Abstract
Free full text

Factors influencing quantitative isolation of varicella-zoster virus.
Abstract
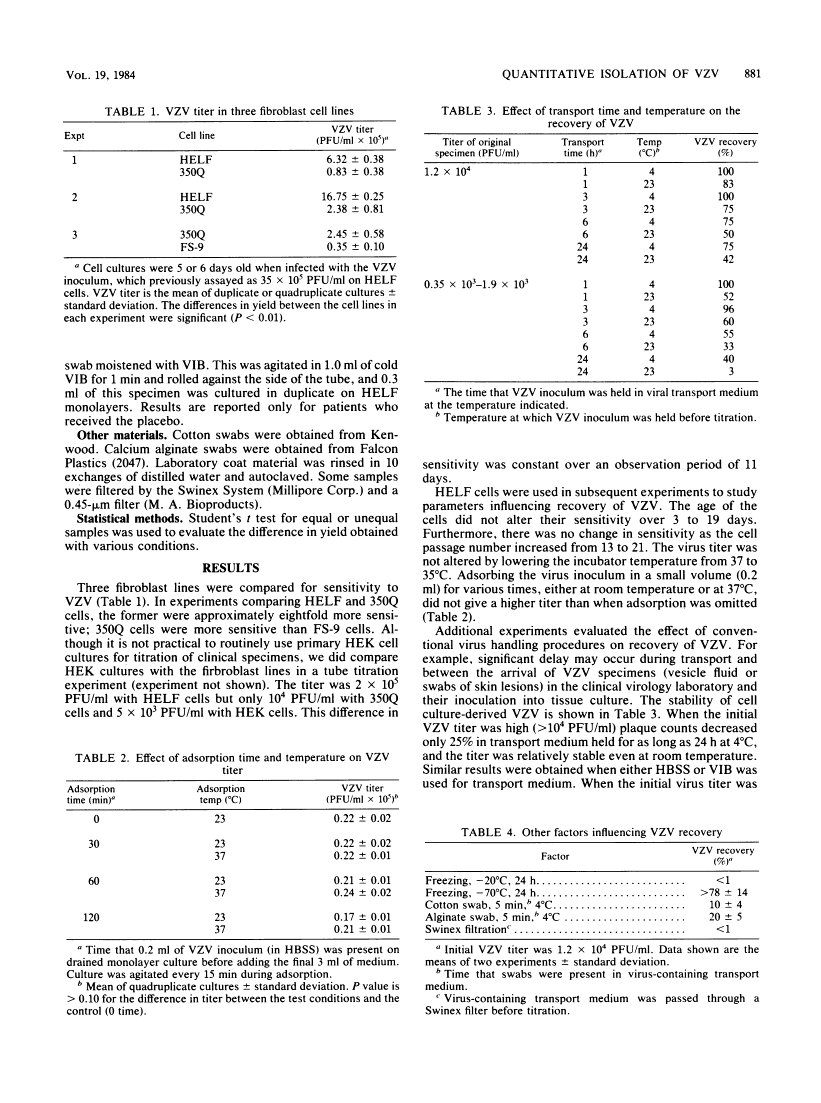
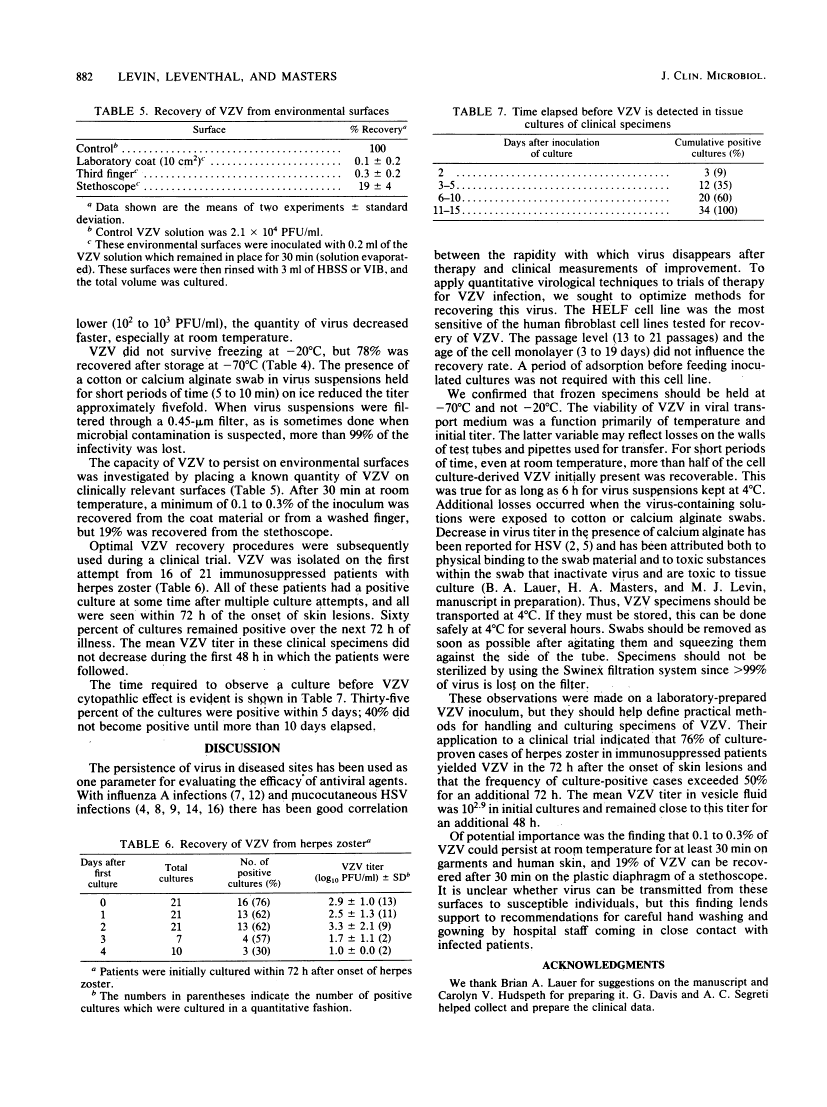
Optimal conditions are described for the recovery of cell culture-derived varicella-zoster virus (VZV). Of the cells tested, human embryonic lung fibroblasts were the most sensitive. Storage and handling procedures were examined to determine the stability of VZV in viral transport medium. When the initial VZV titer was high (2 X 10(4) PFU/ml) 40% of the VZV survived room temperature for 24 h and 75% of the VZV remained viable for this long at 4 degrees C. Recovery was 5- to 10-fold less at lower initial VZV titers (less than 2 X 10(3) PFU/ml). Other factors which influenced VZV recovery included freezing at -20 degrees C, the use of cotton or calcium alginate swabs, and filtration to remove bacterial contaminants. The tissue culture methods described were used in a reconstruction experiment to demonstrate that VZV could be recovered from a laboratory coat or human skin (0.1 to 0.3% of input VZV) or from a stethoscope (19% of input VZV) as late as 30 min after inoculation. During a clinical trial using optimal VZV recovery procedures, 76% of the patients with herpes zoster yielded VZV when first cultured, and 60% remained culture positive for an additional 48 h.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (707K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B, Braun C, Balfour HH., Jr Acyclovir therapy for acute herpes zoster. Lancet. 1982 Jul 17;2(8290):118–121. [Abstract] [Google Scholar]
- Bettoli EJ, Brewer PM, Oxtoby MJ, Zaidi AA, Guinan ME. The role of temperature and swab materials in the recovery of herpes simplex virus from lesions. J Infect Dis. 1982 Mar;145(3):399–399. [Abstract] [Google Scholar]
- Bryson YJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, Johnson BL, Wiesmeier E, Growdon W, Creagh-Kirk T, et al. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. [Abstract] [Google Scholar]
- Corey L, Nahmias AJ, Guinan ME, Benedetti JK, Critchlow CW, Holmes KK. A trial of topical acyclovir in genital herpes simplex virus infections. N Engl J Med. 1982 Jun 3;306(22):1313–1319. [Abstract] [Google Scholar]
- Crane LR, Gutterman PA, Chapel T, Lerner AM. Incubation of swab materials with herpes simplex virus. J Infect Dis. 1980 Apr;141(4):531–531. [Abstract] [Google Scholar]
