge reads, and filter raw tags. Clean reads were clustered into operational taxonomic units (OTUs) at 97% identity, with the most frequent OTU sequence designated as the representative for each OTU. For diversity analysis, we normalized the data and calculated α- and β-diversity indices using QIIME software. The Linear Discriminant Analysis (LDA) Effect Size (LEfSe) algorithm was used to identify differences in the relative abundance of taxa at different classification levels across multiple time points (LDA scores ≥
≥ 4). Additionally, unweighted Unifrac-based principal coordinate analysis (PCoA) and analysis of similarity (Anosim) [35] were performed using the ade4 and vegan packages [36, 37] in R software (v2.15.3; https://cran.r-project.org/). Finally, the predicted functions of the OTUs were inferred using the PICRUSt method [38].
4). Additionally, unweighted Unifrac-based principal coordinate analysis (PCoA) and analysis of similarity (Anosim) [35] were performed using the ade4 and vegan packages [36, 37] in R software (v2.15.3; https://cran.r-project.org/). Finally, the predicted functions of the OTUs were inferred using the PICRUSt method [38].
Venous blood sampling and processing
A total of 15 mL of venous blood was drawn from the right jugular vein of each of the 10 marked calves concurrently with the collection of nasopharyngeal swabs at time points A, B, and C. Approximately 0.5 mL of the blood sample was immediately transferred to an ethylenediaminetetraacetic acid (EDTA) anticoagulant tube for RBTs, which were performed using a PE-6800VET automatic animal blood cell analyzer from Pukang Electronic (Shenzhen, China). Simultaneously, 2 mL of the blood sample was equally distributed into 2 cryopreservation tubes (5 mL), each prefilled with 3 mL of ET111-01 Trizol Reagent (Transgen; Beijing, China), and then vigorously shaken. These tubes, containing the whole blood samples, were promptly stored in dry ice and dispatched to Novogene Biotech for transcriptome sequencing. The remaining blood sample was placed into 3 non-anticoagulant vacuum blood collection tubes and left undisturbed at room temperature for at least 3 h, followed by centrifugation at 1500 ×
× g for 10 min to separate the serum. Afterward, 300 μL of the serum was stored in dry ice and sent to Novogene Biotech for untargeted metabolome sequencing using liquid chromatography-mass spectrometry (LC–MS). The rest of the serum was preserved at
g for 10 min to separate the serum. Afterward, 300 μL of the serum was stored in dry ice and sent to Novogene Biotech for untargeted metabolome sequencing using liquid chromatography-mass spectrometry (LC–MS). The rest of the serum was preserved at −
− 80 °C for subsequent laboratory research.
80 °C for subsequent laboratory research.
Whole blood transcriptome sequencing and analysis
Concisely, total RNA was extracted from the white blood cells of each sample using ET111-01 Trizol Reagent, following the conventional phenol/chloroform phase separation method. The RNA concentration and integrity were then quantified using a NanoDrop ND-2000 spectrophotometer and an Agilent Bioanalyzer 2100 (Agilent Technologies; Santa Clara, CA, USA). Only samples with an RNA integrity number (RIN) exceeding 0.8 were selected for subsequent sequencing. Complementary DNA libraries were constructed using an Illumina TruSeq RNA Sample Prep Kit (Illumina; San Diego, CA, USA), resulting in an average fragment size of 150 bp, excluding adaptors. The libraries’ quality and integrity were assessed using an Agilent 2100 Bioanalyzer and an ABI StepOnePlus Real-Time PCR System (Thermo Fisher Scientific; Waltham, MA, USA). Results indicated that all library samples presented a narrow distribution with a peak size of around 275 bp and maintained an effective concentration of no less than 1.5 nM, thus meeting the criteria for library selection. Alignment of RNA-Seq FASTQ files with the bovine genome was performed using the Hisat2 algorithm [39], with reference data from the Ensembl Bovine Genome Database (http://ftp.ensembl.org/pub/release-105/fasta/bos_taurus/). The Cufflinks tool [40] processed the resulting binary alignment/map (BAM) format output files to evaluate transcript abundance and identify potential mRNA isoforms. Additionally, StringTie (v1.3.3b) [41] was used to assemble the mapped reads from each sample using a reference-based strategy. Transcript expression levels were quantified in terms of fragments per kilobase million (FPKM), and principal component analysis (PCA) was executed based on these FPKM values. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the differentially expressed genes (DEGs) was performed using DESeq2 software [42] and the ClusterProfiler R package [43]. Benjamin-Hochberg’s method was employed to control the false discovery rate.
Untargeted LC–MS and metabolomic analysis
The initiation of the LC–MS procedure began with the resuspension of 100 μL of serum, which was vortexed in 400 μL of pre-chilled 80% methanol and 0.1% formic acid. The mixture was then incubated on ice for 5 min and subsequently subjected to centrifugation at 15,000 ×
× g for 20 min at 4 °C. The supernatant obtained was diluted to a final methanol concentration of 53% using LC–MS grade water and centrifuged once more under the same conditions. Following this, the supernatant was introduced into an LC–MS/MS system comprising a Vanquish UHPLC system (Thermo Fisher Scientific; Waltham, MA, USA) coupled with an Orbitrap Q Exactive™ HF-X mass spectrometer (Thermo Fisher Scientific; Waltham, MA, USA) for analysis. The raw data obtained from the UHPLC-MS/MS were processed using Compound Discoverer 3.1 (CD3.1; Thermo Fisher; Waltham, MA, USA) software, which facilitated peak alignment, peak selection, and quantification of each metabolite. Subsequently, the metabolites were annotated within the KEGG database (https://www.genome.jp/kegg/pathway.html) and the Lipid Maps database (http://www.lipidmaps.org/) using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/). The metaX software was used for the analysis of differentially expressed metabolites (DE Metas), and the functions of metabolites and metabolic pathways were analyzed using MetaboAnalyst 5.0.
g for 20 min at 4 °C. The supernatant obtained was diluted to a final methanol concentration of 53% using LC–MS grade water and centrifuged once more under the same conditions. Following this, the supernatant was introduced into an LC–MS/MS system comprising a Vanquish UHPLC system (Thermo Fisher Scientific; Waltham, MA, USA) coupled with an Orbitrap Q Exactive™ HF-X mass spectrometer (Thermo Fisher Scientific; Waltham, MA, USA) for analysis. The raw data obtained from the UHPLC-MS/MS were processed using Compound Discoverer 3.1 (CD3.1; Thermo Fisher; Waltham, MA, USA) software, which facilitated peak alignment, peak selection, and quantification of each metabolite. Subsequently, the metabolites were annotated within the KEGG database (https://www.genome.jp/kegg/pathway.html) and the Lipid Maps database (http://www.lipidmaps.org/) using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/). The metaX software was used for the analysis of differentially expressed metabolites (DE Metas), and the functions of metabolites and metabolic pathways were analyzed using MetaboAnalyst 5.0.
Laboratory examination of serum
In addition to field observation and omics analysis, we measured various hematological indicators using a Hitachi 7180 automatic biochemical analyzer from Olympus Corporation (Tokyo, Japan) and enzyme-linked immunosorbent assay (ELISA) kits sourced from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Specifically, we assessed liver function indicators, including ALB, globulin (GLB), total protein (TP), the ALB-to-GLB ratio (A/G), alanine aminotransferase (ALT), AST, the ALT to AST ratio (ALT/AST), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), indirect BIL (IBIL), direct BIL (DBIL), and total BIL (TBIL). Renal function indicators, including blood urea nitrogen (BUN) and creatinine (CRE); glucose/lipid metabolism indicators, including total cholesterol (TC), glucose (GLU), triglycerides (TG), very low-density lipoprotein cholesterol (VLDL-C), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C); as well as myocardial enzyme activities, including lactate dehydrogenase (LDH) and creatine kinase (CK), were measured using the Hitachi 7180 automatic biochemical analyzer.
Immunological indicators, including immunoglobulin G (IgG; Cat No: # H106-1–2), IgM (Cat No: # H109-1–2), and IgA (Cat No: # H108-1–2); inflammatory-related cytokines, including tumor necrosis factor-α (TNF-α; Cat No: # H052-1–2), interferon-γ (IFN-γ; Cat No: # H025-1–2), interleukin-2 (IL-2; Cat No: # H003-1–2), IL-6 (Cat No: # H007-1–2), IL-8 (Cat No: # H008-1–2), and IL-10 (Cat No: # H009-1–2); stress-related indicators, including heat shock protein 70 kDa (HSP70; Cat No: # H264-2–2), CRP (Cat No: # H126-1–2), and serum amyloid A (SAA; Cat No: # H134); oxidative damage-related indicators, including malondialdehyde (MDA; Cat No: # A003-1–2), nitric oxide (NO; Cat No: # A012-1–2), hydroxyl radical (·OH; Cat No: # A018-1–1), superoxide dismutase (SOD; Cat No: # A001-3–2), catalase (CAT; Cat No: # A007-1–1), glutathione peroxidase (GSH-Px; Cat No: # A005-1–2), total antioxidant capacity (T-AOC; Cat No: # A015-2–1), and nitric oxide synthase (NOS; Cat No: # A014-2–2); as well as serum hormone levels, including adrenocorticotropic hormone (ACTH; Cat No: # H097-1–2), growth hormone (GH; Cat No: # H091-1–2), antidiuretic hormone (ADH; Cat No: # H396-1), and cortisol (COR; Cat No: # H094-1–2), were examined using corresponding ELISA kits. Additionally, the activities of glucose metabolism-related enzymes, including glucose-6-phosphate dehydrogenase (G-6-PDH; Cat No: # A027-1–1), phosphofructose kinase (PFK; Cat No: # H244), and pyruvate kinase (PK; Cat No: # A076-1–1); as well as lipid metabolism serum indicators, including non-esterified fatty acid (NEFA; Cat No: # A042-2–1), acetyl CoA carboxylase (ACC; Cat No: # H232-1–2), and fatty acid synthetase (FAS; Cat No: # H231-1–2), were also measured using corresponding ELISA kits.
