Abstract
Free full text

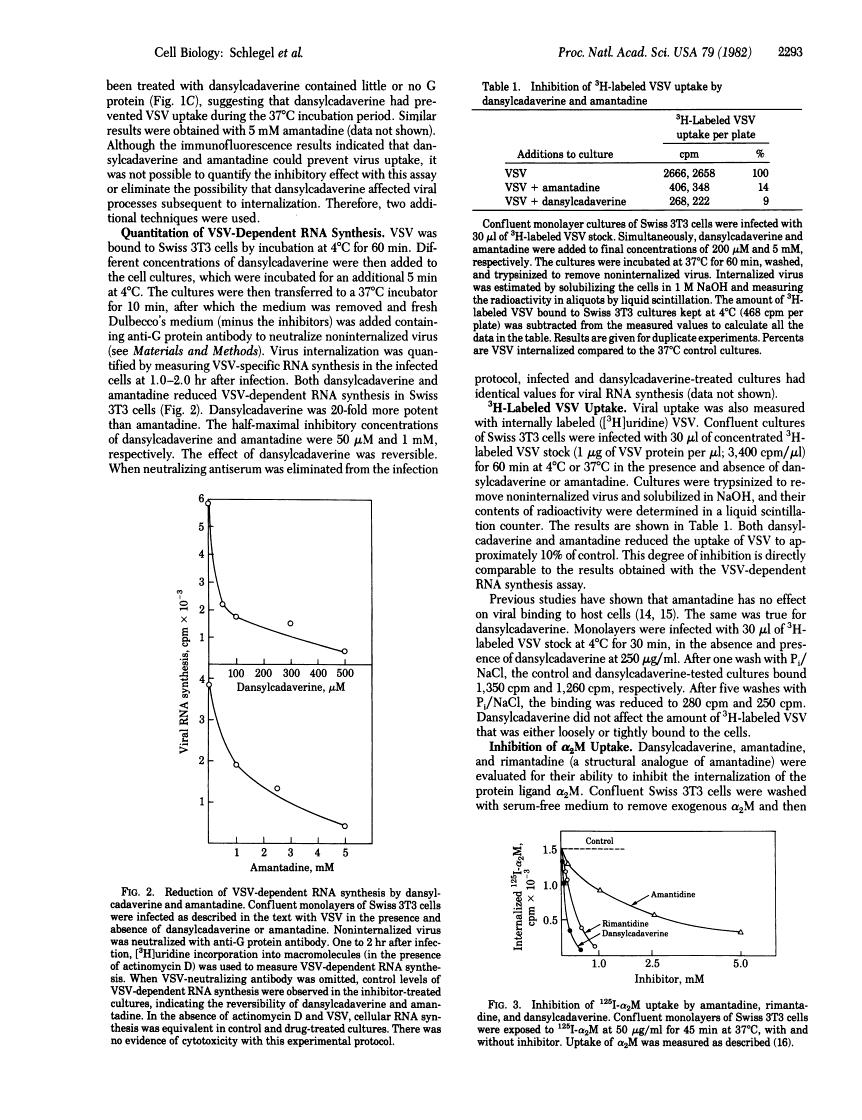
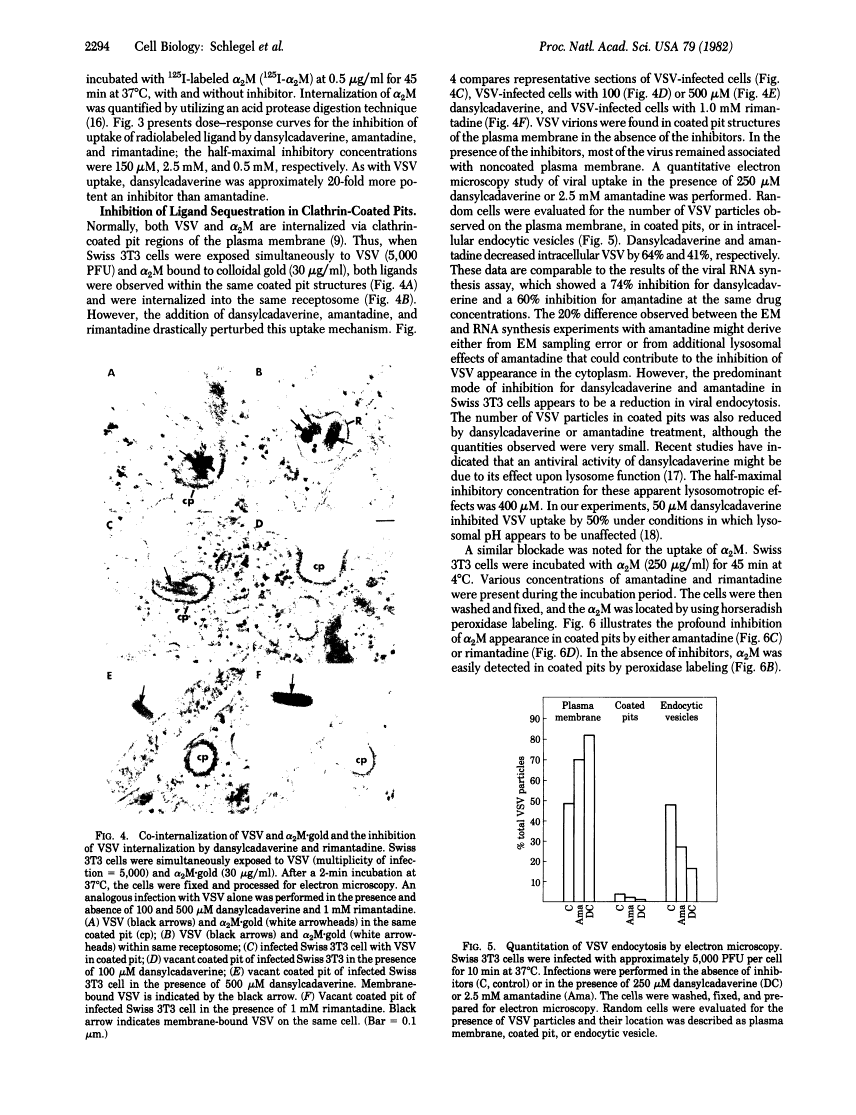