- Knight V, Fedson D, Baldini J, Douglas RG, Couch RB. Amantadine therapy of epidemic influenza a(2) (Hong Kong). Infect Immun. 1970 Feb;1(2):200–204. [Europe PMC free article] [Abstract] [Google Scholar]
- Meyers JD, Wade JC, Mitchell CD, Saral R, Lietman PS, Durack DT, Levin MJ, Segreti AC, Balfour HH., Jr Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am J Med. 1982 Jul 20;73(1A):229–235. [Abstract] [Google Scholar]
- Mitchell CD, Bean B, Gentry SR, Groth KE, Boen JR, Balfour HH., Jr Acyclovir therapy for mucocutaneous herpes simplex infections in immunocompromised patients. Lancet. 1981 Jun 27;1(8235):1389–1392. [Abstract] [Google Scholar]
- Nilsen AE, Aasen T, Halsos AM, Kinge BR, Tjøtta EA, Wikström K, Fiddian AP. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet. 1982 Sep 11;2(8298):571–573. [Abstract] [Google Scholar]
- Prober CG, Kirk LE, Keeney RE. Acyclovir therapy of chickenpox in immunosuppressed children--a collaborative study. J Pediatr. 1982 Oct;101(4):622–625. [Abstract] [Google Scholar]
- Togo Y, Hornick RB, Felitti VJ, Kaufman ML, Dawkins AT, Jr, Kilpe VE, Claghorn JL. Evaluation of therapeutic efficacy of amantadine in patients with naturally occurring A2 influenza. JAMA. 1970 Feb 16;211(7):1149–1156. [Abstract] [Google Scholar]
- Turner R, Shehab Z, Osborne K, Hendley JO. Shedding and survival of herpes simplex virus from 'fever blisters'. Pediatrics. 1982 Oct;70(4):547–549. [Abstract] [Google Scholar]
- Wade JC, Newton B, McLaren C, Flournoy N, Keeney RE, Meyers JD. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double-blind trial. Ann Intern Med. 1982 Mar;96(3):265–269. [Abstract] [Google Scholar]
- Whitley R, Barton N, Collins E, Whelchel J, Diethelm AG. Mucocutaneous herpes simplex virus infections in immunocompromised patients. A model for evaluation of topical antiviral agents. Am J Med. 1982 Jul 20;73(1A):236–240. [Abstract] [Google Scholar]
- Whitley RJ, Soong SJ, Dolin R, Betts R, Linnemann C, Jr, Alford CA., Jr Early vidarabine therapy to control the complications of herpes zoster in immunosuppressed patients. N Engl J Med. 1982 Oct 14;307(16):971–975. [Abstract] [Google Scholar]
