Abstract
Free full text

Anaerobic incubation enhances the colony formation of a polA recB strain of Escherichia coli K-12.
Abstract
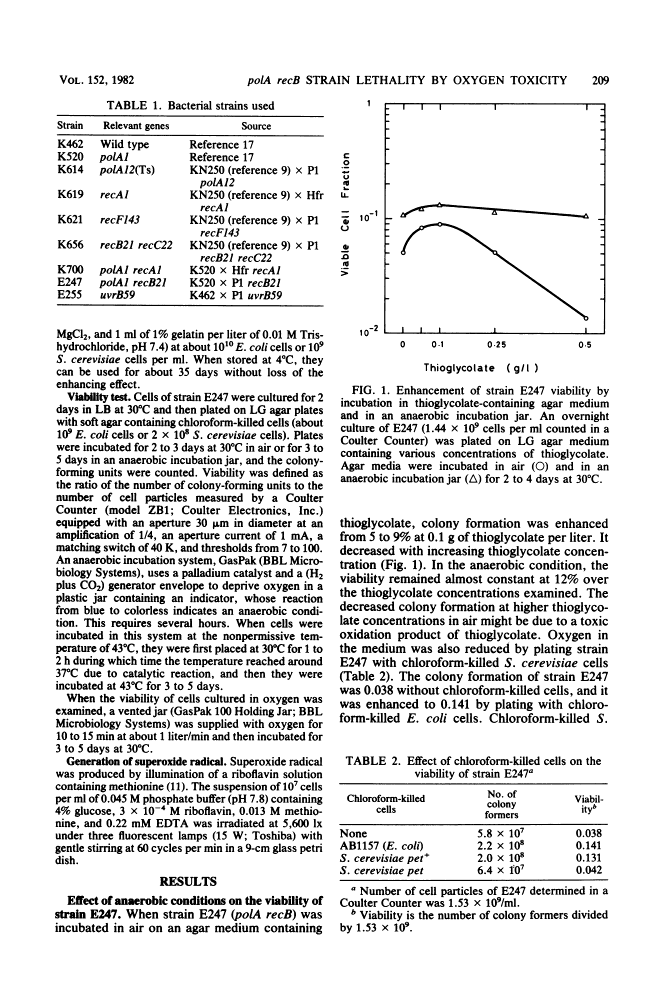
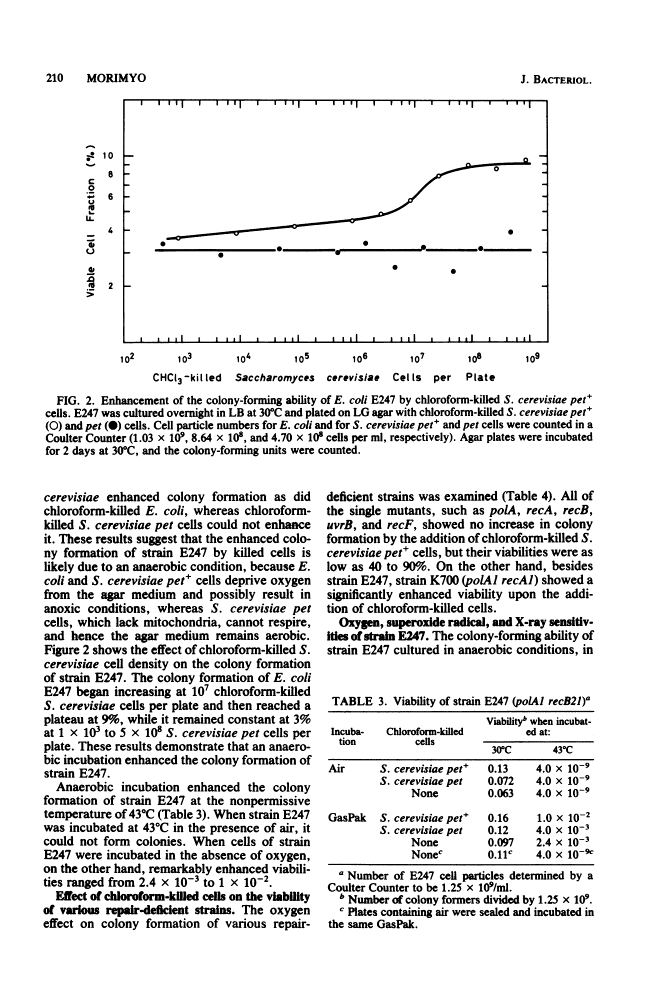
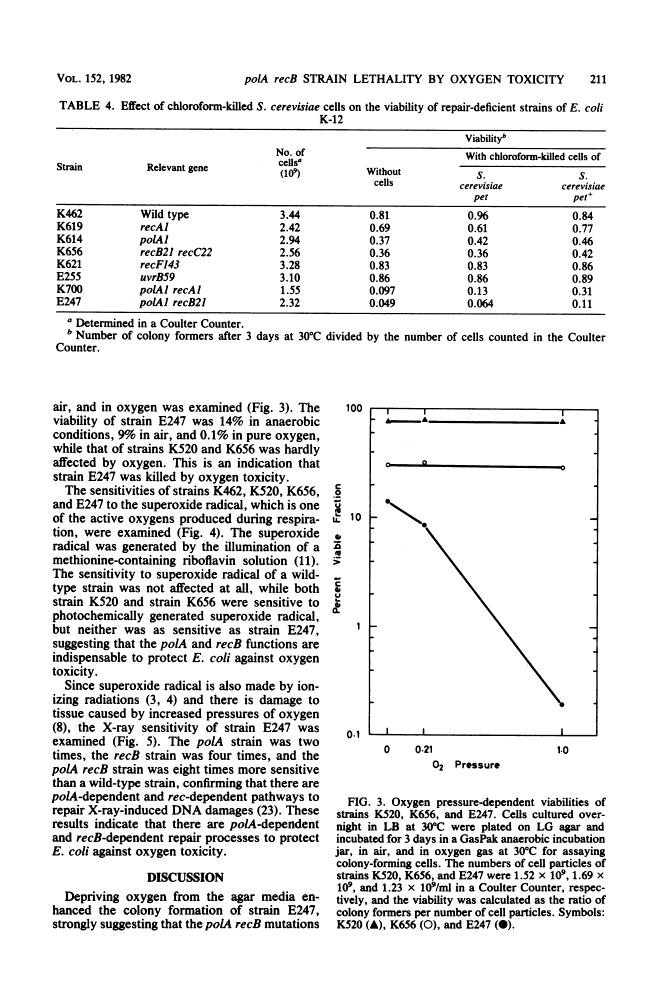
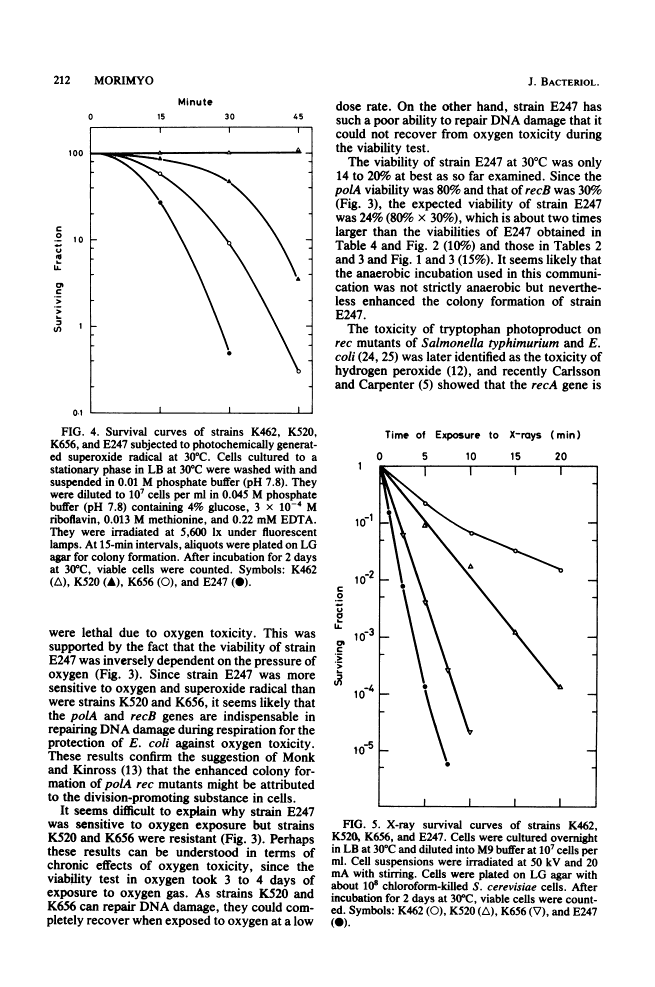
Escherichia coli strain E247 (polA1 recB21) has reduced colony formation (even at the permissive temperature of 30 degrees C) because of a poor suppressor mutation (sup-126). The colony formation was enhanced in the absence of oxygen about 3-fold at 30 degrees C and 10(6)-fold at 43 degrees C, suggesting that a polA recB strain was inviable due to oxygen toxicity. Colony formation was also increased by incubation in an agar medium containing the reducing agent thioglycolate and incubation in the presence of chloroform-killed Saccharomyces cerevisiae pet+ cells, but not pet cells. Since the E247 strain viability was inversely dependent on the oxygen pressure and since the strain was more sensitive to superoxide radical than either the polA or the recB mutant, it seems likely that the polA and recB genes play a role in repairing DNA damage during respiration.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (865K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler HI, Fisher WD, Hardigree AA, Stapleton GE. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1966 Feb;91(2):737–742. [Europe PMC free article] [Abstract] [Google Scholar]
- Adler HI, Carrasco A, Crow W, Gill JS. Cytoplasmic membrane fraction that promotes septation in an Escherichia coli lon mutant. J Bacteriol. 1981 Aug;147(2):326–332. [Europe PMC free article] [Abstract] [Google Scholar]
- Ander S. Untersuchungen an UV- und röntgenbestrahltem Wasser. Strahlentherapie. 1967 Jan;132(1):135–142. [Abstract] [Google Scholar]