Statistical analysis
Data are presented as outcomes (average means/least square means) ±
± standard error means (SEM) and analyzed using SPSS 26 software (IBM; Armonk, NYC, USA) and Novomagic (http://magic.novogene.com) unless otherwise stated. Field data, including THI, rectal temperature, and clinical scores, were analyzed using a generalized linear mixed model (GLMM). In this model, THI, rectal temperature, and score were set as the targets, with “T” (temperature), “RH” (relative humidity), “THI” (temperature-humidity index), “Time” (morning or afternoon), and “RT” (rectal temperature, for clinical scores) as fixed factors, and “Day” as a repeated measurement. Calves were treated as a random effect. Factors with a p value
standard error means (SEM) and analyzed using SPSS 26 software (IBM; Armonk, NYC, USA) and Novomagic (http://magic.novogene.com) unless otherwise stated. Field data, including THI, rectal temperature, and clinical scores, were analyzed using a generalized linear mixed model (GLMM). In this model, THI, rectal temperature, and score were set as the targets, with “T” (temperature), “RH” (relative humidity), “THI” (temperature-humidity index), “Time” (morning or afternoon), and “RT” (rectal temperature, for clinical scores) as fixed factors, and “Day” as a repeated measurement. Calves were treated as a random effect. Factors with a p value <
< 0.05 were determined to have significant effects on the target outcomes. Differences in RBT and serum indicators among different time points were determined using the Student t-test or one-way analysis of variance (ANOVA) for parametric data, and the Mann–Whitney U test or Kruskal–Wallis rank sum test for nonparametric data. A p value
0.05 were determined to have significant effects on the target outcomes. Differences in RBT and serum indicators among different time points were determined using the Student t-test or one-way analysis of variance (ANOVA) for parametric data, and the Mann–Whitney U test or Kruskal–Wallis rank sum test for nonparametric data. A p value <
< 0.05 was considered statistically significant for both field and serum data. In microbiome analysis, classification levels were considered to have significantly different relative abundances between different time points when p
0.05 was considered statistically significant for both field and serum data. In microbiome analysis, classification levels were considered to have significantly different relative abundances between different time points when p <
< 0.05. In transcriptome analysis, genes were recognized as differentially expressed when an adjusted p value
0.05. In transcriptome analysis, genes were recognized as differentially expressed when an adjusted p value ≤
≤ 0.05 and |log2(FoldChange)|≥
0.05 and |log2(FoldChange)|≥ 1, and KEGG pathways were identified as significantly enriched by DEGs when an adjusted p value
1, and KEGG pathways were identified as significantly enriched by DEGs when an adjusted p value <
< 0.05. In metabolism analysis, metabolites were identified as DE Metas when variable importance in the projection (VIP)
0.05. In metabolism analysis, metabolites were identified as DE Metas when variable importance in the projection (VIP) >
> 1, p
1, p <
< 0.05, and |log2(FoldChange)
0.05, and |log2(FoldChange) ≥
≥ 1|. Graphs were generated using Origin 2024 (Originlab; Northampton, MA, USA) and Adobe Illustrator (Adobe Systems Incorporated; San Jose, CA, USA).
1|. Graphs were generated using Origin 2024 (Originlab; Northampton, MA, USA) and Adobe Illustrator (Adobe Systems Incorporated; San Jose, CA, USA).
Results
Within the first month after arrival, 2 experimental calves, identified as No. 17 and 45, passed away. Since these calves were not among the 10 pre-selected for sample collection, their field data were excluded from further analysis.
We observed significant fluctuations in environmental temperatures. Specifically, the average afternoon temperature (19.167 ±
± 0.321 °C) was notably higher (p
0.321 °C) was notably higher (p <
< 0.001) than the average morning temperature (15.067
0.001) than the average morning temperature (15.067 ±
± 0.321 °C), with a significant downward trend over time (p
0.321 °C), with a significant downward trend over time (p <
< 0.001) (Fig. 2A). In contrast, relative humidity levels were significantly lower (p
0.001) (Fig. 2A). In contrast, relative humidity levels were significantly lower (p <
< 0.001) in the afternoon (50.717
0.001) in the afternoon (50.717 ±
± 1.047%) compared to the morning (68.350
1.047%) compared to the morning (68.350 ±
± 1.047%), but these levels did not show significant changes across different dates (p
1.047%), but these levels did not show significant changes across different dates (p =
= 0.113) (Fig. 2B). The THI levels in the afternoon (35.763
0.113) (Fig. 2B). The THI levels in the afternoon (35.763 ±
± 0.599) were significantly greater (p
0.599) were significantly greater (p <
< 0.001) than those in the morning (28.153
0.001) than those in the morning (28.153 ±
± 0.599), and similarly exhibited a decreasing pattern over the dates (p
0.599), and similarly exhibited a decreasing pattern over the dates (p <
< 0.001) (Fig. 2C).
0.001) (Fig. 2C).
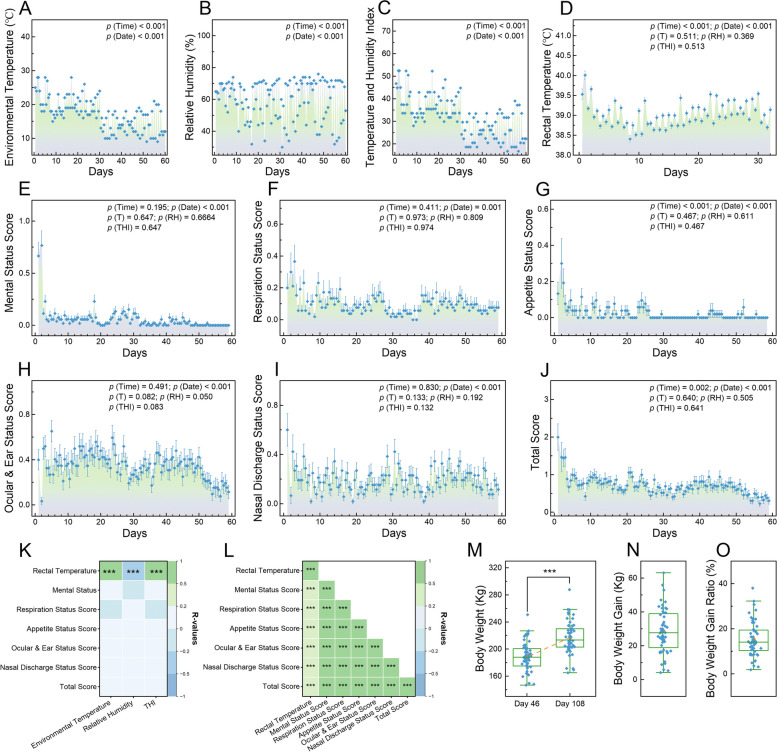
Assessing post-arrival respiratory health and growth performance in calves. Dot plots depicting the variations in environmental temperature (A), relative humidity level (B), and THI level (C) within the enclosure over the 60 days following the calves’ arrival. D Dot plot showing the bi-daily rectal temperature measurements for the calves during the first 30 days post-arrival. E–J Dot plots illustrating changes in scores for the 5 respiratory health scores and their summation (total score), assessed bi-daily over 60 days post-arrival using our established criteria. K Heatmap of Spearman’s rank correlations between environmental factors and average health-related indicators for the 52 calves at each time point. L Heatmap of Spearman’s correlations among respiratory health-related scores, compiling data across all points for all calves. M Boxplot of calf body weights on days 46 and 108 post-arrival. Boxplots depicting the increase in calf body weights from day 46 to day 108 (N) and these increases are expressed as percentages to their initial body weights (O). In A–J, a GLMM was used to analyze the data. In K and L, asterisks indicate significance levels of relevance (***, p <
< 0.001), with pane color denoting the strength and direction of relevance (green for positive, blue for negative). In M–O, the yellow dot denotes average values. In M, a paired Student t-test was used to assess differences between 2 days (***, p
0.001), with pane color denoting the strength and direction of relevance (green for positive, blue for negative). In M–O, the yellow dot denotes average values. In M, a paired Student t-test was used to assess differences between 2 days (***, p <
< 0.001). GLMM, generalized linear mixed model; THI, temperature and humidity index
0.001). GLMM, generalized linear mixed model; THI, temperature and humidity index
Assessing respiratory health and growth performance in calves post-arrival
During the initial 30-day period following their arrival, calves exhibited significant fluctuations in rectal temperature across different days (p <
< 0.001). The afternoon rectal temperatures (39.2
0.001). The afternoon rectal temperatures (39.2 ±
± 0.017 °C) were significantly higher (p
0.017 °C) were significantly higher (p <
< 0.001) than the morning temperatures (38.847
0.001) than the morning temperatures (38.847 ±
± 0.017 °C), and this difference was independent of environmental temperature, relative humidity, or THI levels (p
0.017 °C), and this difference was independent of environmental temperature, relative humidity, or THI levels (p ≥
≥ 0.574) (Fig. 2D). In the subsequent 60-day period post-arrival, significant differences were observed across different dates in the 5 assessed respiratory-related scores and their aggregate (p
0.574) (Fig. 2D). In the subsequent 60-day period post-arrival, significant differences were observed across different dates in the 5 assessed respiratory-related scores and their aggregate (p ≤
≤ 0.001; Fig. 2E–J). The afternoon appetite status score (0.012
0.001; Fig. 2E–J). The afternoon appetite status score (0.012 ±
± 0.004) and the total score (0.853
0.004) and the total score (0.853 ±
± 0.103) were markedly lower (p
0.103) were markedly lower (p ≤
≤ 0.002) compared to the morning scores (0.041
0.002) compared to the morning scores (0.041 ±
± 0.005 and 0.923
0.005 and 0.923 ±
± 0.141, respectively), while the other 4 scores remained consistent between afternoon and morning (p
0.141, respectively), while the other 4 scores remained consistent between afternoon and morning (p ≥
≥ 0.195) (Fig. 2E–J). The ocular and ear status scores showed a tendency to be influenced by environmental temperature (p
0.195) (Fig. 2E–J). The ocular and ear status scores showed a tendency to be influenced by environmental temperature (p =
= 0.082), relative humidity (p
0.082), relative humidity (p =
= 0.050), and THI levels (p
0.050), and THI levels (p =
= 0.083). However, none of the other 4 scores or the total score was affected by these factors (p
0.083). However, none of the other 4 scores or the total score was affected by these factors (p ≥
≥ 0.132) (Fig. 2E–J). All scores and the total score were significantly affected by rectal temperature (p
0.132) (Fig. 2E–J). All scores and the total score were significantly affected by rectal temperature (p ≤
≤ 0.016), except for the respiration status score (p
0.016), except for the respiration status score (p =
= 0.284) (Fig. 2E–J). Spearman’s rank correlation analysis revealed no correlations between the environmental factors and clinical scores (p
0.284) (Fig. 2E–J). Spearman’s rank correlation analysis revealed no correlations between the environmental factors and clinical scores (p >
> 0.05; Fig. 2K). However, significant positive correlations were found among the respiratory health-related indicators (p
0.05; Fig. 2K). However, significant positive correlations were found among the respiratory health-related indicators (p <
< 0.001, 0.2
0.001, 0.2 <
< R
R <
< 1), including both rectal temperature and clinical scores (Fig. 2L).
1), including both rectal temperature and clinical scores (Fig. 2L).
On the 46th day post-arrival, the average body weight of the calves was recorded at 188.56 ±
± 2.92 kg, which was significantly less (p
2.92 kg, which was significantly less (p <
< 0.001) than their weight of 217.04
0.001) than their weight of 217.04 ±
± 3.38 kg on the 108th day post-arrival (Fig. 2M). The weight increase from day 46 to day 108 amounted to an average of 28.48
3.38 kg on the 108th day post-arrival (Fig. 2M). The weight increase from day 46 to day 108 amounted to an average of 28.48 ±
± 1.83 kg per calf (Fig. 2N), representing a growth of 15.34
1.83 kg per calf (Fig. 2N), representing a growth of 15.34 ±
± 1.08% from their initial body weights (Fig. 2O). We also evaluated the relationship between the body weight gain ratio from day 46 to day 108 post-arrival and other health indicators, including rectal temperature and respiratory-related scores. The results demonstrated a trend toward a negative correlation between the body weight gain ratio and the ocular and ear status score (p
1.08% from their initial body weights (Fig. 2O). We also evaluated the relationship between the body weight gain ratio from day 46 to day 108 post-arrival and other health indicators, including rectal temperature and respiratory-related scores. The results demonstrated a trend toward a negative correlation between the body weight gain ratio and the ocular and ear status score (p =
= 0.07,
0.07, −
− 0.5
0.5 <
< R
R < −
< − 0.2). Meanwhile, rectal temperature and the other examined scores did not significantly impact the body-weight gain ratio (p
0.2). Meanwhile, rectal temperature and the other examined scores did not significantly impact the body-weight gain ratio (p >
> 0.100).
