Abstract
Free full text

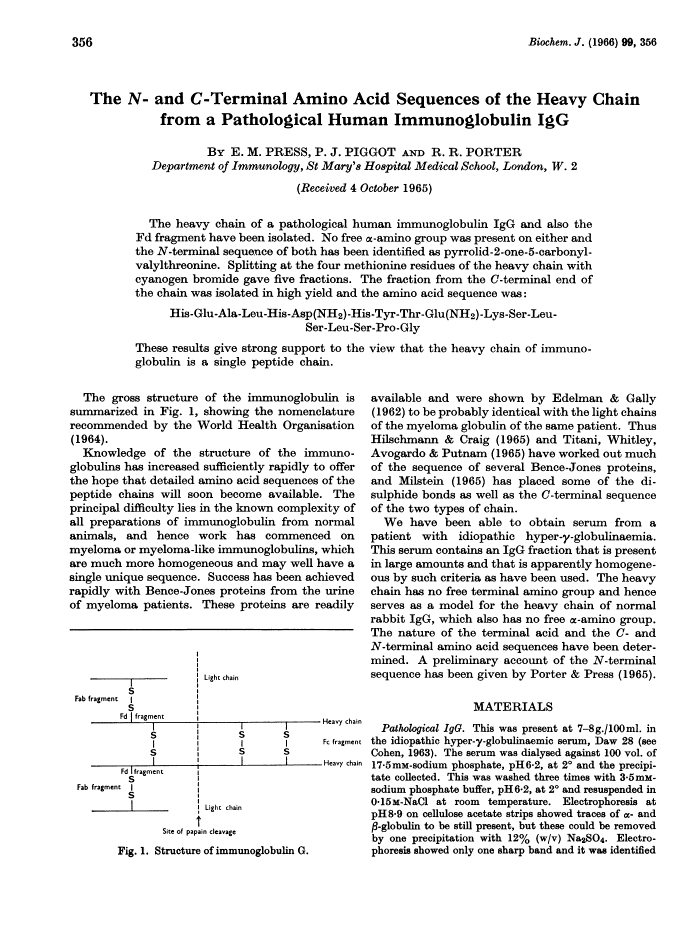
The N- and C-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG
Abstract
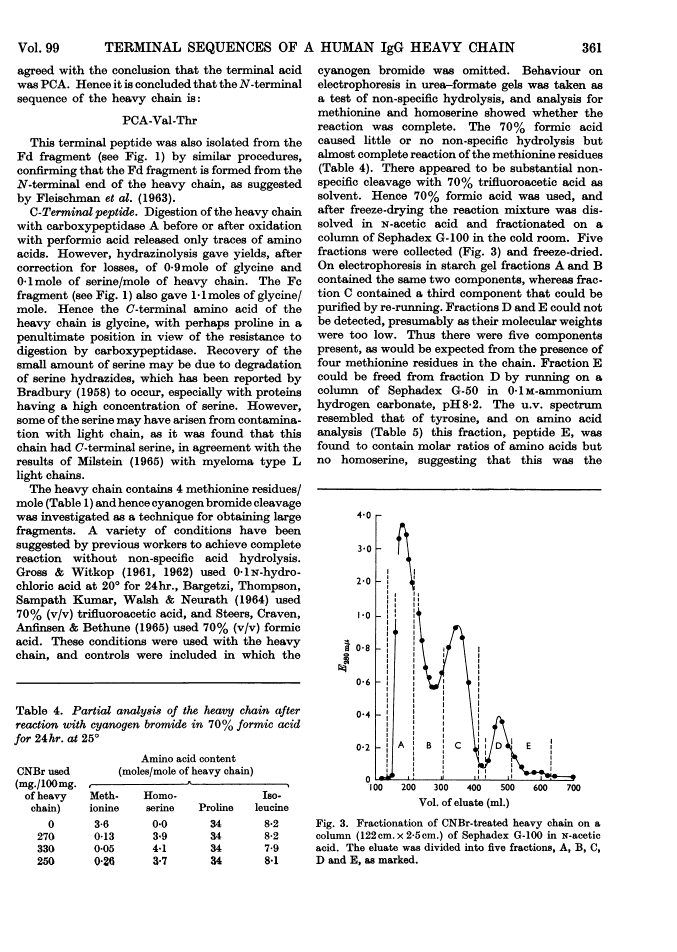
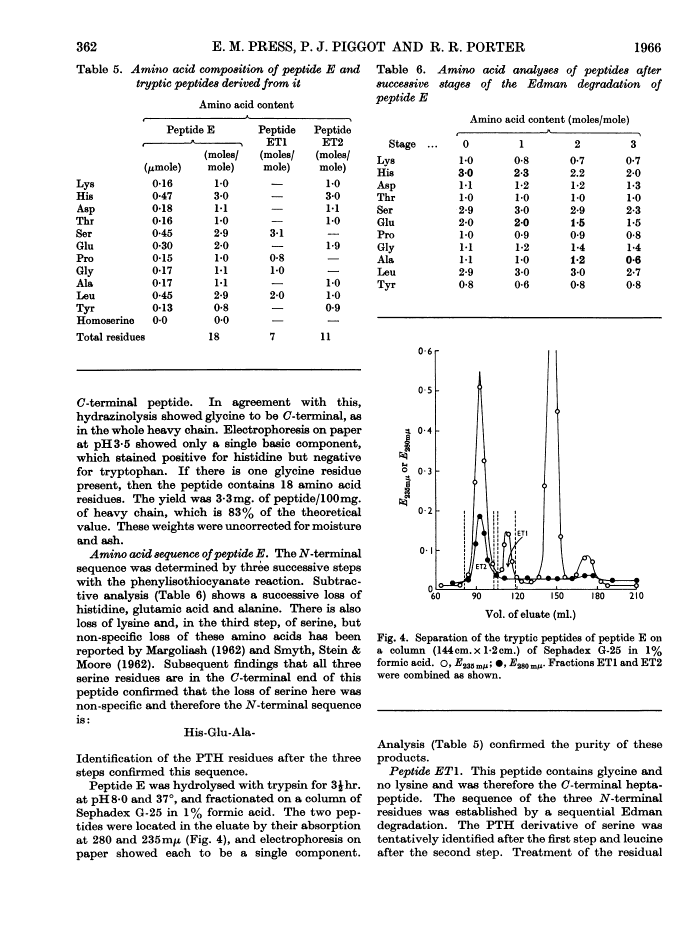
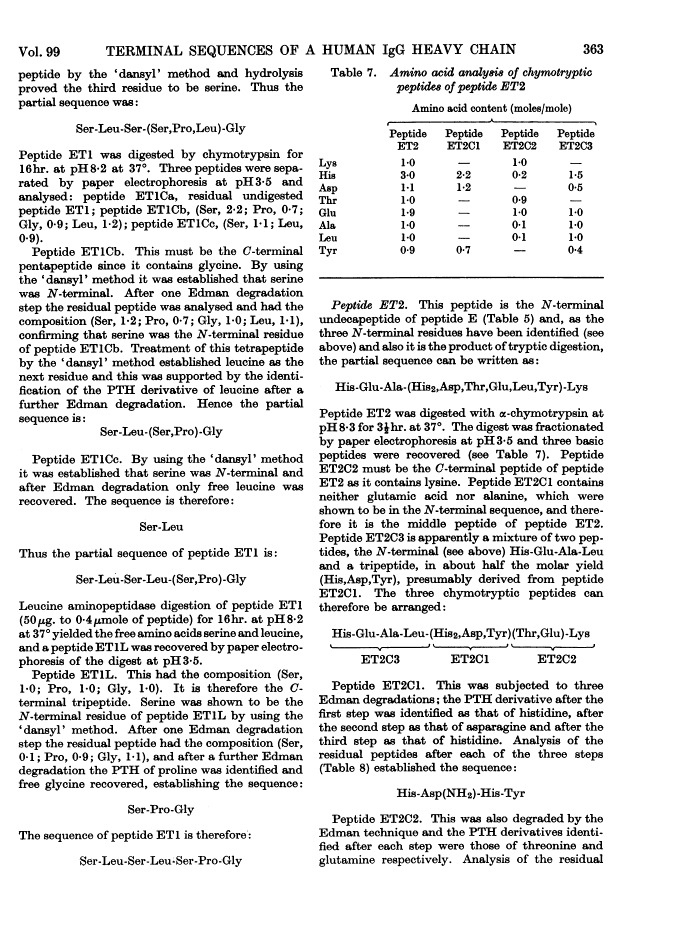
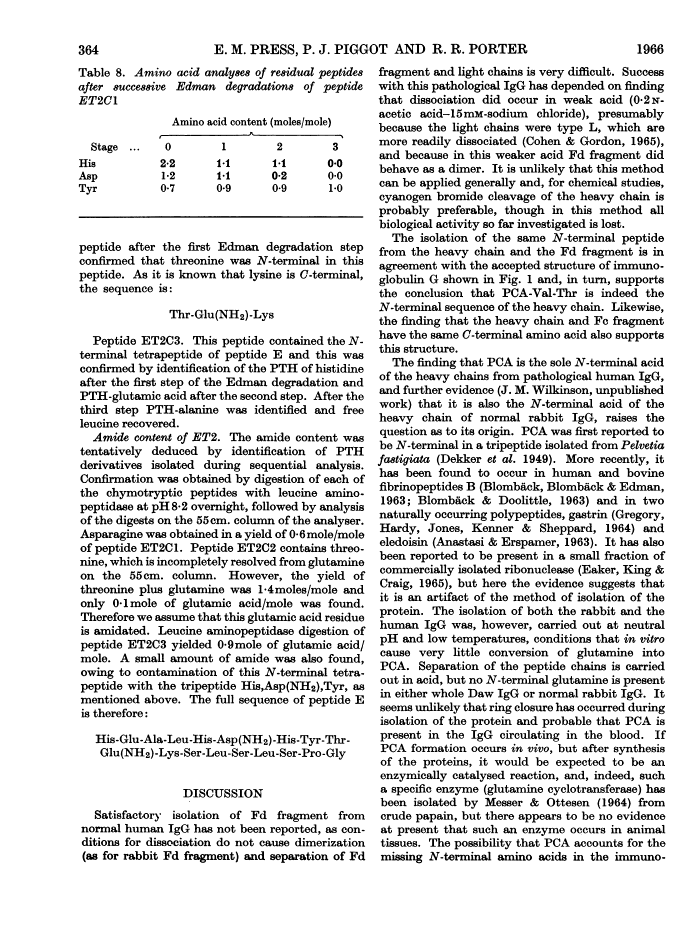
The heavy chain of a pathological human immunoglobulin IgG and also the Fd fragment have been isolated. No free α-amino group was present on either and the N-terminal sequence of both has been identified as pyrrolid-2-one-5-carbonylvalylthreonine. Splitting at the four methionine residues of the heavy chain with cyanogen bromide gave five fractions. The fraction from the C-terminal end of the chain was isolated in high yield and the amino acid sequence was: His-Glu-Ala-Leu-His-Asp(NH2)-His-Tyr-Thr-Glu(NH2)-Lys-Ser-Leu-Ser-Leu-Ser-Pro-Gly These results give strong support to the view that the heavy chain of immunoglobulin is a single peptide chain.
Full text
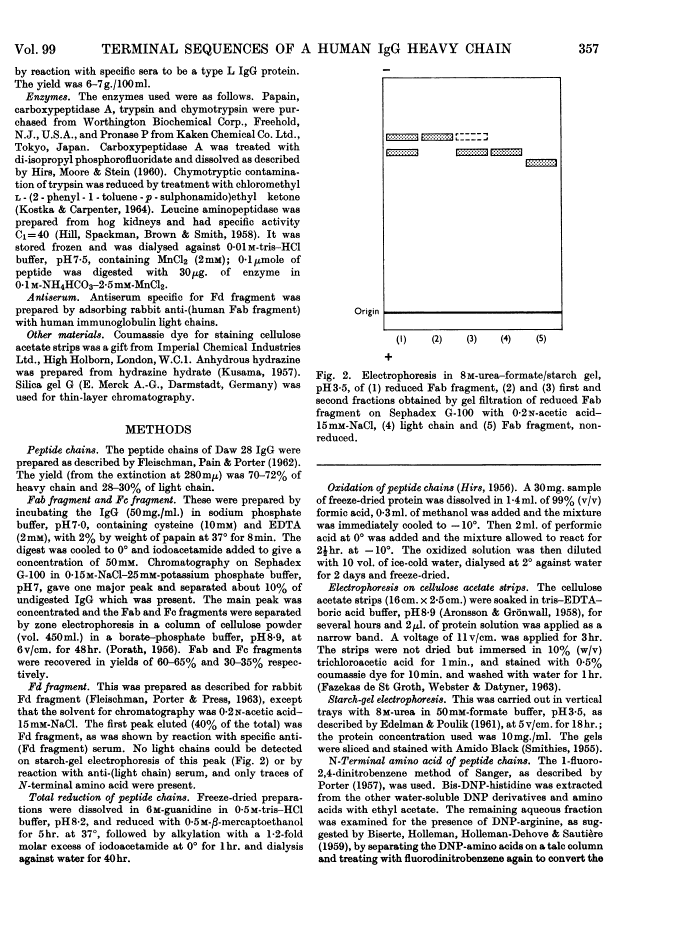
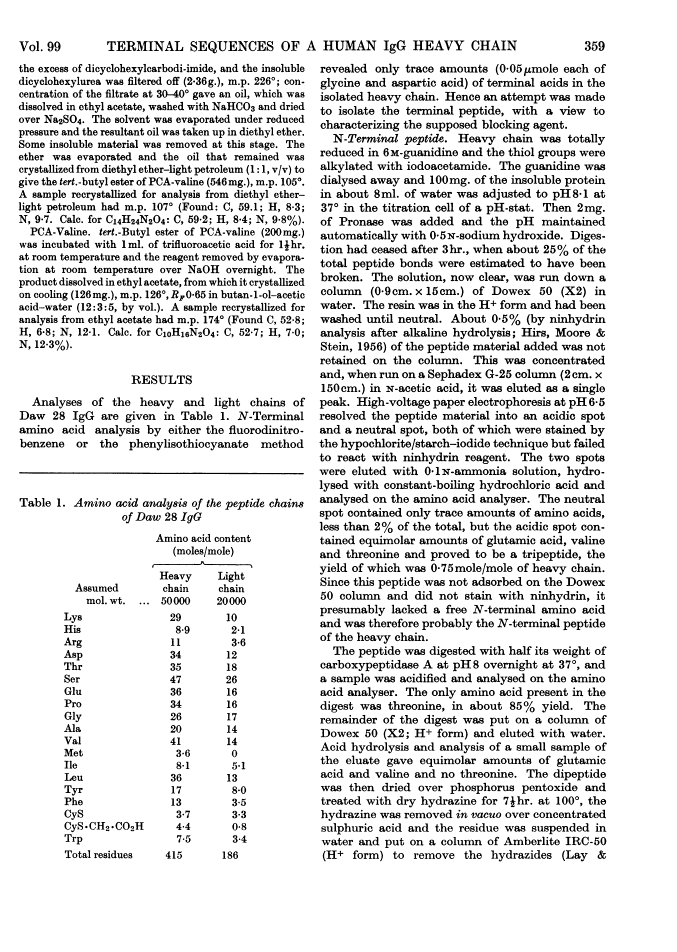
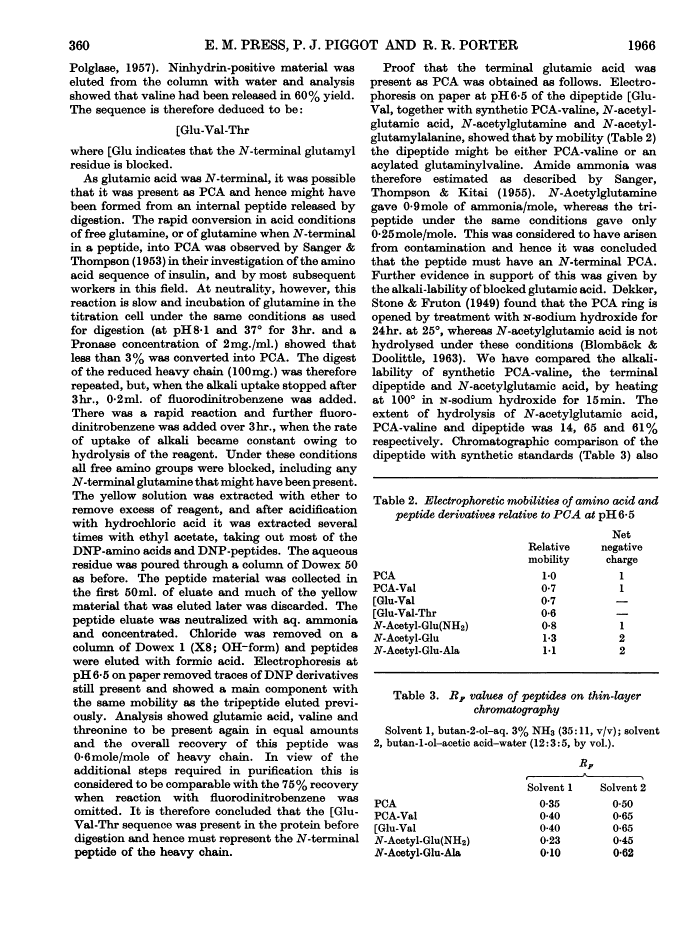
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANASTASI A, ERSPAMER V. The isolation and amino acid sequence of eledoisin, the active endecapeptide of the posterior salivary glands of Eledone. Arch Biochem Biophys. 1963 Apr;101:56–65. [Abstract] [Google Scholar]
- ARONSSON T, GRONWALL A. Electrophoretic separation of serum protein into twelve fractions. Scand J Clin Lab Invest. 1958;10(3):348–348. [Abstract] [Google Scholar]
