Abstract
Free full text

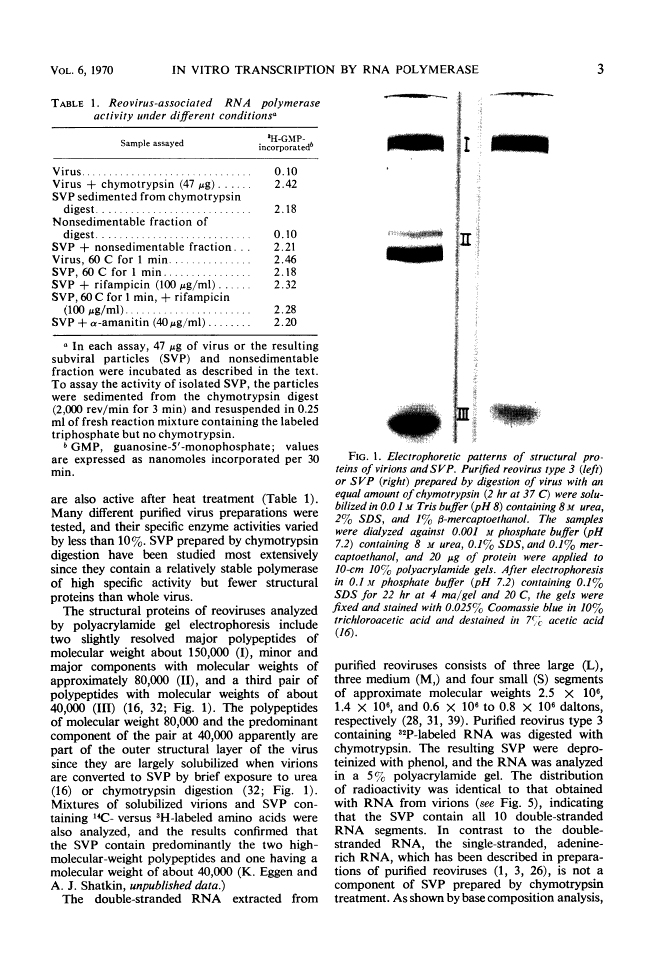
Transcription In Vitro by Reovirus-Associated Ribonucleic Acid-Dependent Polymerase 1
Abstract
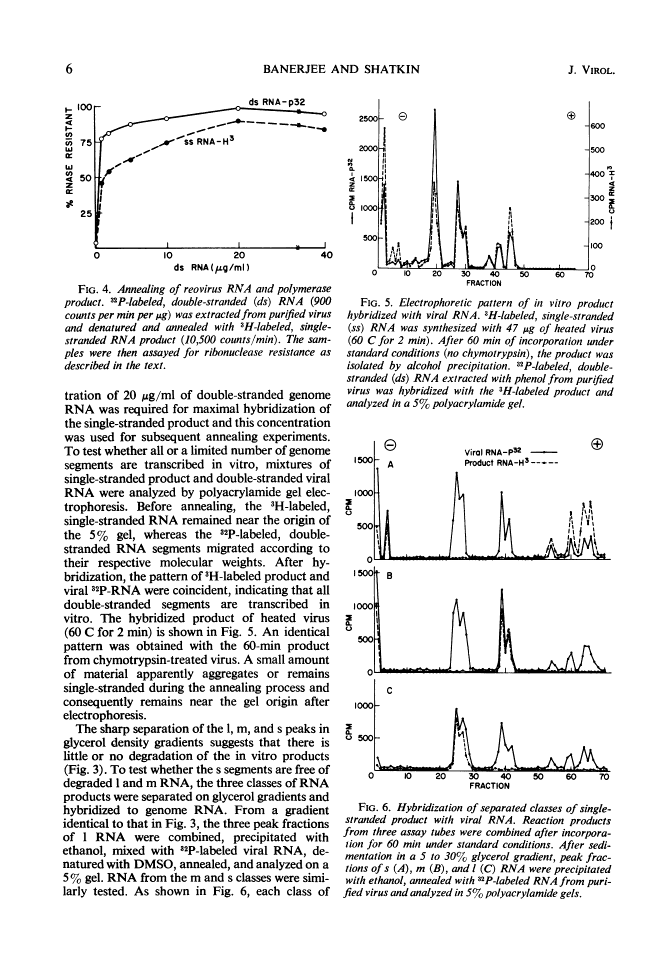
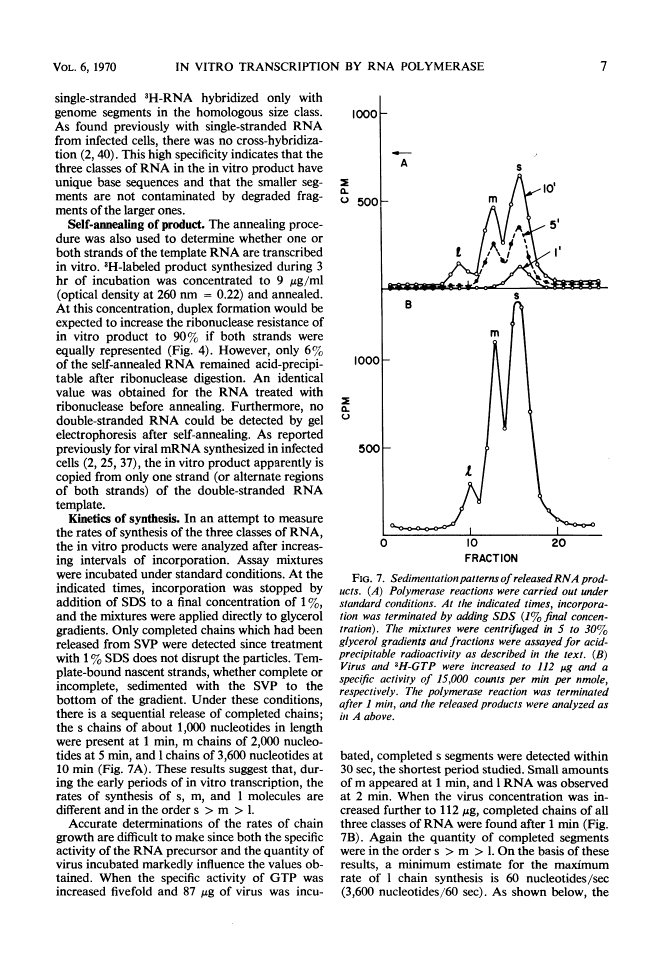
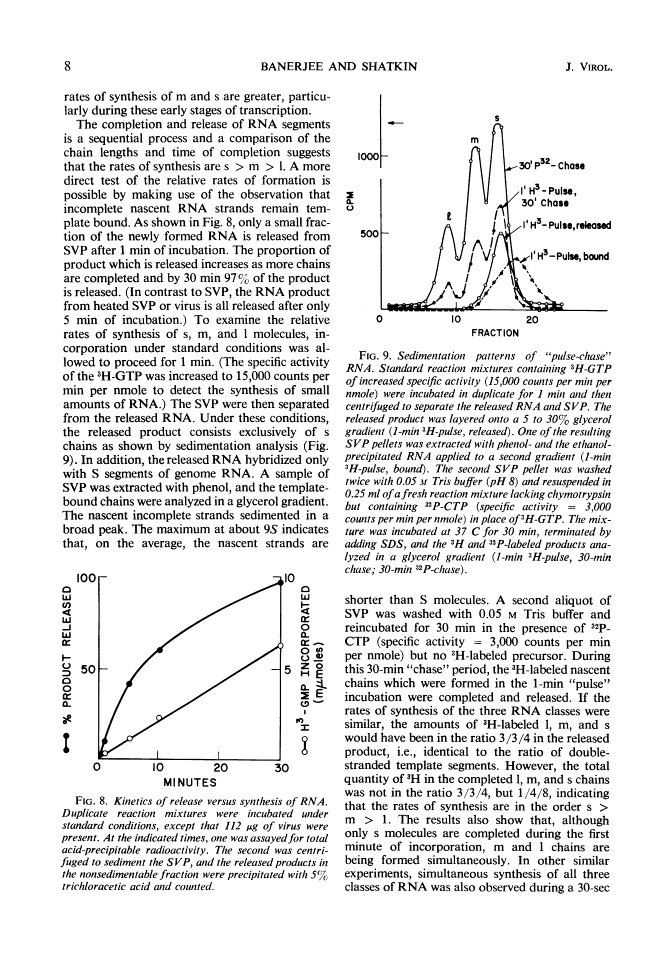
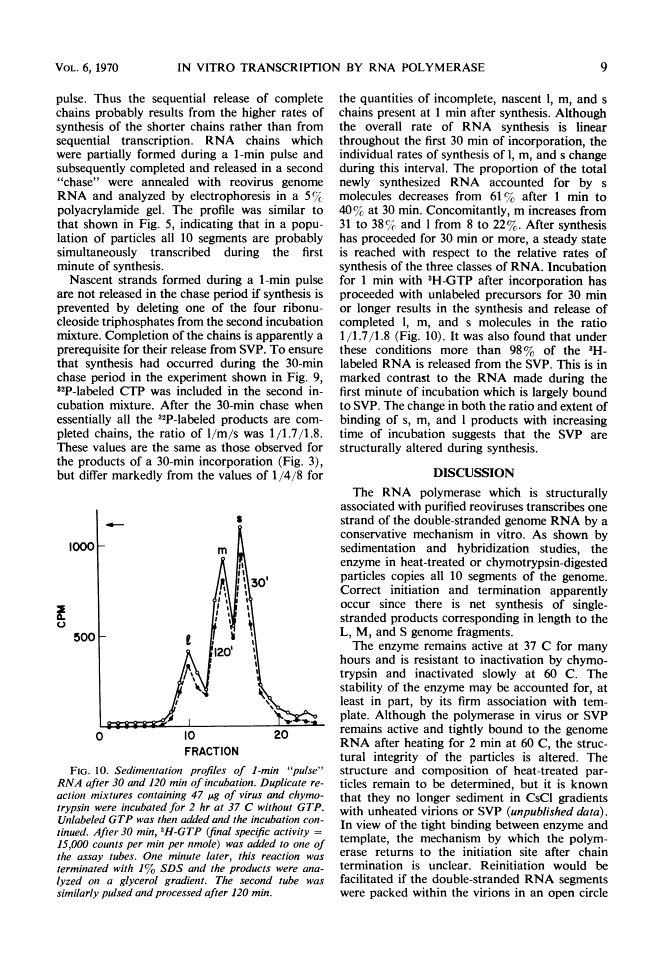
Digestion of purified reovirus type 3 with chymotrypsin degrades 70% of the viral protein and converts the virions to subviral particles (SVP). The SVP contain 3 of the 6 viral structural proteins and all 10 double-stranded ribonucleic acid (RNA) genome segments but not adenine-rich, single-stranded RNA. An RNA polymerase which is structurally associated with SVP transcribes one strand of each genome segment by a conservative mechanism in vitro. The single-stranded products include large (1.2 × 106 daltons), medium (0.7 × 106 daltons), and small (0.4 × 106 daltons) molecules which hybridize exclusively with the corresponding genome segments. The enzyme obtained by heating virions at 60 C synthesizes similar products. Kinetic and pulse-chase studies indicate that the different-sized products are synthesized simultaneously but at rates which are in the order: small > medium > large.