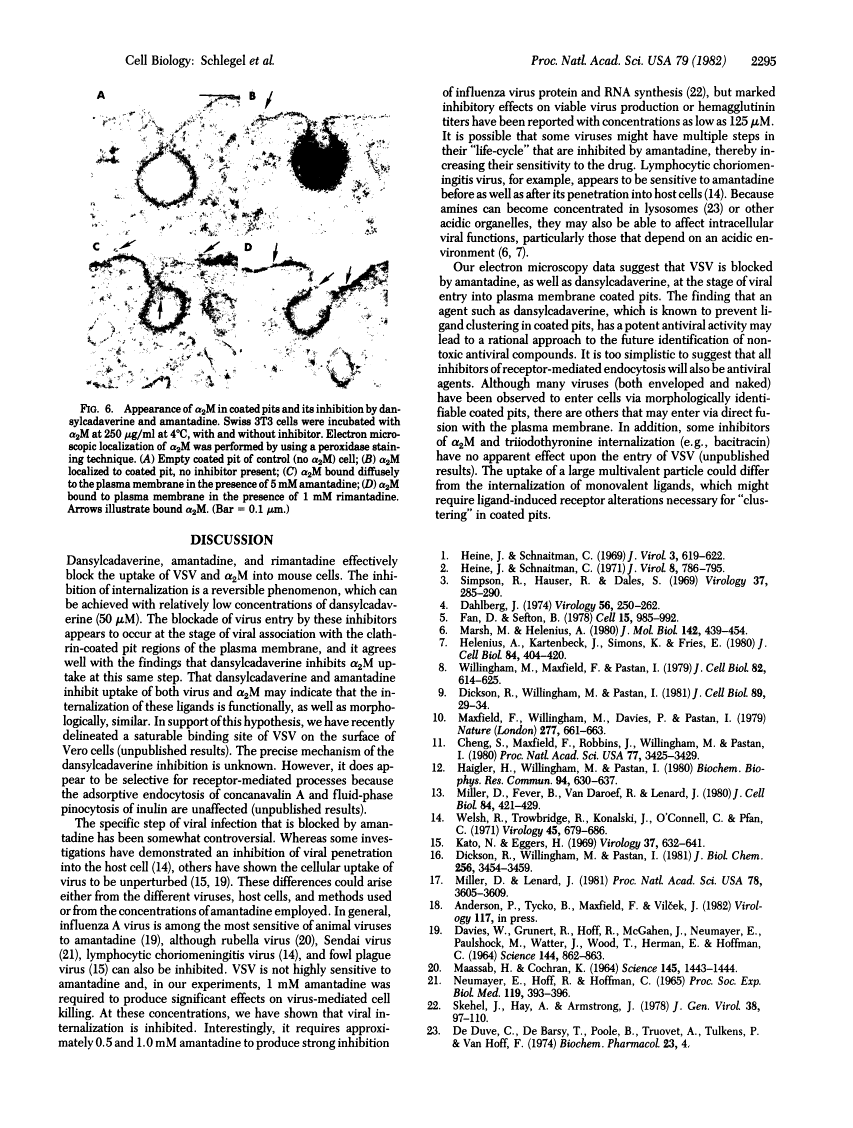
Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin.