0.100).
Profiling and analyzing the nasopharyngeal microbiota of calves
A total of 594,066,796 bases and 2,592,602 paired-end raw reads were generated from nasopharyngeal swabs collected at time points A, B, and C, averaging 86,420 ±
± 4029 paired-end reads per sample (Supplementary Table 3). After data refinement, 2,359,423 effective tags were produced and clustered into 13,363 OUTs at a 97% sequence similarity threshold (Supplementary Table 4). Specifically, the analysis revealed 8939 OTUs at time point A, 5203 OTUs at time point B, and 6163 OTUs at time point C, with 2648 OTUs shared across all 3 time points (Fig. 3A). Rarefaction analysis indicated a plateau at approximately 70,000 sequences per sample, suggesting sufficient sequencing depth to characterize the nasopharyngeal microbiota (Supplementary Fig. 1A). At time point A, the average species count was 2954
4029 paired-end reads per sample (Supplementary Table 3). After data refinement, 2,359,423 effective tags were produced and clustered into 13,363 OUTs at a 97% sequence similarity threshold (Supplementary Table 4). Specifically, the analysis revealed 8939 OTUs at time point A, 5203 OTUs at time point B, and 6163 OTUs at time point C, with 2648 OTUs shared across all 3 time points (Fig. 3A). Rarefaction analysis indicated a plateau at approximately 70,000 sequences per sample, suggesting sufficient sequencing depth to characterize the nasopharyngeal microbiota (Supplementary Fig. 1A). At time point A, the average species count was 2954 ±
± 238.32 per sample, significantly higher than the 2132.90
238.32 per sample, significantly higher than the 2132.90 ±
± 65.80 species at time point B (p
65.80 species at time point B (p =
= 0.003) and the 1848.80
0.003) and the 1848.80 ±
± 201.11 species at time point C (p
201.11 species at time point C (p =
= 0.012). The species counts at time points B and C did not differ significantly (p
0.012). The species counts at time points B and C did not differ significantly (p =
= 0.238) (Supplementary Fig. 1B). The Shannon diversity index for samples from time point A (7.43
0.238) (Supplementary Fig. 1B). The Shannon diversity index for samples from time point A (7.43 ±
± 0.54) did not significantly differ from time point B (6.95
0.54) did not significantly differ from time point B (6.95 ±
± 0.30; p
0.30; p =
= 0.197) but was significantly higher compared to time point C (3.72
0.197) but was significantly higher compared to time point C (3.72 ±
± 0.50; p
0.50; p <
< 0.001) (Fig. 3B). The Simpson diversity index followed similar trends to the Shannon index (Supplementary Fig. 1C). Spearman’s rank correlation analysis revealed 12 significant negative correlations (p
0.001) (Fig. 3B). The Simpson diversity index followed similar trends to the Shannon index (Supplementary Fig. 1C). Spearman’s rank correlation analysis revealed 12 significant negative correlations (p <
< 0.05,
0.05, −
− 1
1 <
< R
R < −
< − 0.2) and 2 negative correlation trends (p
0.2) and 2 negative correlation trends (p <
< 0.1,
0.1, −
− 1
1 <
< R
R < −
< − 0.5) between species numbers at time points A, B, and C, and respiratory health indicators as well as body weight gain ratios in the 10 marked calves (Fig. 3C). PCoA and Anosim demonstrated significant differences in the β diversity of nasopharyngeal microbiota among the 3 time points (p
0.5) between species numbers at time points A, B, and C, and respiratory health indicators as well as body weight gain ratios in the 10 marked calves (Fig. 3C). PCoA and Anosim demonstrated significant differences in the β diversity of nasopharyngeal microbiota among the 3 time points (p <
< 0.001; Fig. 3D).
0.001; Fig. 3D).
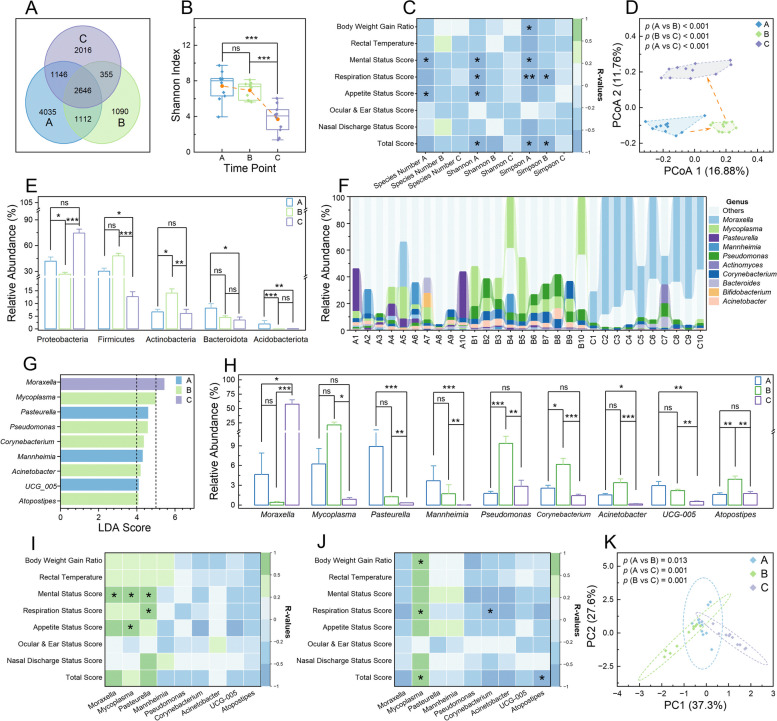
Analysis of nasopharyngeal microbial communities in calves. A Venn diagram representing shared and unique operational taxonomic units (OTUs) among different time points. B Boxplot illustrating the Shannon diversity indices within the microbial communities of each sample group. C Heatmap depicting Spearman’s rank correlations between microbiota indices and respiratory health and growth performance across sample groups. D Principal coordinate analysis plot based on unweighted Unifrac distances, showing the variance in nasopharyngeal microbial communities among the sample. Bar charts displaying the relative abundances of microbial phyla (E) and genera (H) with Linear Discriminant Analysis (LDA) scores exceeding 4 for each sample group. F Percentage bar chart showing the relative abundances of the 10 most abundant genera in each sample group. G Bar chart representing the identified genera with LDA scores over 4. Heatmaps of Spearman’s rank correlations between the 9 genera with high LDA scores (> 4) in time points A (I) and B (J), correlated with respiratory and growth metrics. K Principal component analysis plot of enriched KEGG pathways across microbial communities in each sample group. In B, the yellow dot denotes average values, and a one-way analysis of variance was used to assess differences among groups (ns, non-significant; ***, p
4) in time points A (I) and B (J), correlated with respiratory and growth metrics. K Principal component analysis plot of enriched KEGG pathways across microbial communities in each sample group. In B, the yellow dot denotes average values, and a one-way analysis of variance was used to assess differences among groups (ns, non-significant; ***, p <
< 0.001). In E and H, data are expressed as outcomes
0.001). In E and H, data are expressed as outcomes ±
± standard error means, and a Kruskal–Wallis rank sum test was employed to examine differences among groups (ns, non-significant; *, p
standard error means, and a Kruskal–Wallis rank sum test was employed to examine differences among groups (ns, non-significant; *, p <
< 0.05; **, p
0.05; **, p <
< 0.01; ***, p
0.01; ***, p <
< 0.001). In C, I, and J, correlations were calculated using clinical data from the 10 marked calves; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p
0.001). In C, I, and J, correlations were calculated using clinical data from the 10 marked calves; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p <
< 0.05; **, p
0.05; **, p <
< 0.01). In D and K, the analysis of similarities algorithm was employed to analyze the differences among groups
0.01). In D and K, the analysis of similarities algorithm was employed to analyze the differences among groups
A total of 1346 genera across 78 phyla were annotated from the obtained OTUs (Supplementary Table 5). At the phylum level, the 5 most abundant phyla were Proteobacteria, Firmicutes, Actinobacteria, Bacteroidota, and Acidobacteriota (Supplementary Fig. 1D). LEfSe analysis identified 21 phyla with significant relative abundances at different time points (p <
< 0.05) (Supplementary Table 6), and 5 of these phyla exhibited large effect sizes with LDA scores exceeding 4 (Supplementary Fig. 1E and Fig. 3E). At the genus level, the 10 most prevalent genera included Moraxella, Mycoplasma, Pasteurella, Mannheimia, Pseudomonas, Actinomyces, Corynebacterium, Bacteroides, Bifidobacterium, and Acinetobacter (Fig. 3F). LEfSe analysis revealed 170 genera with significant differences in relative abundance over time points (p
0.05) (Supplementary Table 6), and 5 of these phyla exhibited large effect sizes with LDA scores exceeding 4 (Supplementary Fig. 1E and Fig. 3E). At the genus level, the 10 most prevalent genera included Moraxella, Mycoplasma, Pasteurella, Mannheimia, Pseudomonas, Actinomyces, Corynebacterium, Bacteroides, Bifidobacterium, and Acinetobacter (Fig. 3F). LEfSe analysis revealed 170 genera with significant differences in relative abundance over time points (p <
< 0.05) (Supplementary Table 6). Among these, Mycoplasma, Mannheimia, Pseudomonas, Acinetobacter, Pasteurella, Moraxella, UCG_005, Atopostipes, and Corynebacterium showed LDA scores above 4 (Fig. 3G and H). Spearman’s rank correlation analysis demonstrated significant positive correlations between certain genera, including Moraxella, Mycoplasma, as well as Pasteurella, and respiratory health-related indicators at time points A and B (p
0.05) (Supplementary Table 6). Among these, Mycoplasma, Mannheimia, Pseudomonas, Acinetobacter, Pasteurella, Moraxella, UCG_005, Atopostipes, and Corynebacterium showed LDA scores above 4 (Fig. 3G and H). Spearman’s rank correlation analysis demonstrated significant positive correlations between certain genera, including Moraxella, Mycoplasma, as well as Pasteurella, and respiratory health-related indicators at time points A and B (p <
< 0.05, 0.2
0.05, 0.2 <
< R
R <
< 1) (Fig. 3I and J). However, similar correlations were absent at time point C (Supplementary Fig. 1F). Additionally, a total of 327 KEGG pathways were enriched in the genes annotated from the obtained OTUs (Supplementary Table 7 and Supplementary Fig. 1G). PCA and Anosim demonstrated that the functional profile of the nasopharyngeal microbial community underwent significant alterations across different time points (p
1) (Fig. 3I and J). However, similar correlations were absent at time point C (Supplementary Fig. 1F). Additionally, a total of 327 KEGG pathways were enriched in the genes annotated from the obtained OTUs (Supplementary Table 7 and Supplementary Fig. 1G). PCA and Anosim demonstrated that the functional profile of the nasopharyngeal microbial community underwent significant alterations across different time points (p ≤
≤ 0.013) (Fig. 3K). At last, a heatmap analysis highlighted the variability in the top 20 functions across the 30 samples (Supplementary Fig. 1H).