- Carlsson J, Carpenter VS. The recA+ gene product is more important than catalase and superoxide dismutase in protecting Escherichia coli against hydrogen peroxide toxicity. J Bacteriol. 1980 Apr;142(1):319–321. [Europe PMC free article] [Abstract] [Google Scholar]
- De Lucia P, Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. [Abstract] [Google Scholar]
- Fisher WD, Adler HI, Shull FW, Jr, Cohen A. Properties of a cell fraction that repairs damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1969 Feb;97(2):500–505. [Europe PMC free article] [Abstract] [Google Scholar]
- Horiuchi T, Nagata T. Mutations affecting growth of the Escherichia coli cell under a condition of DNA polymerase I-deficiency. Mol Gen Genet. 1973;123(1):89–110. [Abstract] [Google Scholar]
- Kapp DS, Smith KC. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. [Europe PMC free article] [Abstract] [Google Scholar]
- Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969 Sep 10;36(6):891–897. [Abstract] [Google Scholar]
- McCormick JP, Fischer JR, Pachlatko JP, Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. [Abstract] [Google Scholar]
- Monk M, Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. [Europe PMC free article] [Abstract] [Google Scholar]
- Monk M, Kinross J, Town C. Deoxyribonucleic acid synthesis in recA and recB derivatives of an Escherichia coli K-12 strain with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1973 Jun;114(3):1014–1017. [Europe PMC free article] [Abstract] [Google Scholar]
- Monk M, Peacey M, Gross JD. Repair of damage induced by ultraviolet light in DNA polymerase-defective Escherichia coli cells. J Mol Biol. 1971 Jun 14;58(2):623–630. [Abstract] [Google Scholar]
- Morimyo M, Shimazu Y. Evidence that the gene uvrB is indispensable for a polymerase I deficient strain of Escherichia coli K-12. Mol Gen Genet. 1976 Sep 23;147(3):243–250. [Abstract] [Google Scholar]
