Abstract
Free full text

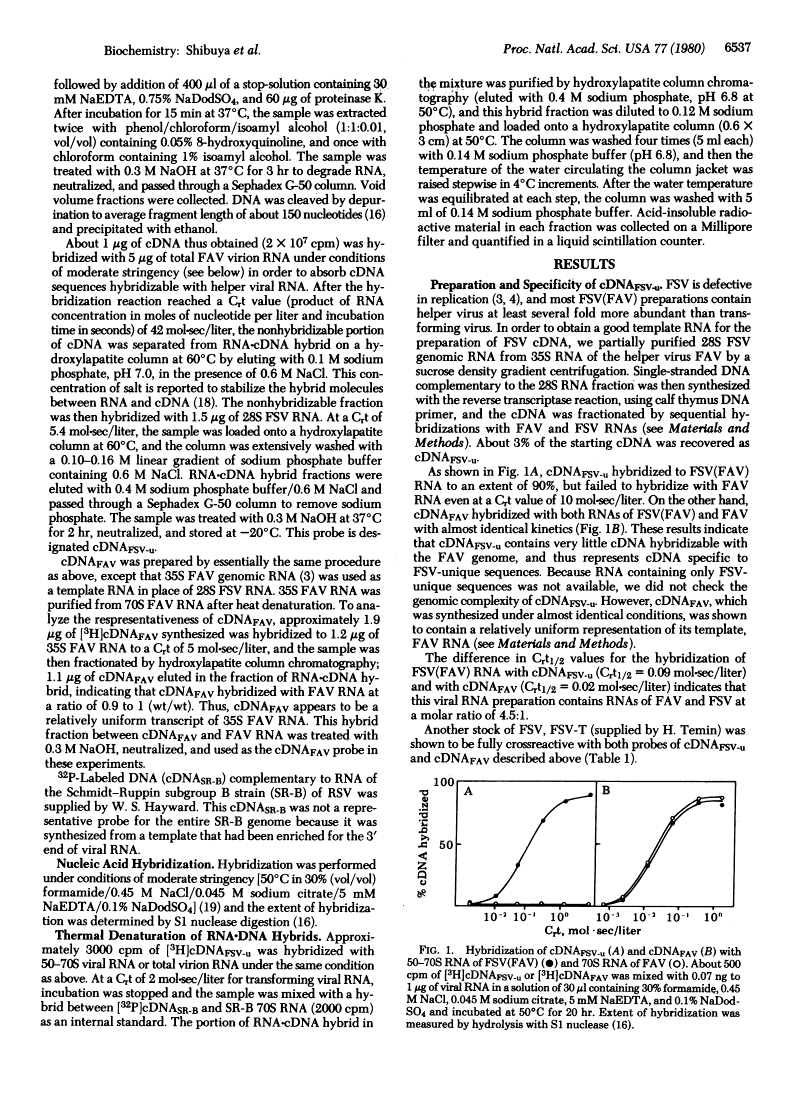
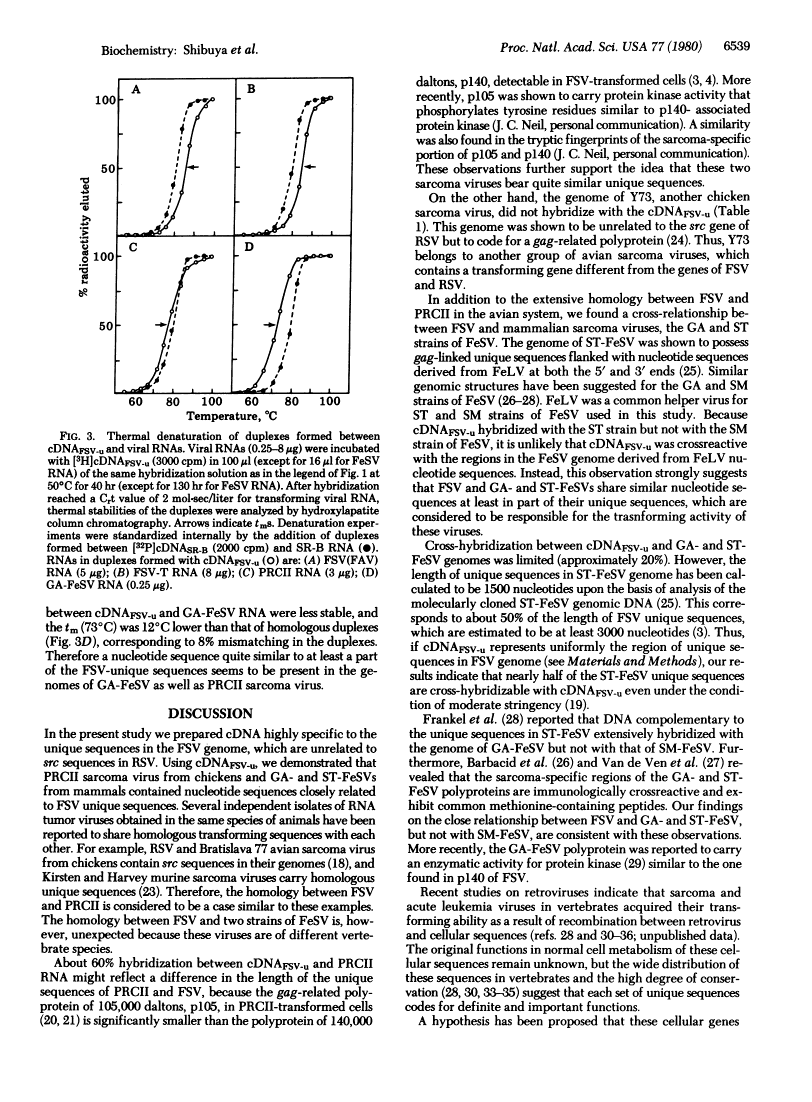
Homology exists among the transforming sequences of avian and feline sarcoma viruses.