Abstract
The entry of many animal viruses into their host cells often proceeds via a specialized internalization pathway involving clathrin-coated regions of the plasma membrane. We have examined the effect of dansylcadaverine and amantadine on the entry of vesicular stomatitis virus (VSV) into mouse cells. Both compounds inhibit VSV entry as determined by fluorescence and electron microscopy, 3H-labeled VSV uptake, and VSV-dependent RNA synthesis assays. They also inhibit the uptake of alpha 2-macroglobulin, a protein that binds to specific membrane receptors and follows the same route of internalization. Dansylcadaverine is 20-fold more potent than amantadine in blocking virus and alpha 2-macroglobulin uptake. One cellular target for both of these amine-containing compounds appears to be the clustering of membrane-bound ligands or particles in clathrin-coated pits.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Heine JW, Schnaitman CA. Fusion of vesicular stomatitis virus with the cytoplasmic membrane of L cells. J Virol. 1969 Jun;3(6):619–622. [Europe PMC free article] [Abstract] [Google Scholar]
- Heine JW, Schnaitman CA. Entry of vesicular stomatitis virus into L cells. J Virol. 1971 Nov;8(5):786–795. [Europe PMC free article] [Abstract] [Google Scholar]
- Simpson RW, Hauser RE, Dales S. Viropexis of vesicular stomatitis virus by L cells. Virology. 1969 Feb;37(2):285–290. [Abstract] [Google Scholar]
- Dahlberg JE. Quantitative electron microscopic analysis of the penetration of VSV into L cells. Virology. 1974 Mar;58(1):250–262. [Abstract] [Google Scholar]
- Fan DP, Sefton BM. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978 Nov;15(3):985–992. [Abstract] [Google Scholar]
- Marsh M, Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. [Abstract] [Google Scholar]
- Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. [Europe PMC free article] [Abstract] [Google Scholar]
- Willingham MC, Maxfield FR, Pastan IH. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979 Sep;82(3):614–625. [Europe PMC free article] [Abstract] [Google Scholar]
- Dickson RB, Willingham MC, Pastan I. alpha 2-macroglobulin adsorbed to colloidal gold: a new probe in the study of receptor-mediated endocytosis. J Cell Biol. 1981 Apr;89(1):29–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Maxfield FR, Willingham MC, Davies PJ, Pastan I. Amines inhibit the clustering of alpha2-macroglobulin and EGF on the fibroblast cell surface. Nature. 1979 Feb 22;277(5698):661–663. [Abstract] [Google Scholar]
- Cheng SY, Maxfield FR, Robbins J, Willingham MC, Pastan IH. Receptor-mediated uptake of 3,3',5-triiodo-L-thyronine by cultured fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3425–3429. [Europe PMC free article] [Abstract] [Google Scholar]
- Haigler HT, Willingham MC, Pastan I. Inhibitors of 125I-epidermal growth factor internalization. Biochem Biophys Res Commun. 1980 May 30;94(2):630–637. [Abstract] [Google Scholar]
- Miller DK, Feuer BI, Vanderoef R, Lenard J. Reconstituted G protein-lipid vesicles from vesicular stomatitis virus and their inhibition of VSV infection. J Cell Biol. 1980 Feb;84(2):421–429. [Europe PMC free article] [Abstract] [Google Scholar]
- Welsh RM, Trowbridge RS, Kowalski JB, O'Connell CM, Peau CJ. Amantadine hydrochloride inhibition of early and late stages of lymphocytic choriomenigitis virus-cell interactions. Virology. 1971 Sep;45(3):679–686. [Abstract] [Google Scholar]