0.013) (Fig. 3K). At last, a heatmap analysis highlighted the variability in the top 20 functions across the 30 samples (Supplementary Fig. 1H).
Measurement and analysis of whole blood transcriptomes and immune response of calves
The transcriptome sequencing process generated a total of 207.98 Gbp of raw data and 201.45 Gbp of clean data, with an average of 6.93 ±
± 0.39 Gbp raw bases and 6.72
0.39 Gbp raw bases and 6.72 ±
± 0.35 Gbp clean bases per sample. After thorough quality control measures, a total of 1,342,968,544 clean reads (150 bp) were produced, averaging 44,765,618.13
0.35 Gbp clean bases per sample. After thorough quality control measures, a total of 1,342,968,544 clean reads (150 bp) were produced, averaging 44,765,618.13 ±
± 2,355,600.62 reads per sample (Supplementary Table 8). Following the assembly process, we annotated and quantified a total of 24,816 genes across the 30 samples (Supplementary Table 9). Of these, 20,539 genes were expressed at all 3 time points (Fig. 4A). PCA and Anosim demonstrated significant differences (p
2,355,600.62 reads per sample (Supplementary Table 8). Following the assembly process, we annotated and quantified a total of 24,816 genes across the 30 samples (Supplementary Table 9). Of these, 20,539 genes were expressed at all 3 time points (Fig. 4A). PCA and Anosim demonstrated significant differences (p ≤
≤ 0.005) in the gene expression files of white blood cells among time points A, B, and C (Fig. 4B). At time point A compared to B, we identified 883 downregulated genes and 1553 upregulated genes (|log2(FoldChange)|≥
0.005) in the gene expression files of white blood cells among time points A, B, and C (Fig. 4B). At time point A compared to B, we identified 883 downregulated genes and 1553 upregulated genes (|log2(FoldChange)|≥ 1 and adjusted p
1 and adjusted p <
< 0.05) (Fig. 4C and Supplementary Table 10), which were mapped to 314 KEGG pathways (Supplementary Table 11). Six of these pathways were significantly enriched (adjusted p
0.05) (Fig. 4C and Supplementary Table 10), which were mapped to 314 KEGG pathways (Supplementary Table 11). Six of these pathways were significantly enriched (adjusted p <
< 0.05), including ribosome, oxidative phosphorylation, thermogenesis, Parkinson disease, Huntington disease, and non-alcoholic fatty liver disease (Fig. 4D). Moving on to time point B compared to C, there were 594 downregulated and 707 upregulated genes (|log2(FoldChange)|≥
0.05), including ribosome, oxidative phosphorylation, thermogenesis, Parkinson disease, Huntington disease, and non-alcoholic fatty liver disease (Fig. 4D). Moving on to time point B compared to C, there were 594 downregulated and 707 upregulated genes (|log2(FoldChange)|≥ 1 and adjusted p
1 and adjusted p <
< 0.05) (Fig. 4E and Supplementary Table 10). These genes were associated with 274 KEGG pathways (Supplementary Table 11), with ribosome and platelet activation pathways being significantly enriched (adjusted p
0.05) (Fig. 4E and Supplementary Table 10). These genes were associated with 274 KEGG pathways (Supplementary Table 11), with ribosome and platelet activation pathways being significantly enriched (adjusted p <
< 0.05) (Fig. 4F). Additionally, when comparing time points A to C, we found 362 downregulated and 602 upregulated genes (|log2(FoldChange)|≥
0.05) (Fig. 4F). Additionally, when comparing time points A to C, we found 362 downregulated and 602 upregulated genes (|log2(FoldChange)|≥ 1 and adjusted p
1 and adjusted p <
< 0.05) (Fig. 4G and Supplementary Table 10). These genes corresponded to 279 KEGG pathways (Supplementary Table 11), with 9 pathways showing significant enrichment (adjusted p
0.05) (Fig. 4G and Supplementary Table 10). These genes corresponded to 279 KEGG pathways (Supplementary Table 11), with 9 pathways showing significant enrichment (adjusted p <
< 0.05), including oxidative phosphorylation, ribosome, thermogenesis, Parkinson disease, Huntington disease, non-alcoholic fatty liver disease, Alzheimer disease, cardiac muscle contraction, and proteasome (Fig. 4H). Among these DEGs, 31 genes were consistently altered across all 3 comparisons (Fig. 5I). Of these shared DEGs, 20 were annotated, and their FPKMs in each sample were shown in Supplementary Fig. 2A and B.
0.05), including oxidative phosphorylation, ribosome, thermogenesis, Parkinson disease, Huntington disease, non-alcoholic fatty liver disease, Alzheimer disease, cardiac muscle contraction, and proteasome (Fig. 4H). Among these DEGs, 31 genes were consistently altered across all 3 comparisons (Fig. 5I). Of these shared DEGs, 20 were annotated, and their FPKMs in each sample were shown in Supplementary Fig. 2A and B.
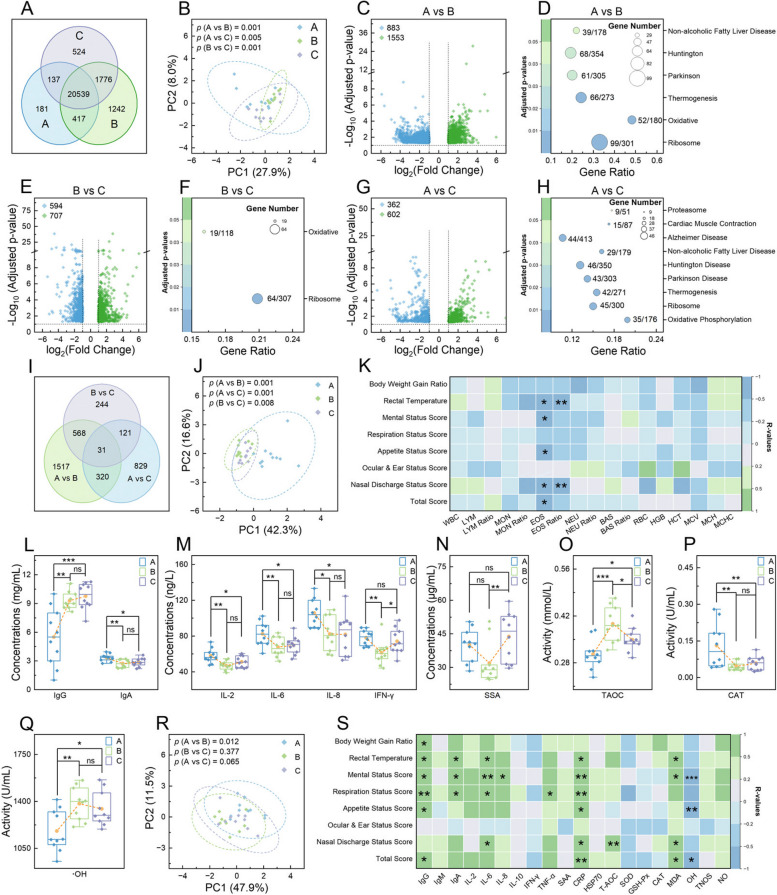
Analysis of white cell composition and their transcriptome in calves. A Venn diagram showing shared and unique genes among different time points. Principal component analysis plots of the mapped genes (B), assessed indicators in routine blood test (RBT) (J), and measured serum indicators (R), respectively. Volcano plots representing differentially expressed genes (DEGs) at 3 time points: A vs B (C), B vs C (E), and A vs C (G). Bubble charts detailing the KEGG pathways significantly enriched by DEGs at 3 time points: A vs B (D), B vs C (F), and A vs C (H). I Venn diagram of the shared and unique DEGs identified across all comparisons. Heatmaps of Spearman’s rank correlations between respiratory health indicators and growth performance with RBT indicators at time point A (K), as well as with considered serum indicators at time point A (S). L–Q Boxplots of selected serum indicators with significant differences across time points. In B, J, and R, the analysis of similarities algorithm was employed to analyze the differences among groups. In C, E, and G, upregulated genes are denoted by green dots and downregulated genes by blue dots, with significance determined by adjusted p <
< 0.05 and |log2 (fold change)|≥
0.05 and |log2 (fold change)|≥ 1. In D, F, and H, the ratio of genes enriched in a pathway to the total number of genes in that pathway is indicated by numbers next to the bubbles; bubble size represents the count of enriched genes, and color indicates significance levels based on adjusted p values. In K and S, correlations were calculated using clinical data from the 10 marked calves; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p
1. In D, F, and H, the ratio of genes enriched in a pathway to the total number of genes in that pathway is indicated by numbers next to the bubbles; bubble size represents the count of enriched genes, and color indicates significance levels based on adjusted p values. In K and S, correlations were calculated using clinical data from the 10 marked calves; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p <
< 0.05; **, p
0.05; **, p <
< 0.01; ***, p
0.01; ***, p <
< 0.001). In L–Q, the yellow dot denotes average values, and one-way analysis of variance was used to assess difference levels among groups (ns, non-significant; *, p
0.001). In L–Q, the yellow dot denotes average values, and one-way analysis of variance was used to assess difference levels among groups (ns, non-significant; *, p <
< 0.05; **, p
0.05; **, p <
< 0.01; ***, p
0.01; ***, p <
< 0.001)
0.001)
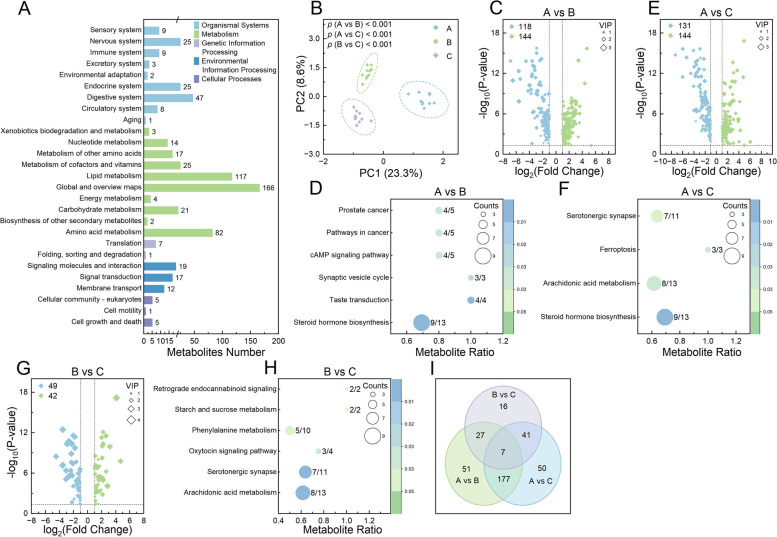
Analysis of serum metabolomic profiling in calves. A Bar chart representing the KEGG pathways into which the identified metabolites were categorized, with the number of metabolites for each pathway displayed alongside the corresponding bars. B Principal component analysis plot of the metabolites identified in each sample group. Volcano plots illustrating differentially expressed metabolites at different time points: A vs B (C), A vs C (E), and B vs C (G). Bubble charts detailing the KEGG pathways significantly enriched by differentially expressed metabolites at 3 time points: A vs B (D), B vs C (F), and A vs C (H). I Venn diagram of the shared and unique differentially expressed metabolites identified across all comparisons. In C, E, and G, upregulated metabolites are indicated by green dots and downregulated metabolites by blue dots; dot sizes denote the variable importance in the projection (VIP) values, with significance determined by adjusted p <
< 0.05, |log2 (fold change)|≥
0.05, |log2 (fold change)|≥ 1, and VIP
1, and VIP >
> 1. In D, F, and H, the ratio of metabolites enriched in a pathway to the total number of metabolites in that pathway is indicated by numbers adjacent to the bubbles; bubble size represents the count of enriched metabolites, and color indicates the significance levels based on adjusted p values
1. In D, F, and H, the ratio of metabolites enriched in a pathway to the total number of metabolites in that pathway is indicated by numbers adjacent to the bubbles; bubble size represents the count of enriched metabolites, and color indicates the significance levels based on adjusted p values
The outcomes of the RBT are presented in Table 1. PCA and Anosim revealed significant differences in these RBT parameters among different time points (p ≤
≤ 0.008; Fig. 4J). Notable differences were observed in white blood cell counts (WBC), lymphocyte counts (LYM), and the ratios of LYM (LYM Ratio) and monocytes (Mon Ratio) (p
0.008; Fig. 4J). Notable differences were observed in white blood cell counts (WBC), lymphocyte counts (LYM), and the ratios of LYM (LYM Ratio) and monocytes (Mon Ratio) (p ≤
≤ 0.043). Additionally, eosinophil counts (EOS), basophil counts (BAS), the ratio of BAS (BAS Ratio), red blood cell counts (RBC), hemoglobin concentration (HGB), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentrations (MCHC), and hematocrit (HCT) showed significant variations (p