Abstract
Fujinami sarcoma virus (FSV) of chickens does not contain nucleotide sequences related to the src gene of Rous sarcoma virus, but it carries unique sequences of at least 3000 bases, which are likely to code for the transforming protein of this virus. Using radioactive DNA complementary to FSV-unique sequences, we investigated the relatedness of FSV to other sarcoma-leukemia retroviruses in vertebrates. Under conditions of moderate stringency, no cross-hybridization was detected between FSV cDNA and RNAs of Rous sarcoma virus, Y73 avian sarcoma virus, several representative avian acute leukemia viruses, or Abelson murine leukemia virus. This cDNA, however, did hybridize with RNA of PRCII sarcoma virus of chickens to the extent of 56%. In addition, FSV cDNA was found to hybridize with RNAs of Gardner-Arnstein and Snyder-Theilen strains of feline sarcoma virus to the extent of 27% and 19%, respectively, but not with RNA of McDonough feline sarcoma virus. Studies on thermal denaturation of hybrids showed that the melting temperatures of the heteroduplexes of the FSV cDNA with RNAs of PRCII and Gardner-Arnstein feline sarcoma virus were 7 degrees C and 12 degrees C lower, respectively, compared with the melting temperature of the homologous hybrid of FSV, and suggested less than 10% mismatching in both heteroduplexes. These results indicate that nucleotide sequences closely related to at least a part of FSV-unique sequences are present in the genomes of other sarcoma viruses obtained in chickens and in cats.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hanafusa T, Wang LH, Anderson SM, Karess RE, Hayward WS, Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee WH, Bister K, Pawson A, Robins T, Moscovici C, Duesberg PH. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. [Europe PMC free article] [Abstract] [Google Scholar]
- Purchio AF, Erikson E, Brugge JS, Erikson RL. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. [Europe PMC free article] [Abstract] [Google Scholar]
- Collett MS, Erikson RL. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. [Europe PMC free article] [Abstract] [Google Scholar]
- CARR JG, CAMPBELL JG. Three new virus-induced fowl sarcomata. Br J Cancer. 1958 Dec;12(4):631–635. [Europe PMC free article] [Abstract] [Google Scholar]
- Gardner MB, Rongey RW, Arnstein P, Estes JD, Sarma P, Huebner RJ, Rickard CG. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. [Abstract] [Google Scholar]
- Snyder SP, Theilen GH. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. [Abstract] [Google Scholar]
- Itohara S, Hirata K, Inoue M, Hatsuoka M, Sato A. Isolation of a sarcoma virus from a spontaneous chicken tumor. Gan. 1978 Dec;69(6):825–830. [Abstract] [Google Scholar]
- Reynolds FH, Jr, Sacks TL, Deobagkar DN, Stephenson JR. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. [Europe PMC free article] [Abstract] [Google Scholar]
- Khan AS, Deobagkar DN, Stephenson JR. Isolation and characterization of a feline sarcoma virus-coded precursor polyprotein. Competition immunoassay for nonstructural components. J Biol Chem. 1978 Dec 25;253(24):8894–8901. [Abstract] [Google Scholar]
- McDonough SK, Larsen S, Brodey RS, Stock ND, Hardy WD., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [Abstract] [Google Scholar]
- Sarma PS, Sharar AL, McDonough S. The SM strain of feline sarcoma virus. Biologic and antigenic characterization of virus. Proc Soc Exp Biol Med. 1972 Sep;140(4):1365–1368. [Abstract] [Google Scholar]
- Hayward WS. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. [Europe PMC free article] [Abstract] [Google Scholar]
- Taylor JM, Illmensee R, Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. [Abstract] [Google Scholar]
- Stehelin D, Guntaka RV, Varmus HE, Bishop JM. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. [Abstract] [Google Scholar]
- Hayward WS, Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. [Europe PMC free article] [Abstract] [Google Scholar]
- Ullman JS, McCarthy BJ. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. II. Effects of deamination of cytosine. Biochim Biophys Acta. 1973 Feb 4;294(1):416–424. [Abstract] [Google Scholar]
- Shih TY, Williams DR, Weeks MO, Maryak JM, Vass WC, Scolnick EM. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. [Europe PMC free article] [Abstract] [Google Scholar]
- Kawai S, Yoshida M, Segawa K, Sugiyama H, Ishizaki R, Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. [Europe PMC free article] [Abstract] [Google Scholar]