- Nagata T, Horiuchi T. Isolation and characterization of a temperature-sensitive amber suppressor mutant of Escherichia coli K12. Mol Gen Genet. 1973;123(1):77–88. [Abstract] [Google Scholar]
- Okazaki R, Arisawa M, Sugino A. Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2954–2957. [Europe PMC free article] [Abstract] [Google Scholar]
- Rupp WD, Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. [Abstract] [Google Scholar]
- Smith KC, Meun DH. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. [Abstract] [Google Scholar]
- Town CD, Smith KC, Kaplan HS. DNA polymerase required for rapid repair of x-ray--induced DNA strand breaks in vivo. Science. 1971 May 21;172(3985):851–854. [Abstract] [Google Scholar]
- Town CD, Smith KC, Kaplan HS. Repair of X-ray damage to bacterial DNA. Curr Top Radiat Res Q. 1973 Jul;8(4):351–399. [Abstract] [Google Scholar]
- Yoakum G, Eisenstark A. Toxicity of L-Tryptophan photoproduct on recombinationless (rec) mutants of Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):653–655. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoakum G, Ferron W, Eisenstark A, Webb RB. Inhibition of replication gap closure in Escherichia coli by near-ultraviolet light photoproducts of L-tryptophan. J Bacteriol. 1974 Jul;119(1):62–69. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.152.1.208-214.1982
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc221393?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/152/1/208
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/152/1/208
Citations & impact
Impact metrics
Citations of article over time
Article citations
How Microbes Defend Themselves From Incoming Hydrogen Peroxide.
Front Immunol, 12:667343, 27 Apr 2021