0.043). Additionally, eosinophil counts (EOS), basophil counts (BAS), the ratio of BAS (BAS Ratio), red blood cell counts (RBC), hemoglobin concentration (HGB), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentrations (MCHC), and hematocrit (HCT) showed significant variations (p ≤
≤ 0.002). Spearman’s rank correlation analysis identified significant associations between some of these RBT indicators and respiratory health indicators as well as growth performance at each time point (p
0.002). Spearman’s rank correlation analysis identified significant associations between some of these RBT indicators and respiratory health indicators as well as growth performance at each time point (p <
< 0.05 and 0.5
0.05 and 0.5 <|R|<
<|R|< 1; Fig. 4K and Supplementary Fig. 2C and D). Immunoglobulin levels varied across time points, with IgG and IgA showing significant fluctuations (p
1; Fig. 4K and Supplementary Fig. 2C and D). Immunoglobulin levels varied across time points, with IgG and IgA showing significant fluctuations (p ≤
≤ 0.006), whereas IgM levels remained consistent (p
0.006), whereas IgM levels remained consistent (p =
= 0.299) (Fig. 4L and Supplementary Fig. 2E). Inflammatory cytokines such as IL-2, IL-6, IL-8, and IFN-γ exhibited significant differences over time points (p
0.299) (Fig. 4L and Supplementary Fig. 2E). Inflammatory cytokines such as IL-2, IL-6, IL-8, and IFN-γ exhibited significant differences over time points (p ≤
≤ 0.015; Fig. 4M). However, IL-10 and TNF-α levels did not change significantly (p
0.015; Fig. 4M). However, IL-10 and TNF-α levels did not change significantly (p ≥
≥ 0.188; Supplementary Fig. 2F). The concentration of SAA exhibited a significant alteration (p
0.188; Supplementary Fig. 2F). The concentration of SAA exhibited a significant alteration (p =
= 0.025; Fig. 4N), while CRP and HSP 70 levels remain stable (p
0.025; Fig. 4N), while CRP and HSP 70 levels remain stable (p ≥
≥ 0.256; Supplementary Fig. 2G and H). The activities of T-AOC, CAT, and •OH underwent significant changes across the time points (p
0.256; Supplementary Fig. 2G and H). The activities of T-AOC, CAT, and •OH underwent significant changes across the time points (p ≤
≤ 0.006; Fig. 4O–Q). The other 5 considered oxidant-related indicators, including SOD, GSH-Px, NOS, MDA, and NO, did not show significant changes across the same periods (p
0.006; Fig. 4O–Q). The other 5 considered oxidant-related indicators, including SOD, GSH-Px, NOS, MDA, and NO, did not show significant changes across the same periods (p ≥
≥ 0.144; Supplementary Fig. 2I–K). Further PCA and Anosim on these 20 serum indicators demonstrated significant differences between time points A and B (p
0.144; Supplementary Fig. 2I–K). Further PCA and Anosim on these 20 serum indicators demonstrated significant differences between time points A and B (p =
= 0.012). However, these indicators at time points A and C (p
0.012). However, these indicators at time points A and C (p =
= 0.065), as well as B and C (p
0.065), as well as B and C (p =
= 0.377), were not significantly different (Fig. 4R). Finally, Spearman’s rank correlation analysis underscored significant correlations between these serum indicators and respiratory health indicators as well as growth performance at each time point (p
0.377), were not significantly different (Fig. 4R). Finally, Spearman’s rank correlation analysis underscored significant correlations between these serum indicators and respiratory health indicators as well as growth performance at each time point (p <
< 0.05 and 0.5
0.05 and 0.5 <|R|<
<|R|< 1; Fig. 4S and Supplementary Fig. 2L and M).
1; Fig. 4S and Supplementary Fig. 2L and M).
Table 1
Results of the blood routine test measurement
| Indicators | Time point A | Time point B | Time point C |
|---|---|---|---|
| WBC (109/L) | 10.53 ± ± 3.67a 3.67a | 8.04 ± ± 3.05ab 3.05ab | 7.10 ± ± 2.80b 2.80b |
| LYM (109/L) | 6.11 ± ± 1.47a 1.47a | 4.25 ± ± 1.56b 1.56b | 4.10 ± ± 1.66b 1.66b |
| LYM Ratio (%) | 60.84 ± ± 10.31a 10.31a | 53.16 ± ± 6.41b 6.41b | 57.20 ± ± 6.66ab 6.66ab |
| MON (109/L) | 0.55 ± ± 0.29 0.29 | 0.81 ± ± 0.53 0.53 | 0.72 ± ± 0.27 0.27 |
| MON Ratio (%) | 5.74 ± ± 4.00b 4.00b | 9.62 ± ± 2.67a 2.67a | 10.23 ± ± 2.70a 2.70a |
| EOS (109/L) | 0.12 ± ± 0.04a 0.04a | 0.06 ± ± 0.03b 0.03b | 0.05 ± ± 0.05b 0.05b |
| EOS Ratio (%) | 1.22 ± ± 0.52 0.52 | 3.68 ± ± 3.03 3.03 | 2.61 ± ± 2.67 2.67 |
| NEU (109/L) | 3.71 ± ± 2.94 2.94 | 2.69 ± ± 1.19 1.19 | 2.11 ± ± 1.08 1.08 |
| NEU Ratio (%) | 31.77 ± ± 11.27 11.27 | 33.35 ± ± 6.37 6.37 | 30.10 ± ± 7.46 7.46 |
| BAS (109/L) | 0.04 ± ± 0.05a 0.05a | 0.01 ± ± 0.01b 0.01b | 0.01 ± ± 0.01b 0.01b |
| BAS Ratio (%) | 0.42 ± ± 0.35a 0.35a | 0.19 ± ± 0.20b 0.20b | 0.13 ± ± 0.13b 0.13b |
| RBC (1012/L) | 6.08 ± ± 0.67b 0.67b | 9.01 ± ± 1.41a 1.41a | 6.47 ± ± 2.31b 2.31b |
| HGB (g/L) | 127.56 ± ± 13.94a 13.94a | 103.40 ± ± 13.77b 13.77b | 84.50 ± ± 28.51c 28.51c |
| HCT (%) | 22.19 ± ± 2.94b 2.94b | 31.87 ± ± 4.93a 4.93a | 23.95 ± ± 8.36ab 8.36ab |
| MCV (fL) | 36.49 ± ± 1.78 1.78 | 35.47 ± ± 2.81 2.81 | 36.95 ± ± 4.10 4.10 |
| MCH (pg) | 21.19 ± ± 3.56a 3.56a | 11.55 ± ± 0.68b 0.68b | 13.05 ± ± 1.10b 1.10b |
| MCHC (g/L) | 581.11 ± ± 108.86a 108.86a | 326.00 ± ± 18.59b 18.59b | 354.70 ± ± 21.24b 21.24b |
Note: Results are expressed as the average values ±
± the standard deviation mean. Different superscripts (a-c) in each row indicate the significant differences (p
the standard deviation mean. Different superscripts (a-c) in each row indicate the significant differences (p <
< 0.05 or adjusted p
0.05 or adjusted p <
< 0.05) among groups, which were calculated using a one-way analysis of variance for parametric analysis or a Kruskal–Wallis rank sum test for nonparametric analysis
0.05) among groups, which were calculated using a one-way analysis of variance for parametric analysis or a Kruskal–Wallis rank sum test for nonparametric analysis
WBC White blood cell counts, LYM Lymphocyte counts, LYM Ratio the ratio of LYM to WBC, MON Monocyte counts, MON Ratio the ratio of MON to WBC, EOS Eosinophil counts, EOS Ratio the ratio of EOS to WBC, NEU neutrophil counts, NUE Ratio the ratio of NEU to WBC, BAS Basophil counts, BAS Ratio the ratio of BAS to WBC, RBC Red blood cell counts, HGB Hemoglobin concentration, HCT Hematocrit, MCV Mean corpuscular volume, MCH Mean corpuscular hemoglobin, MCHC Mean corpuscular hemoglobin concentrations
Characterizing and analyzing the metabolic status in calves
In the untargeted LC–MS metabolomic analysis, 620 metabolites were identified in the positive ion model and 472 in the negative ion mode (Supplementary Table 12). Of these 1092 detected metabolites, 396 were categorized into 27 KEGG pathways (Fig. 5A), and 215 were mapped onto 23 lipid maps (Supplementary Fig. 3A). Significant differences in metabolite profiles at various time points were revealed by PCA and Anosim (p <
< 0.001; Fig. 5B). Specifically, between time points A and B, 118 metabolites were significantly downregulated and 144 were upregulated at time point B (VIP
0.001; Fig. 5B). Specifically, between time points A and B, 118 metabolites were significantly downregulated and 144 were upregulated at time point B (VIP >
> 1, p
1, p <
< 0.05, and |log2(FoldChange)|≥
0.05, and |log2(FoldChange)|≥ 1|; Supplementary Table 13 and Fig. 5C). These 262 DE Metas were significantly enriched in 6 KEGG pathways, which include steroid hormone biosynthesis, taste transduction, synaptic vesicle cycle, cAMP signaling pathway, pathways in cancer, and prostate cancer (p
1|; Supplementary Table 13 and Fig. 5C). These 262 DE Metas were significantly enriched in 6 KEGG pathways, which include steroid hormone biosynthesis, taste transduction, synaptic vesicle cycle, cAMP signaling pathway, pathways in cancer, and prostate cancer (p ≤
≤ 0.027; Fig. 5D). Comparatively, from time points A to C, there were 131 metabolites significantly downregulated and 144 upregulated (VIP
0.027; Fig. 5D). Comparatively, from time points A to C, there were 131 metabolites significantly downregulated and 144 upregulated (VIP >
> 1, p
1, p <
< 0.05, and |log2(FoldChange)|≥
0.05, and |log2(FoldChange)|≥ 1|; Supplementary Table 13 and Fig. 5E). These 275 DE Metas were significantly enriched in 4 KEGG pathways, including steroid hormone biosynthesis, arachidonic acid metabolism, ferroptosis, and serotonergic synapse (p
1|; Supplementary Table 13 and Fig. 5E). These 275 DE Metas were significantly enriched in 4 KEGG pathways, including steroid hormone biosynthesis, arachidonic acid metabolism, ferroptosis, and serotonergic synapse (p ≤
≤ 0.037; Fig. 5F). Transitioning from time points B to C, it was observed that 49 metabolites were significantly downregulated and 42 upregulated (VIP
0.037; Fig. 5F). Transitioning from time points B to C, it was observed that 49 metabolites were significantly downregulated and 42 upregulated (VIP >
> 1, p
1, p <
< 0.05, and |log2(FoldChange)|≥
0.05, and |log2(FoldChange)|≥ 1|; Supplementary Table 13 and Fig. 5G). These 91 DE Metas were significantly enriched in 6 KEGG pathways, including arachidonic acid metabolism, serotonergic synapse, oxytocin signaling pathway, phenylalanine metabolism, starch and sucrose metabolism, and retrograde endocannabinoid signaling (p
1|; Supplementary Table 13 and Fig. 5G). These 91 DE Metas were significantly enriched in 6 KEGG pathways, including arachidonic acid metabolism, serotonergic synapse, oxytocin signaling pathway, phenylalanine metabolism, starch and sucrose metabolism, and retrograde endocannabinoid signaling (p ≤
≤ 0.046; Fig. 5H). Among these DE Metas, 7 were consistently altered across all 3 comparisons (Fig. 5I), namely, 1-methylhistamine, linustatin, β-muricholic acid, N-phenylacetylglutamine, 16(R)-HETE, trehalose, and 19(R)-hydroxy-prostaglandin E2, with their relative expression levels shown in Supplementary Fig. 3B.