- Kato N, Eggers HJ. Inhibition of uncoating of fowl plague virus by l-adamantanamine hydrochloride. Virology. 1969 Apr;37(4):632–641. [Abstract] [Google Scholar]
- Dickson RB, Willingham MC, Pastan I. Binding and internalization of 125I-alpha 2-macroglobulin by cultured fibroblasts. J Biol Chem. 1981 Apr 10;256(7):3454–3459. [Abstract] [Google Scholar]
- Miller DK, Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3605–3609. [Europe PMC free article] [Abstract] [Google Scholar]
- DAVIES WL, GRUNERT RR, HAFF RF, MCGAHEN JW, NEUMAYER EM, PAULSHOCK M, WATTS JC, WOOD TR, HERMANN EC, HOFFMANN CE. ANTIVIRAL ACTIVITY OF 1-ADAMANTANAMINE (AMANTADINE). Science. 1964 May 15;144(3620):862–863. [Abstract] [Google Scholar]
- MAASSAB HF, COCHRAN KW. RUBELLA VIRUS: INHIBITION IN VITRO BY AMANTADINE HYDROCHLORIDE. Science. 1964 Sep 25;145(3639):1443–1444. [Abstract] [Google Scholar]
- NEUMAYER EM, HAFF RF, HOFFMAN CE. ANTIVIRAL ACTIVITY OF AMANTADINE HYDROCHLORIDE IN TISSUE CULTURE AND IN OVO. Proc Soc Exp Biol Med. 1965 Jun;119:393–396. [Abstract] [Google Scholar]
- Skehel JJ, Hay AJ, Armstrong JA. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978 Jan;38(1):97–110. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.79.7.2291
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc346178?pdf=render
Citations & impact
Impact metrics
Article citations
Pulmonary Delivery of Anti-microRNA Oligonucleotide and Glycyrrhizic Acid Using Ternary Peptide Micelles for the Treatment of Acute Lung Injury.
Biomater Res, 28:0107, 08 Nov 2024
Cited by: 0 articles | PMID: 39524248
Macropinocytosis Is the Principal Uptake Mechanism of Antigen-Presenting Cells for Allergen-Specific Virus-like Nanoparticles.
Vaccines (Basel), 12(7):797, 18 Jul 2024
Cited by: 0 articles | PMID: 39066435 | PMCID: PMC11281386
The sulfonadyns: a class of aryl sulfonamides inhibiting dynamin I GTPase and clathrin mediated endocytosis are anti-seizure in animal models.
RSC Med Chem, 14(8):1492-1511, 26 Apr 2023
Cited by: 1 article | PMID: 37593570 | PMCID: PMC10429932
Amantadine and Rimantadine Inhibit Hepatitis A Virus Replication through the Induction of Autophagy.
J Virol, 96(18):e0064622, 30 Aug 2022
Cited by: 7 articles | PMID: 36040176 | PMCID: PMC9517723
Soluble C-Type Lectin-Receptor Ligands Stimulate ROS Production in Dendritic Cells and Potentiate Killing of MRSA as Well as the MRSA Induced IL-12 Production.
Front Immunol, 13:845881, 21 Mar 2022
Cited by: 2 articles | PMID: 35386713 | PMCID: PMC8977849
Go to all (102) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Inhibitors of endocytosis perturb phospholipid metabolism in rabbit neutrophils and other cells.
Proc Natl Acad Sci U S A, 80(7):1929-1932, 01 Apr 1983
Cited by: 9 articles | PMID: 6572951 | PMCID: PMC393724
Role of cell membrane composition in receptor-mediated internalization of vesicular stomatitis virus in human HEp-2 cells.
J Biol Chem, 260(9):5265-5270, 01 May 1985
Cited by: 4 articles | PMID: 2985585
The effect of lipophilic amines on the growth of hepatitis A virus in Frp/3 cells.
Arch Virol, 96(3-4):289-296, 01 Jan 1987
Cited by: 21 articles | PMID: 2821967
Studies on the effects of dansylcadaverine and related compounds on receptor-mediated endocytosis in cultured cells.
Diabetes Care, 7 Suppl 1:35-41, 01 May 1984
Cited by: 17 articles | PMID: 6145551
Review