Full text
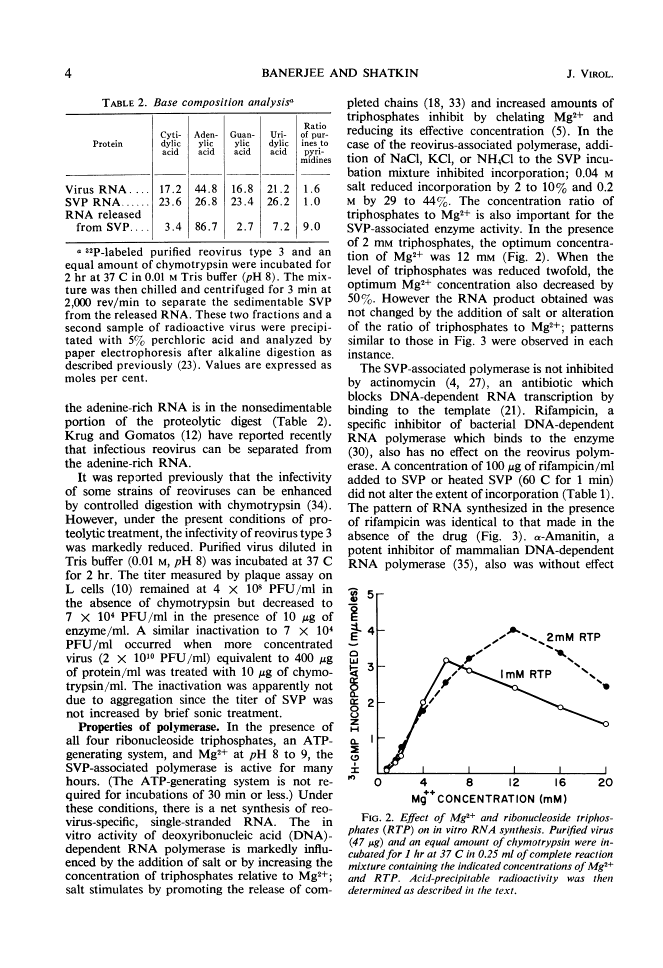
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
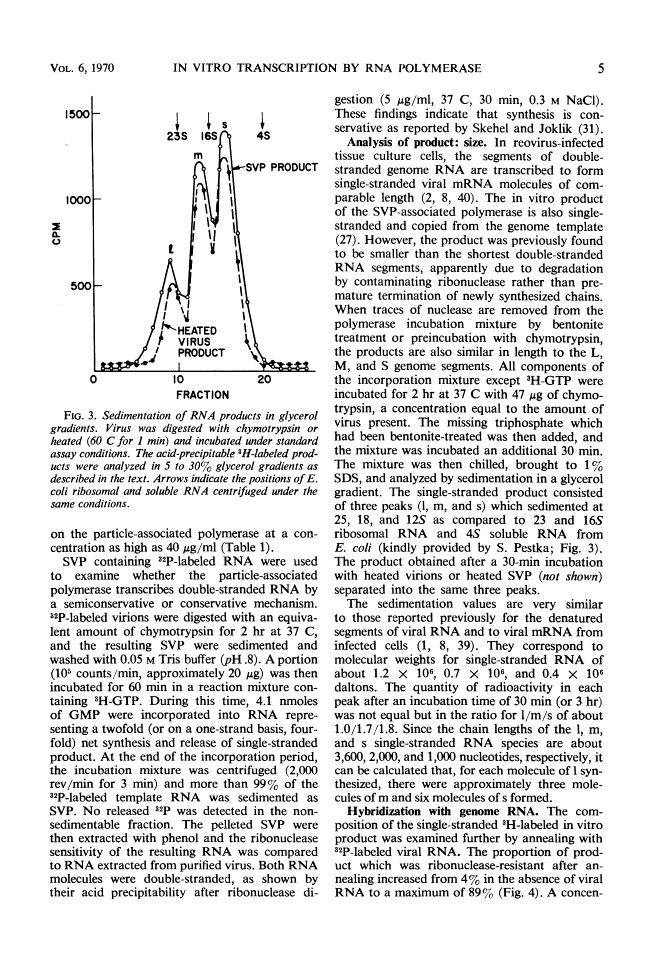
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy AR, Joklik WK. Studies on the A-rich RNA of reovirus. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1389–1395. [Europe PMC free article] [Abstract] [Google Scholar]
- Bellamy AR, Joklik WK. Studies on reovirus RNA. II. Characterization of reovirus messenger RNA and of the genome RNA segments from which it is transcribed. J Mol Biol. 1967 Oct 14;29(1):19–26. [Abstract] [Google Scholar]
- Bellamy AR, Shapiro L, August JT, Joklik WK. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. [Abstract] [Google Scholar]
- Borsa J, Graham AF. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. [Abstract] [Google Scholar]
- Boyer PD. The inhibition of pyruvate kinase by ATP: a Mg++ buffer system for use in enzyme studies. Biochem Biophys Res Commun. 1969 Mar 10;34(5):702–706. [Abstract] [Google Scholar]
- Burgess RR, Travers AA, Dunn JJ, Bautz EK. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. [Abstract] [Google Scholar]
- FRAENKEL-CONRAT H, SINGER B, TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. [Abstract] [Google Scholar]
- Gomatos PJ. RNA synthesis in reovirus-infected L929 mouse fibroblasts. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1798–1805. [Europe PMC free article] [Abstract] [Google Scholar]
- Gomatos PJ, Tamm I. THE SECONDARY STRUCTURE OF REOVIRUS RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):707–714. [Europe PMC free article] [Abstract] [Google Scholar]
- GOMATOS PJ, TAMM I, DALES S, FRANKLIN RM. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. [Abstract] [Google Scholar]
- Kates JR, McAuslan BR. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. [Europe PMC free article] [Abstract] [Google Scholar]
- Krug RM, Gomatos PJ. Absence of adenine-rich ribonucleic acid from purified infectious reovirus 3. J Virol. 1969 Nov;4(5):642–650. [Europe PMC free article] [Abstract] [Google Scholar]
- Kudo H, Graham AF. Selective inhibition of reovirus induced RNA in L cells. Biochem Biophys Res Commun. 1966 Jul 20;24(2):150–155. [Abstract] [Google Scholar]
- Lewandowski LJ, Kalmakoff J, Tanada Y. Characterization of a Ribonucleic Acid Polymerase Activity Associated with Purified Cytoplasmic Polyhedrosis Virus of the Silkworm Bombyx mori. J Virol. 1969 Dec;4(6):857–865. [Europe PMC free article] [Abstract] [Google Scholar]
- Loening UE. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. [Europe PMC free article] [Abstract] [Google Scholar]