- BARGETZI JP, THOMPSON EO, SAMPATHKUMAR KS, WALSH KA, NEURATH H. THE AMINO- AND CARBOXYL-TERMINAL RESIDUES AND THE SELF-DIGESTION OF BOVINE PANCREATIC CARBOXYPEPTIDASE A. J Biol Chem. 1964 Nov;239:3767–3774. [Abstract] [Google Scholar]
- BRADBURY JH. The kinetics of hydrazinolysis of simple peptides in anhydrous hydrazine. Biochem J. 1958 Mar;68(3):475–482. [Europe PMC free article] [Abstract] [Google Scholar]
- COHEN S. PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL AND PATHOLOGICAL HUMAN GAMMA GLOBULINS. Biochem J. 1963 Nov;89:334–341. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen S, Gordon S. Dissociation of kappa- and lambda-chains from reduced human immunoglobulins. Biochem J. 1965 Nov;97(2):460–465. [Europe PMC free article] [Abstract] [Google Scholar]
- COHEN S, PORTER RB. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. [Abstract] [Google Scholar]
- Crumpton MJ, Wilkinson JM. The immunological activity of some of the chymotryptic peptides of sperm-whale myoglobin. Biochem J. 1965 Mar;94(3):545–556. [Europe PMC free article] [Abstract] [Google Scholar]
- DEKKER CA, STONE D, FRUTON JS. A peptide from a marine alga. J Biol Chem. 1949 Dec;181(2):719–729. [Abstract] [Google Scholar]
- Eaker DL, King TP, Craig LC. Des-lysyl glutamyl and des-lysyl pyroglutamyl ribonucleases. I. Isolation and characterization. Biochemistry. 1965 Aug;4(8):1473–1478. [Abstract] [Google Scholar]