- Sherr CJ, Fedele LA, Oskarsson M, Maizel J, Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. [Europe PMC free article] [Abstract] [Google Scholar]
- Barbacid M, Lauver AV, Devare SG. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. [Europe PMC free article] [Abstract] [Google Scholar]
- Van de Ven WJ, Khan AS, Reynolds FH, Jr, Mason KT, Stephenson JR. Translational products encoded by newly acquired sequences of independently derived feline sarcoma virus isolates are structurally related. J Virol. 1980 Mar;33(3):1034–1045. [Europe PMC free article] [Abstract] [Google Scholar]
- Frankel AE, Gilbert JH, Porzig KJ, Scolnick EM, Aaronson SA. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. [Europe PMC free article] [Abstract] [Google Scholar]
- Van de Ven WJ, Reynolds FH, Jr, Stephenson JR. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. [Abstract] [Google Scholar]
- Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. [Abstract] [Google Scholar]
- Hanafusa H, Halpern CC, Buchhagen DL, Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. [Europe PMC free article] [Abstract] [Google Scholar]
- Karess RE, Hayward WS, Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. [Europe PMC free article] [Abstract] [Google Scholar]
- Spector DH, Varmus HE, Bishop JM. Nucleotide sequences related to the transforming gene of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4102–4106. [Europe PMC free article] [Abstract] [Google Scholar]
- Roussel M, Saule S, Lagrou C, Rommens C, Beug H, Graf T, Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. [Abstract] [Google Scholar]
- Frankel AE, Fischinger PJ. Rate of divergence of cellular sequences homologous to segments of Moloney sarcoma virus. J Virol. 1977 Jan;21(1):153–160. [Europe PMC free article] [Abstract] [Google Scholar]
- Shih TY, Williams DR, Weeks MO, Maryak JM, Vass WC, Scolnick EM. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. [Europe PMC free article] [Abstract] [Google Scholar]
- Collett MS, Erikson E, Purchio AF, Brugge JS, Erikson RL. A normal cell protein similar in structure and function to the avian sarcoma virus transforming gene product. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3159–3163. [Europe PMC free article] [Abstract] [Google Scholar]
- Oppermann H, Levinson AD, Varmus HE, Levintow L, Bishop JM. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.77.11.6536
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc350320?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Overexpression of FES might inhibit cell proliferation, migration, and invasion of osteosarcoma cells.
Cancer Cell Int, 20:102, 30 Mar 2020
Cited by: 5 articles | PMID: 32256211 | PMCID: PMC7106745
Pathological significance and predictive value for biochemical recurrence of c-Fes expression in prostate cancer.
Prostate, 72(2):201-208, 11 May 2011
Cited by: 10 articles | PMID: 21563194
Fes tyrosine kinase expression in the tumor niche correlates with enhanced tumor growth, angiogenesis, circulating tumor cells, metastasis, and infiltrating macrophages.
Cancer Res, 71(4):1465-1473, 15 Dec 2010
Cited by: 14 articles | PMID: 21159660 | PMCID: PMC3041852
Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation.
Cell, 134(5):793-803, 01 Sep 2008
Cited by: 125 articles | PMID: 18775312 | PMCID: PMC2572732
Contributions of F-BAR and SH2 domains of Fes protein tyrosine kinase for coupling to the FcepsilonRI pathway in mast cells.
Mol Cell Biol, 29(2):389-401, 10 Nov 2008
Cited by: 16 articles | PMID: 19001085 | PMCID: PMC2612524
Go to all (70) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Molecular cloning of the Fujinami sarcoma virus genome and its comparison with sequences of other related transforming viruses.
J Virol, 42(3):1007-1016, 01 Jun 1982
Cited by: 32 articles | PMID: 6284986 | PMCID: PMC256934
Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally.
Cell, 24(1):145-153, 01 Apr 1981
Cited by: 63 articles | PMID: 6263483
Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene.
Proc Natl Acad Sci U S A, 77(4):2018-2022, 01 Apr 1980
Cited by: 72 articles | PMID: 6246518 | PMCID: PMC348642
Fusion proteins in retroviral transformation.
Adv Cancer Res, 43:205-239, 01 Jan 1985
Cited by: 4 articles | PMID: 2986428
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: CA 14935
Grant ID: N01-CO-75380
PHS HHS (1)
Grant ID: 18213