- Loh PC, Shatkin AJ. Structural proteins of reoviruses. J Virol. 1968 Nov;2(11):1353–1359. [Europe PMC free article] [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Maitra U, Barash F. DNA-dependent synthesis of RNA by Escherichia coli RNA polymerase: release and reinitiation of RNA chains from DNA templates. Proc Natl Acad Sci U S A. 1969 Oct;64(2):779–786. [Europe PMC free article] [Abstract] [Google Scholar]
- MAYOR HD, JAMISON RM, JORDAN LE, VANMITCHELL M. REOVIRUSES. II. STRUCTURE AND COMPOSITION OF THE VIRION. J Bacteriol. 1965 Jun;89:1548–1556. [Europe PMC free article] [Abstract] [Google Scholar]
- Munyon W, Paoletti E, Grace JT., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. [Europe PMC free article] [Abstract] [Google Scholar]
- Reich E, Goldberg IH. Actinomycin and nucleic acid function. Prog Nucleic Acid Res Mol Biol. 1964;3:183–234. [Abstract] [Google Scholar]
- Roberts JW. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. [Abstract] [Google Scholar]
- SEBRING ED, SALZMAN NP. AN IMPROVED PROCEDURE FOR MEASURING THE DISTRIBUTION OF P32O4--AMONG THE NUCLEOTIDES OF RIBONUCLEIC ACID. Anal Biochem. 1964 May;8:126–129. [Abstract] [Google Scholar]
- Shatkin AJ. Inactivity of purified reovirus RNA as a template for E. coli polymerases in vitro. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1721–1728. [Europe PMC free article] [Abstract] [Google Scholar]
- Shatkin AJ, Rada B. Reovirus-directed ribonucleic acid synthesis in infected L cells. J Virol. 1967 Feb;1(1):24–35. [Europe PMC free article] [Abstract] [Google Scholar]
- Shatkin AJ, Sipe JD. Single-stranded, adenine-rich RNA from purified reoviruses. Proc Natl Acad Sci U S A. 1968 Jan;59(1):246–253. [Europe PMC free article] [Abstract] [Google Scholar]
- Shatkin AJ, Sipe JD. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. [Europe PMC free article] [Abstract] [Google Scholar]
- Shatkin AJ, Sipe JD, Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. [Europe PMC free article] [Abstract] [Google Scholar]
- Sippel A, Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. [Abstract] [Google Scholar]
- Skehel JJ, Joklik WK. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. [Abstract] [Google Scholar]
- Smith RE, Zweerink HJ, Joklik WK. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. [Abstract] [Google Scholar]
- So AG, Davie EW, Epstein R, Tissières A. Effects of cations on DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1739–1746. [Europe PMC free article] [Abstract] [Google Scholar]
- Stirpe F, Fiume L. Studies on the pathogenesis of liver necrosis by alpha-amanitin. Effect of alpha-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967 Nov;105(2):779–782. [Europe PMC free article] [Abstract] [Google Scholar]