Cited by: 44 articles | PMID: 33995399 | PMCID: PMC8115020
Review Free full text in Europe PMC
How Microbes Evolved to Tolerate Oxygen.
Trends Microbiol, 29(5):428-440, 24 Oct 2020
Cited by: 39 articles | PMID: 33109411 | PMCID: PMC8043972
Review Free full text in Europe PMC
Where in the world do bacteria experience oxidative stress?
Environ Microbiol, 21(2):521-530, 19 Nov 2018
Cited by: 129 articles | PMID: 30307099 | PMCID: PMC7301649
Review Free full text in Europe PMC
Replication Fork Breakage and Restart in Escherichia coli.
Microbiol Mol Biol Rev, 82(3):e00013-18, 13 Jun 2018
Cited by: 51 articles | PMID: 29898897 | PMCID: PMC6094043
Review Free full text in Europe PMC
Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli.
Free Radic Biol Med, 120:217-227, 14 Mar 2018
Cited by: 36 articles | PMID: 29550333 | PMCID: PMC5940505
Go to all (20) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Restoration of viability to an Escherichia coli mutant deficient in the 5'----3' exonuclease of DNA polymerase I.
J Bacteriol, 160(2):706-710, 01 Nov 1984
Cited by: 13 articles | PMID: 6094486 | PMCID: PMC214794
Role of DNA polymerase I in postreplication repair: a reexamination with Escherichia coli delta polA.
J Bacteriol, 169(10):4559-4564, 01 Oct 1987
Cited by: 21 articles | PMID: 3308845 | PMCID: PMC213821
Excision repair characteristics of recB - res - and uvrC - strains of Escherichia coli.
J Bacteriol, 112(3):1237-1246, 01 Dec 1972
Cited by: 41 articles | PMID: 4344920 | PMCID: PMC251554
Separate branches of the uvr gene-dependent excision repair process in ultraviolet-irradiated Escherichia coli K-12 cells; their dependence upon growth medium and the polA, recA, recB, and exrA genes.
J Bacteriol, 117(2):717-725, 01 Feb 1974
Cited by: 40 articles | PMID: 4590484 | PMCID: PMC285565