- EDELMAN GM, GALLY JA. The nature of Bence-Jones proteins. Chemical similarities to polypetide chains of myeloma globulins and normal gamma-globulins. J Exp Med. 1962 Aug 1;116:207–227. [Europe PMC free article] [Abstract] [Google Scholar]
- EDELMAN GM, POULIK MD. Studies on structural units of the gamma-globulins. J Exp Med. 1961 May 1;113:861–884. [Europe PMC free article] [Abstract] [Google Scholar]
- ERIKSSON S, SJOQUIST J. Quantitative determination of N-terminal amino acids in some serum proteins. Biochim Biophys Acta. 1960 Dec 4;45:290–296. [Abstract] [Google Scholar]
- FLEISCHMAN JB, PAIN RH, PORTER RR. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [Abstract] [Google Scholar]
- FLEISCHMAN JB, PORTER RR, PRESS EM. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. [Europe PMC free article] [Abstract] [Google Scholar]
- FRAENKEL-CONRAT H, HARRIS JI, LEVY AL. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. [Abstract] [Google Scholar]
- GRAY WR, HARTLEY BS. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. [Abstract] [Google Scholar]
- GREGORY H, HARDY PM, JONES DS, KENNER GW, SHEPPARD RC. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. [Abstract] [Google Scholar]
- GROSS E, WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [Abstract] [Google Scholar]
- Hilschmann N, Craig LC. Amino acid sequence studies with Bence-Jones proteins. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1403–1409. [Europe PMC free article] [Abstract] [Google Scholar]
- HIRS CH. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [Abstract] [Google Scholar]