- VASQUEZ C, TOURNIER P. The morphology of reovirus. Virology. 1962 Aug;17:503–510. [Abstract] [Google Scholar]
- Watanabe Y, Graham AF. Structural units of reovirus ribonucleic acid and their possible functional significance. J Virol. 1967 Aug;1(4):665–677. [Europe PMC free article] [Abstract] [Google Scholar]
- Watanabe Y, Kudo H, Graham AF. Selective inhibition of reovirus ribonucleic acid synthesis by cycloheximide. J Virol. 1967 Feb;1(1):36–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Watanabe Y, Millward S, Graham AF. Regulation of transcription of the Reovirus genome. J Mol Biol. 1968 Aug 28;36(1):107–123. [Abstract] [Google Scholar]
- Watanabe Y, Prevec L, Graham AF. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Citations & impact
Impact metrics
Citations of article over time
Article citations
Cell Entry-Independent Role for the Reovirus μ1 Protein in Regulating Necroptosis and the Accumulation of Viral Gene Products.
J Virol, 93(11):e00199-19, 15 May 2019
Cited by: 7 articles | PMID: 30894465 | PMCID: PMC6532083
Cationic liposome-mediated delivery of reovirus enhances the tumor cell-killing efficiencies of reovirus in reovirus-resistant tumor cells.
Int J Pharm, 524(1-2):238-247, 04 Apr 2017
Cited by: 6 articles | PMID: 28389364
A putative ATPase mediates RNA transcription and capping in a dsRNA virus.
Elife, 4:e07901, 04 Aug 2015
Cited by: 25 articles | PMID: 26240998 | PMCID: PMC4522710
Aquareovirus NS80 Initiates Efficient Viral Replication by Retaining Core Proteins within Replication-Associated Viral Inclusion Bodies.
PLoS One, 10(5):e0126127, 04 May 2015
Cited by: 12 articles | PMID: 25938226 | PMCID: PMC4418822
Discovery of m(7)G-cap in eukaryotic mRNAs.
Proc Jpn Acad Ser B Phys Biol Sci, 91(8):394-409, 01 Jan 2015
Cited by: 112 articles | PMID: 26460318 | PMCID: PMC4729855
Review Free full text in Europe PMC
Go to all (92) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transcription by infectious subviral particles of reovirus.
J Virol, 10(4):698-706, 01 Oct 1972
Cited by: 77 articles | PMID: 4673489 | PMCID: PMC356523
Unilateral synthesis of reovirus double-stranded ribonucleic acid by a cell-free replicase system.
J Virol, 8(2):190-196, 01 Aug 1971
Cited by: 30 articles | PMID: 5115915 | PMCID: PMC356230
Reovirus-specific ribonucleic acid from polysomes of infected L cells.
J Virol, 9(1):61-69, 01 Jan 1972
Cited by: 18 articles | PMID: 5061989 | PMCID: PMC356262
[Structure and multiplication mechanism of reovirus].
Tanpakushitsu Kakusan Koso, 15(7):764-776, 01 Jun 1970
Cited by: 0 articles | PMID: 4915333
Review