0.046; Fig. 5H). Among these DE Metas, 7 were consistently altered across all 3 comparisons (Fig. 5I), namely, 1-methylhistamine, linustatin, β-muricholic acid, N-phenylacetylglutamine, 16(R)-HETE, trehalose, and 19(R)-hydroxy-prostaglandin E2, with their relative expression levels shown in Supplementary Fig. 3B.
Differences in the levels of ACTH, COR, GH, and ADH were significant at different time points (p ≤
≤ 0.025; Fig. 6A and B). Similarly, the concentration of GLU and the activity of PK showed significant fluctuations (p
0.025; Fig. 6A and B). Similarly, the concentration of GLU and the activity of PK showed significant fluctuations (p <
< 0.001; Fig. 6C and D), whereas the activities of G-6-PDH and PFK remained consistent (p
0.001; Fig. 6C and D), whereas the activities of G-6-PDH and PFK remained consistent (p ≥
≥ 0.149; Supplementary Fig. 3C and D). In terms of lipid metabolism, the activities of FAS and ACC, along with the concentrations of NEFA, TC, HDL-C, LDL-C, and VLDL-C, exhibited significant variations (p
0.149; Supplementary Fig. 3C and D). In terms of lipid metabolism, the activities of FAS and ACC, along with the concentrations of NEFA, TC, HDL-C, LDL-C, and VLDL-C, exhibited significant variations (p ≤
≤ 0.013; Fig. 6E and F). However, the concentration of TG was stable (p
0.013; Fig. 6E and F). However, the concentration of TG was stable (p =
= 0.096; Supplementary Fig. 3E). Protein metabolism indicators also changed significantly: ALB and GLB concentrations and their ratio varied notably (p
0.096; Supplementary Fig. 3E). Protein metabolism indicators also changed significantly: ALB and GLB concentrations and their ratio varied notably (p ≤
≤ 0.005; Fig. 6G and H), while TP levels did not (p
0.005; Fig. 6G and H), while TP levels did not (p =
= 0.110; Supplementary Fig. 3F). The enzymatic activities of AST, ALT, and ALP shifted significantly over time points (p
0.110; Supplementary Fig. 3F). The enzymatic activities of AST, ALT, and ALP shifted significantly over time points (p ≤
≤ 0.006; Fig. 6I), but GGT activity and the ALT/AST ratio did not (p
0.006; Fig. 6I), but GGT activity and the ALT/AST ratio did not (p ≥
≥ 0.296; Supplementary Fig. 3G and H). BIL levels, including TBIL, DBIL, and IBIL, along with renal function indicators BUN and CRE, also varied significantly (p
0.296; Supplementary Fig. 3G and H). BIL levels, including TBIL, DBIL, and IBIL, along with renal function indicators BUN and CRE, also varied significantly (p ≤
≤ 0.037; Fig. 6J and K). Regarding myocardial enzyme indicators, LDH activity showed significant variation (p
0.037; Fig. 6J and K). Regarding myocardial enzyme indicators, LDH activity showed significant variation (p =
= 0.029; Fig. 6L), while CK activity remained unchanged (p
0.029; Fig. 6L), while CK activity remained unchanged (p =
= 0.217; Supplementary Fig. 3I). PCA and Anosim confirmed these significant differences across different time points (p
0.217; Supplementary Fig. 3I). PCA and Anosim confirmed these significant differences across different time points (p ≤
≤ 0.007; Fig. 6M). Lastly, Spearman’s rank correlation analysis revealed significant associations between calves’ performance metrics and these hematological indicators (p
0.007; Fig. 6M). Lastly, Spearman’s rank correlation analysis revealed significant associations between calves’ performance metrics and these hematological indicators (p <
< 0.05 and 0.5
0.05 and 0.5 ≤|R|≤
≤|R|≤ 1; Fig. 6N–P).
1; Fig. 6N–P).
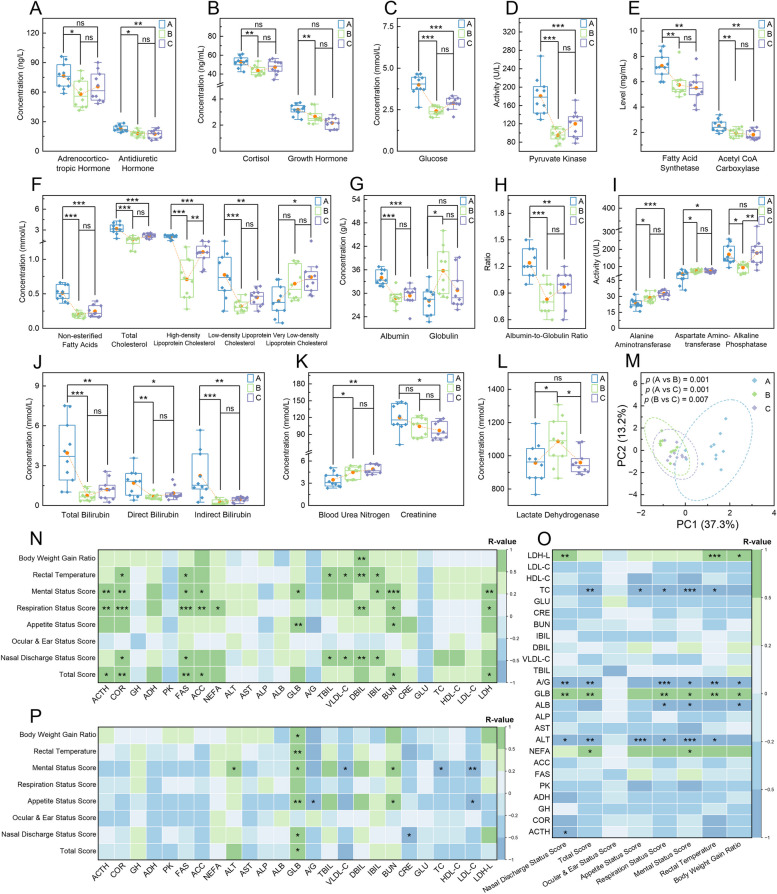
Analysis of the metabolic serum indicators in calves. A–L Boxplots of the considered hematological indicators with significant differences among different time points. M Principal component analysis plot of all these assessed indicators in each sample group. Heatmaps of Spearman’s rank correlations between respiratory health indicators and growth performance with the considered hematological indicators at time points A (N), B (O), and C (P). In A–L, the yellow dot denotes average values, and a one-way analysis of variance was used to assess difference levels among groups (ns, non-significant; *, p <
< 0.05; **, p
0.05; **, p <
< 0.01; ***, p
0.01; ***, p <
< 0.001). In N–P, correlations were calculated using clinical data from the 10 marked calves; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p
0.001). In N–P, correlations were calculated using clinical data from the 10 marked calves; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p <
< 0.05; **, p
0.05; **, p <
< 0.01; ***, p
0.01; ***, p <
< 0.001)
0.001)
Joint analysis of the nasopharyngeal microbiome, whole blood transcriptome, and serum metabolome in calves
Spearman’s correlation analysis identified significant associations between the shared DEGs and DE Mates across the 3 comparisons (p <
< 0.05 and 0.5
0.05 and 0.5 <|R|<
<|R|< 1). It also showed significant correlations between the shared DE Metas and the shared nasopharyngeal genera with LDA
1). It also showed significant correlations between the shared DE Metas and the shared nasopharyngeal genera with LDA ≥
≥ 4 across these time points (p
4 across these time points (p <
< 0.05 and 0.5
0.05 and 0.5 <|R|<
<|R|< 1; Fig. 7A). Additionally, a total of 56 shared KEGG pathways were enriched by DEGs and DE Metas in the A vs B comparison, 41 pathways in A vs C, and 42 pathways in B vs C (Fig. 7B–D and Supplementary Table 14).
1; Fig. 7A). Additionally, a total of 56 shared KEGG pathways were enriched by DEGs and DE Metas in the A vs B comparison, 41 pathways in A vs C, and 42 pathways in B vs C (Fig. 7B–D and Supplementary Table 14).