- HIRS CH, MOORE S, STEIN WH. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [Abstract] [Google Scholar]
- HIRS CH, MOORE S, STEIN WH. The sequence of the amino acid residues in performic acid-oxidized ribonuclease. J Biol Chem. 1960 Mar;235:633–647. [Abstract] [Google Scholar]
- KATZ AM, DREYER WJ, ANFINSEN CB. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [Abstract] [Google Scholar]
- KOSTKA V, CARPENTER FH. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [Abstract] [Google Scholar]
- LAY WP, POLGLASE WJ. Terminal amino acids of human and bovine gamma globulin. Can J Biochem Physiol. 1957 Jan;35(1):39–44. [Abstract] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [Abstract] [Google Scholar]
- MESSER M, OTTESEN M. ISOLATION AND PROPERTIES OF GLUTAMINE CYCLOTRANSFERASE OF DRIED PAPAYA LATEX. Biochim Biophys Acta. 1964 Nov 22;92:409–411. [Abstract] [Google Scholar]
- MOORE S, STEIN WH. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [Abstract] [Google Scholar]
- PORATH J. Methodological studies of zone-electrophoresis in vertical columns. I. Fractionation in cellulose powder columns of substances of low molecular weight exemplified by amino acids and related compounds. Biochim Biophys Acta. 1956 Oct;22(1):151–175. [Abstract] [Google Scholar]
- Sie HG, Hablanian A. Depletion of glycogen synthetase and increase of glucose 6-phosphate dehydrogenase in livers of ethionine-treated mice. Biochem J. 1965 Oct;97(1):32–36. [Europe PMC free article] [Abstract] [Google Scholar]