- Yeager AS, Morris JE, Prober CG. Storage and transport of cultures for Herpes simplex virus, type 2. Am J Clin Pathol. 1979 Dec;72(6):977–979. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Clinical Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jcm.19.6.880-883.1984
Read article for free, from open access legal sources, via Unpaywall:
https://jcm.asm.org/content/jcm/19/6/880.full.pdf
Free to read at intl-jcm.asm.org
http://intl-jcm.asm.org/cgi/content/abstract/19/6/880
Free after 4 months at intl-jcm.asm.org
http://intl-jcm.asm.org/cgi/reprint/19/6/880.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jcm.19.6.880-883.1984
Article citations
Homotypic Dengue Virus Reinfections in Nicaraguan Children.
J Infect Dis, 214(7):986-993, 16 Mar 2016
Cited by: 75 articles | PMID: 26984144 | PMCID: PMC5021223
Performance evaluation of Puritan® universal transport system (UniTranz-RT™) for preservation and transport of clinical viruses.
J Med Virol, 87(10):1796-1805, 06 May 2015
Cited by: 5 articles | PMID: 26243168
Development of a varicella-zoster virus neutralization assay using a glycoprotein K antibody enzyme-linked immunosorbent spot assay.
J Virol Methods, 200:10-14, 31 Jan 2014
Cited by: 8 articles | PMID: 24486923
Herpes simplex virus and varicella-zoster virus: why do these human alphaherpesviruses behave so differently from one another?
Rev Med Virol, 15(6):393-406, 01 Nov 2005
Cited by: 24 articles | PMID: 16173110
Review
Rapid contamination of the environments with varicella-zoster virus DNA from a patient with herpes zoster.
J Med Virol, 63(1):64-66, 01 Jan 2001
Cited by: 19 articles | PMID: 11130889
Go to all (12) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human Embryonic Stem Cell-Derived Neurons Are Highly Permissive for Varicella-Zoster Virus Lytic Infection.
J Virol, 92(1):e01108-17, 14 Dec 2017
Cited by: 14 articles | PMID: 29046461 | PMCID: PMC5730782
Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C.
Infect Immun, 19(1):199-203, 01 Jan 1978
Cited by: 85 articles | PMID: 203532 | PMCID: PMC414067
Replication of varicella-zoster virus in human skin organ culture.
J Virol, 79(17):11501-11506, 01 Sep 2005
Cited by: 30 articles | PMID: 16103201 | PMCID: PMC1193618
Immunization of the elderly to boost immunity against varicella-zoster virus (VZV) as assessed by VZV skin test reaction.
Arch Virol Suppl, (17):161-172, 01 Jan 2001
Cited by: 7 articles | PMID: 11339545
Review