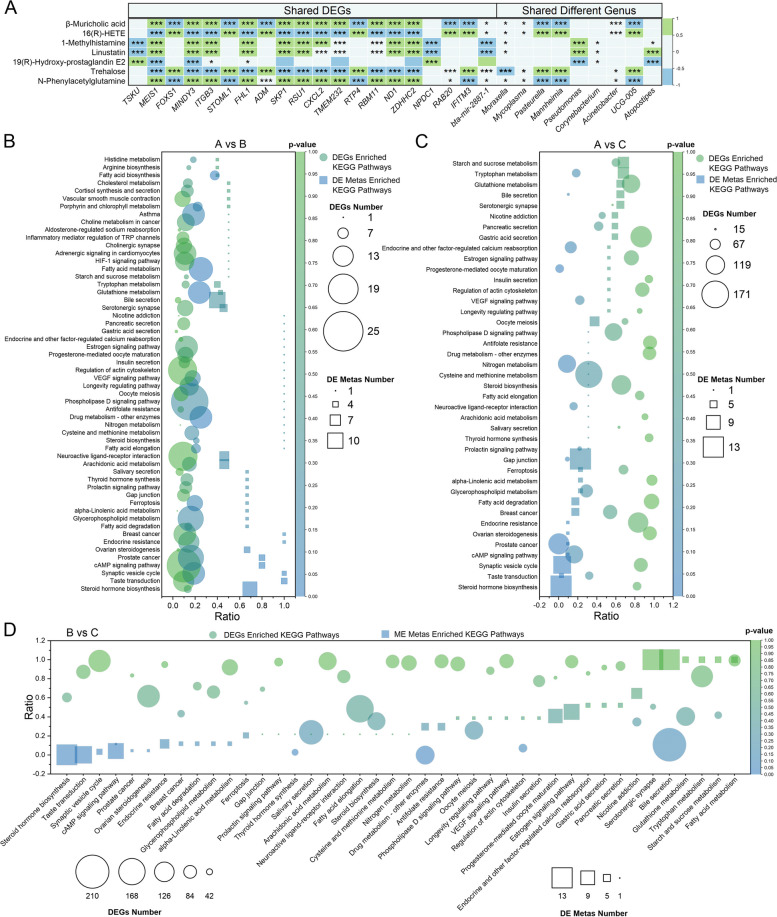
Relationship among the whole blood transcriptome, serum metabolism, and nasopharyngeal microbiome in calves. A Heatmap of Spearman’s rank correlations between the whole blood transcriptome, serum metabolism, and nasopharyngeal microbiome. Bubble charts detailing the shared KEGG pathways enriched by DEGs and DE Metas across time points: A vs B (B), B vs C (C), and A vs C (D). In A, correlations were calculated using sequencing data from the 10 marked calves at all 3 time points; pane colors denote the strength and direction of relevance (green for positive, blue for negative), and asterisks indicate significance levels of relevance (*, p <
< 0.05; **, p
0.05; **, p <
< 0.01; ***, p
0.01; ***, p <
< 0.001). In B, C, and D, the ratio of genes/metabolites enriched in a pathway to the total number of genes/metabolites in that pathway is indicated by “Ratio”; bubble shape represents the type of KEGG pathways (circle for DEGs enriched KEGG pathways, square for DE Metas enriched KEGG pathways), size represents the count of enriched genes, and color indicates significance levels based on p values. DEGs, differentially expressed genes; DE Metas, differentially expressed metabolites
0.001). In B, C, and D, the ratio of genes/metabolites enriched in a pathway to the total number of genes/metabolites in that pathway is indicated by “Ratio”; bubble shape represents the type of KEGG pathways (circle for DEGs enriched KEGG pathways, square for DE Metas enriched KEGG pathways), size represents the count of enriched genes, and color indicates significance levels based on p values. DEGs, differentially expressed genes; DE Metas, differentially expressed metabolites
Discussion
Road transportation, an integral part of the cattle industry, poses significant challenges to the health and performance of newly received cattle, raising both animal welfare concerns and economic issues [1, 24]. Although numerous studies have investigated the effects of transportation on calf respiratory health [16, 44], they have often focused on only a subset of these impacts [20]. In this study, we conducted a comprehensive assessment of the respiratory health of transported beef calves by continuously monitoring their clinical performances twice daily during the first 60 days following a 3000-km road transportation. Additionally, we analyzed the calves’ nasopharyngeal microbial communities and immunometabolic status at 3 time points: before transportation, 30 days after, and 60 days post-transportation. Given the extensive range of indicators in our study, it is impractical to discuss each outcome in detail within the limited space, especially since many have been thoroughly reviewed in recent literature [3, 11, 20]. Therefore, we will focus our discussion on the most significant aspects of our findings.
Environmental factors, particularly ambient temperature, are well-recognized for influencing calves’ physiological conditions. Hill et al. observed a 0.0325 ±
± 0.00035 °C increase in calf temperature per 1 °C rise in ambient temperature [45]. Conversely, Bernardini et al. found that transported calves’ rectal temperatures remained stable within an ambient range of 8–28 °C [29]. In our study, calves’ rectal temperatures were unaffected by environmental temperature within a range of 9–28 °C (Fig. 2D), corroborating Bernardini et al.’s finding [29]. Consistent with the observations by Hill et al., who reported the lowest rectal temperatures of calves at 8:00 and the highest between 17:00 and 22:00 [45], our study also detected significantly higher rectal temperatures in transported calves during the afternoon (Fig. 2D). Beyond ambient temperature, the effects of relative humidity and THI levels are also commonly evaluated in cattle studies. For instance, Zeng et al. recently documented significant effects of THI levels on dairy cow rectal temperatures [46]. However, our observations revealed no significant effects of relative humidity or THI levels on the rectal temperature of transported calves (Fig. 2D). This discrepancy may be due to the relatively low THI levels (<
0.00035 °C increase in calf temperature per 1 °C rise in ambient temperature [45]. Conversely, Bernardini et al. found that transported calves’ rectal temperatures remained stable within an ambient range of 8–28 °C [29]. In our study, calves’ rectal temperatures were unaffected by environmental temperature within a range of 9–28 °C (Fig. 2D), corroborating Bernardini et al.’s finding [29]. Consistent with the observations by Hill et al., who reported the lowest rectal temperatures of calves at 8:00 and the highest between 17:00 and 22:00 [45], our study also detected significantly higher rectal temperatures in transported calves during the afternoon (Fig. 2D). Beyond ambient temperature, the effects of relative humidity and THI levels are also commonly evaluated in cattle studies. For instance, Zeng et al. recently documented significant effects of THI levels on dairy cow rectal temperatures [46]. However, our observations revealed no significant effects of relative humidity or THI levels on the rectal temperature of transported calves (Fig. 2D). This discrepancy may be due to the relatively low THI levels (< 55) maintained throughout our experiment (Fig. 2C) [47, 48], which also informs the facility manager’s decision to purchase beef calves each October. These findings suggest that the significant outcomes detected in our study are unlikely to be caused by alterations in environmental factors, as confirmed by Spearman’s correlation analysis (Fig. 2K).
55) maintained throughout our experiment (Fig. 2C) [47, 48], which also informs the facility manager’s decision to purchase beef calves each October. These findings suggest that the significant outcomes detected in our study are unlikely to be caused by alterations in environmental factors, as confirmed by Spearman’s correlation analysis (Fig. 2K).
Behavioral indicators are frequently used to evaluate calves’ response to transportation in studies aiming to understand recovery and how calves physiologically coped with the experience [20]. In recent decades, numerous clinical scoring systems have been developed and widely used to assess the respiratory health of calves [17, 18]. However, except for our previous study [49], only 1 study has scored the clinical performances of transported calves, and it did not provide detailed information on its scoring methodology [50]. In this research, we observed that calves exhibited elevated rectal temperatures and higher scores across all metrics upon arrival (Fig. 2D–J). These indicators decreased progressively over the following week, suggesting the calves’ adaptability to the new surroundings at this facility. By approximately the 7th day, rectal temperatures stabilized around 39.0 °C, and both mental and appetite scores generally approached zero. These patterns imply that calves typically require about 1 week to acclimate to the new environment, highlighting the necessity for increased care during the initial week post-arrival. Notably, the modified scoring system we used was based on Maier’s system for dairy calves [18]. Considering that its effectiveness has not been verified yet, additional experiments aiming to address this issue should be conducted as soon as possible.
It is also noteworthy that these calves received BSG during their first 6 days at the facility, based on the staff’s prior experiences. Mudawaroch et al. have documented that brown sugar and herbal medicines can enhance goat productivity [51], while Lin et al. revealed the antioxidative and anti-inflammation properties of brown sugar longan ginger tea [52]. The facility staff has also observed significant improvements in calves with BSG administration. This suggests that the adaptive feeding period might be extended without BSG, warranting further investigation to validate its beneficial effects on calves and the underlying mechanisms, which probably involve the anti-inflammatory and antimicrobial effects of curcumin [53]. Moreover, our findings raise questions about the adequacy of existing studies that have monitored calf behavior for only 24 or 48 h post-arrival [54, 55]. These results suggest that newly received calves subjected to 3000-km of road transportation may require at least 1 week to acclimate to the new facility, irrespective of other conditions.
Current research on the duration of transportation-induced effects on calves is limited. Feedlot managers typically allocate a 1-month adaptation period for newly arrived veal calves, a practice informed by their previous experiences. This aligns with the existing literature on calf respiratory health post-transport, which predominantly examines the first-month post-arrival [5, 12, 24, 56, 57]. We observed a reduction in respiration status, ocular and ear, and nasal discharge scores within the first-week post-arrival, indicating a potential gradual decline in the risk of BRD during this period (Fig. 2F, H, and I). Although we did not assess the immunometabolic status of these calves on the 7th day post-arrival, significant differences were observed in nasopharyngeal microbial community composition and structure (Fig. 3D), white blood cell profiles (Table 1 and Fig. 4J), and immunometabolic markers (Figs. (Figs.4B,4B, R, R,5B,5B, and and6M)6M) between time point A and B. The relative abundance of respiratory tract pathogens such as Pasteurella and Mannheimia tended to decrease at time point B compared to A, whereas the relative abundance of Mycoplasma tended to increase, corroborating findings from previous research [26, 58]. Additionally, significant changes in various immune, inflammatory, oxidative, and stress response indicators were observed at time points A versus B (Table 1, Figs. Figs.4L–Q4L–Q and and6A–L),6A–L), consistent with earlier studies [5, 10, 27]. These observations indicate an improvement in respiratory health and immunometabolic status over the 30 days post-arrival.
Despite most hematological indicators showing no significant changes between time points B and C (Table 1, Figs. Figs.4L–Q4L–Q and and6A–L),6A–L), PCA and Anosim revealed significant differences in the immunometabolic and nasopharyngeal microbial profiles (Figs. (Figs.4B,4B, R, R,5B,5B, and and6M).6M). Notably, the relative abundance of pathogenic genera such as Pasteurella, Mannheimia, and Mycoplasma, which are strongly associated with BRD risk [59–61], decreased nearly to 0 by time point C (Fig. 2H). This trend aligns with findings from Johnson et al., who tracked nasopharyngeal microbiome dynamics in calves with clinical BRD symptoms from diagnosis to recovery [62]. These results suggest an improvement in the respiratory health of transported calves during the second-month post-arrival. However, these respiratory health-related symptoms did not resolve entirely and remained variable throughout the second month (Fig. 2F, H, and I). Given the normal mental and appetite scores (Fig. 2E and G) and the recovered immunometabolic status (Figs. (Figs.4,4, ,5,5, and and6)6) of calves during the second month, these symptoms might be responses to environmental irritants or interactions with other unrecovered individuals. Therefore, during this period, it is essential to improve ventilation and air quality in the pens as much as possible and to relocate calves that show clear signs of infection to prevent recurrent cross-infection.