- SANGER F, THOMPSON EOP. The amino-acid sequence in the glycyl chain of insulin. II. The investigation of peptides from enzymic hydrolysates. Biochem J. 1953 Feb;53(3):366–374. [Europe PMC free article] [Abstract] [Google Scholar]
- SANGER F, THOMPSON EO, KITAI R. The amide groups of insulin. Biochem J. 1955 Mar;59(3):509–518. [Europe PMC free article] [Abstract] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. [Europe PMC free article] [Abstract] [Google Scholar]
- SMYTH DG, STEIN WH, MOORE S. On the sequence of residues 11 to 18 in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1845–1850. [Abstract] [Google Scholar]
- STEERS E, Jr, CRAVEN GR, ANFINSEN CB, BETHUNE JL. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [Abstract] [Google Scholar]
- Stemke GW, Fischer RJ. Rabbit 19S antibodies with allotypic specificities of the a-locus group. Science. 1965 Dec 3;150(3701):1298–1303. [Abstract] [Google Scholar]
- Titani K, Whitley E, Jr, Avogardo L, Putnam FW. Immunoglobulin structure: partial amino acid sequence of a Bence Jones protein. Science. 1965 Sep 3;149(3688):1090–1092. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj0990356
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1265004?pdf=render
Citations & impact
Impact metrics
Article citations
Evidence of intra- and extracellular modifications of monoclonal IgG polypeptide chains generating charge heterogeneity.
Electrophoresis, 19(5):767-775, 01 May 1998
Cited by: 9 articles | PMID: 9629913
Human recombinant CD4 and CD4-derived synthetic peptides agglutinate immunoglobulin-coated latex particles. Evidence that residues 25-28 and 35-38 of human CD4 form two separate immunoglobulin binding sites.
Mol Immunol, 32(17-18):1399-1404, 01 Dec 1995
Cited by: 2 articles | PMID: 8643109
Pyrrolidone carboxyl peptidase (Pcp): an enzyme that removes pyroglutamic acid (pGlu) from pGlu-peptides and pGlu-proteins.
Proteins, 20(1):34-51, 01 Sep 1994
Cited by: 46 articles | PMID: 7824521
Review
Isolation of carboxyl-termini and blocked amino-termini of viral proteins by high-performance cation-exchange chromatography.
J Chromatogr, 646(1):193-205, 01 Aug 1993
Cited by: 6 articles | PMID: 8408428
Lecture for the Nobel Prize for physiology or medicine 1972: Structural studies of immunoglobulins. 1972.
Scand J Immunol, 34(4):381-389, 01 Oct 1991
Cited by: 5 articles | PMID: 1925407
Go to all (116) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The N-terminal sequence of the heavy chain of rabbit immunoglobulin IgG.
Biochem J, 100(2):303-308, 01 Aug 1966
Cited by: 35 articles | PMID: 4165510 | PMCID: PMC1265136
Comparative studies of two types of bovine immunoglobulin G heavy chains.
Biochem J, 107(4):559-564, 01 Apr 1968
Cited by: 44 articles | PMID: 4173472 | PMCID: PMC1198699
Comparative studies of sheep immunoglobulins IgG1 and IgG2: amino acid sequence of carboxy-terminal cyanogen bromide fragments from their heavy chains.
Eur J Immunol, 5(4):291-293, 01 Apr 1975
Cited by: 6 articles | PMID: 824134