Previous studies have highlighted significant changes in the nasopharyngeal microbiota and serum metabolites of calves before and after transportation [5, 6, 63], which are considered to be attributed to the stress induced by transportation. Based on these findings, we hypothesized that by time point B, the calves would have recovered to their baseline status at time point A, and we expected the relative abundance of Pasteurella, Mannheimia, and Mycoplasma, as well as the levels of hematological indicators, to be consistent between time points A and B. However, contrary to our expectations, we observed a higher abundance of these pathogens (Fig. 2H) and higher levels of immune, stress, inflammatory, and oxidative indicators at time point A, which were significantly higher than those at time point B (Table 1, Figs. Figs.4L–Q4L–Q and and6A–L).6A–L). These unexpected findings suggest that the health status of the calves at time point B was significantly different from that at time point A. Ideally, to establish an accurate baseline, we would have collected samples at the calves’ original farms before they were transported a short distance to the enclosure, where they were subsequently mixed for 3 days. However, due to a lack of authorization from the farmers to sample these calves at the original farm before purchase confirmation, we were unable to establish an accurate baseline. Considering that commingling can significantly affect calf respiratory health [64, 65], it is likely that the calves were already experiencing stress at time point A [66, 67]. Therefore, the baseline established at time point A likely reflected a moderately stressed state following commingling, rather than an unstressed status. This issue must be considered in further studies aiming to explore the effects of transportation.
Several factors, such as age, body weight, origin farm, pre-transportation diet, transportation duration, and post-transportation management, have been found to impact the health and performance of calves after transportation [19, 57, 68]. Therefore, the optimization of management practices before, during, and after transportation has been widely applied to enhance the health and productivity of transported veal calves. For instance, Marcato et al. discovered that feeding milk before 6-h road transportation can improve the behavioral and hematological indicators of veal calves [57]. Initially, we believed that management during the adaptive feeding period after transportation played a more critical role in maintaining the health status of transported calves [6]. However, in this study, more significant associations were observed between these indicators and the post-transportation performances of calves at time point A compared to B or C (Figs. (Figs.3C3C and I, I,4K4K and S, and and6N–P).6N–P). This suggests that improving the health status before transportation, such as through vaccinations, immune enhancers, stress reduction, and minimizing pathogen exposure, might be helpful in improving the growth performance of calves subjected to transportation. This might explain why calves who received colostrum showed quick recoveries after arriving at farms [69, 70]. Nonetheless, these correlations require further verification through well-designed studies with accurate baselines and larger sample sizes.
Previous research has separately examined the impact of road transportation on various aspects such as nasopharyngeal microbiota [6, 16, 26, 63], serum metabolism [5], and the blood transcriptome [27] in calves. However, the interactions among calf performance, white blood cells, serum metabolites, and nasopharyngeal microbiota have not been fully explored. Our study found significant correlations among these factors. Specifically, we observed that calf performance was closely linked to white blood cell composition, function, and gene expression profiles (Fig. 4K and S), serum metabolites profiles (Fig. 6N–O), and nasopharyngeal microbiota composition (Fig. 3I and J). Furthermore, our joint analysis highlighted significant correlations among white blood cell composition and gene expression profiles, serum metabolite profiles, and nasopharyngeal microbiota profiles (Fig. 7), indicating a complex network of physiological interactions triggered by road transportation. These findings suggest that road transportation initiates a chain reaction, starting with the activation of the hypothalamic-pituitary-adrenocortical axis, as evidenced by increased levels of circulating hormones and cortisol (Fig. 6A and B). This activation affects white blood cells and serum metabolites, which subsequently influence each other and disturb the equilibrium of nasopharyngeal microflora. These effects are further compounded by exposure to new individuals, environments, and management practices. We summarize the complex interactions among white blood cells, serum metabolites, and nasopharyngeal microflora in Fig. 8.
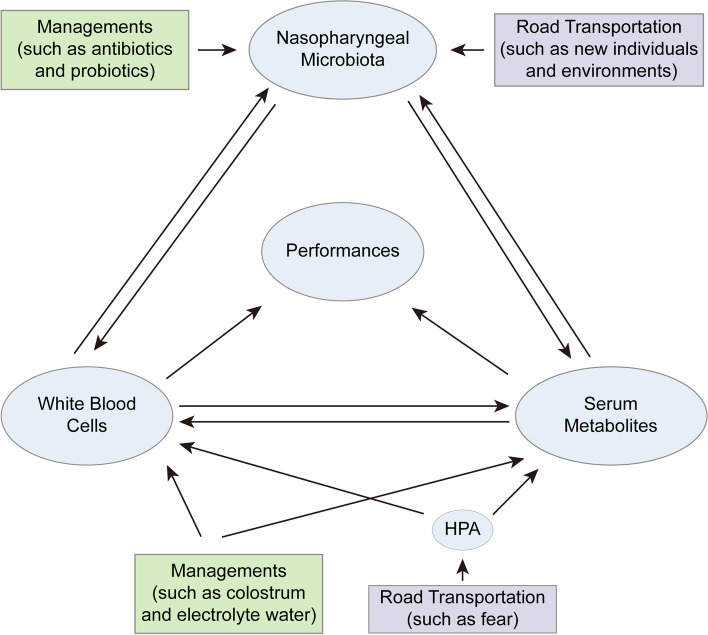
Interactions among white blood cells, serum metabolites, and nasopharyngeal microflora in transported calves. Transportation stress, such as fear, hunger, and dehydration, significantly alters the composition and gene expression profiles of white blood cells, as well as the profiles of serum metabolites. These changes adversely affect the performance of calves and disrupt the balance of their nasopharyngeal microflora. Exposure to unfamiliar individuals and new environments further destabilizes the nasopharyngeal microflora equilibrium in calves. This disruption not only affects the white blood cell composition and gene expression profiles but also alters the serum metabolites profiles, culminating in diminished calf performance. Management practices play a crucial role in mitigating these effects. The administration of colostrum and electrolyte water supplements enhances the immune function of calves, thereby improving their performance and restoring the balance of nasopharyngeal microflora. Similarly, the use of antibiotics and probiotics supplements aids in maintaining the nasopharyngeal microflora’s equilibrium. These practices weaken the negative impacts of transpiration stress on white blood cells and serum metabolites, leading to improved calf performance. HPA, hypothalamic-pituitary-adrenocortical axis
Based on these insights, we propose 3 strategies to reduce the adverse effects of transportation on calves: minimizing stress sources, blocking signal transmission, and mitigating stress-induced consequences. To minimize stress sources, it is essential to avoid psychological stimuli such as handling, bumps, and noise during transport [71]. Additionally, exposure to pathogenic microorganisms can be reduced by enhancing ventilation systems in transport vehicles. To block signal transmission, stress-induced hormones, oxidative stresses, and inflammatory factors can be diluted or neutralized by supplementing electrolytes and immune agents. This approach reduces their impact on respiratory epithelial tissues through peripheral circulation, thereby preventing cytokine storms and tissue damage. Finally, to mitigate the consequences of stress, pathogenic microorganisms that invade the body through damaged epithelium can be eliminated, and the inflammatory response can be reduced by administering antibiotics and providing anti-inflammatory and antioxidant drugs.
Finally, some limitations of this study must be noted. First, this is an observational study with a limited sample size, particularly for high-throughput sequencing and laboratory analyses. Second, we did not collect samples at the original farms, which may make our baseline at time point A unrepresentative of the calves’ unstimulated state. Lastly, we did not explore the underlying molecular mechanisms of these alterations, which warrant further validation through well-designed in vivo and in vitro confirmatory experiments with larger sample size.
Conclusion
Collectively, our results confirm that 60 days of road transportation significantly alter the composition and gene expression profiles of circulating blood cells in calves, affect their metabolic processes, disrupt their nasopharyngeal microbial communities, and result in pronounced respiratory symptoms that persist for at least 60 days. During this period, these symptoms gradually diminish, the disrupted nasopharyngeal microbial communities gradually return to equilibrium, and the abundance of BRD-related pathogens gradually decreases. The counts of white blood cells in the circulatory system recover, and the expression levels of stress-related genes are downregulated. Meanwhile, the metabolic processes shift from a catabolic to an anabolic state, characterized by increased concentrations of anabolic-related metabolites and enzyme activities. These alterations are correlated, and several indicators, such as respiratory scores, eosinophil levels, IL-6, glucose concentrations, and pyruvate kinase activity, can serve as markers of respiratory health in calves. These findings highlight the extensive and fundamental impact of long-distance road transportation on the respiratory health of beef calves, enhancing our understanding of BRD pathogenesis and providing a scientific basis for developing effective prevention and control strategies. However, these results and their underlying molecular mechanisms warrant further validation through well-designed in vivo and in vitro confirmatory experiments with larger sample size.
Acknowledgements
We thank Dr. Jizong Zhang, MS Xinxin Fu, and Xiyu Zhu (Sichuan Agricultural University, Chengdu, China) for their contributions in this work.
Authors’ contributions
Jiancheng Qi, Fangyuan Huang, Linli Gan, and Zhicai Zuo conducted the experiment; Jiancheng Qi, Fangyuan Huang, Linli Gan, and Zhicai Zuo collected and analyzed the data; Jiancheng Qi and Zhicai Zuo wrote the main text of the manuscript; Jiancheng Qi prepared all figures and tables; All authors reviewed the manuscript.
Funding
This investigation was funded by the National Key Research and Development Program of China (2022YFD1601600) and the China Agriculture Research System, a joint venture of the Ministry of Finance and the Ministry of Agriculture and Rural Affairs (Beef Cattle/Yak, CARS-37).
Data availability
The raw data of the 16S rRNA gene microbiome sequence has been uploaded to the SRA database (BioProject: PRJNA1059521 at https://dataview.ncbi.nlm.nih.gov/object/PRJNA1059521?reviewer=uh0gsaubkllp6ulpujk1m3vg72); the raw data of the Whole-Blood transcriptome sequence has been uploaded to the SRA database (BioProject: PRJNA1059657 at https://dataview.ncbi.nlm.nih.gov/object/PRJNA1059657?reviewer=7ko5incs65bb2hujmd87kitkpk); and the raw data of the untargeted metabolomics sequence has been uploaded to the Metabolights database (MTBLS9073; https://www.ebi.ac.uk/metabolights/reviewer3585309a-3b64-4bce-8405-8efc073918c7).
Declarations
This experiment received approval from the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Approval Code: 2021103003; Approval Date: September 5, 2022), following the Regulations for the Administration of Affairs Concerning Experimental Animals issued by the State Science and Technology Commission of China. Veterinarians from the Caijiashan Breeding Company, an intensive beef cattle breeding facility in Quxian County, Dazhou, Sichuan Province, supervised all the experimental procedures.
All authors have consented to the publication of this work.
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiancheng Qi, Fangyuan Huang and Linli Gan contributed equally to this work.
References
Articles from Microbiome are provided here courtesy of BMC
Funding
Funders who supported this work.
China Agriculture Research System, a joint venture of the Ministry of Finance and the Ministry of Agriculture and Rural Affairs (1)
Grant ID: CARS-37
National Key Research and Development Program of China (1)
Grant ID: 2022YFD1601600

 1 and
1 and 

