Abstract
Free full text

Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53.
Abstract
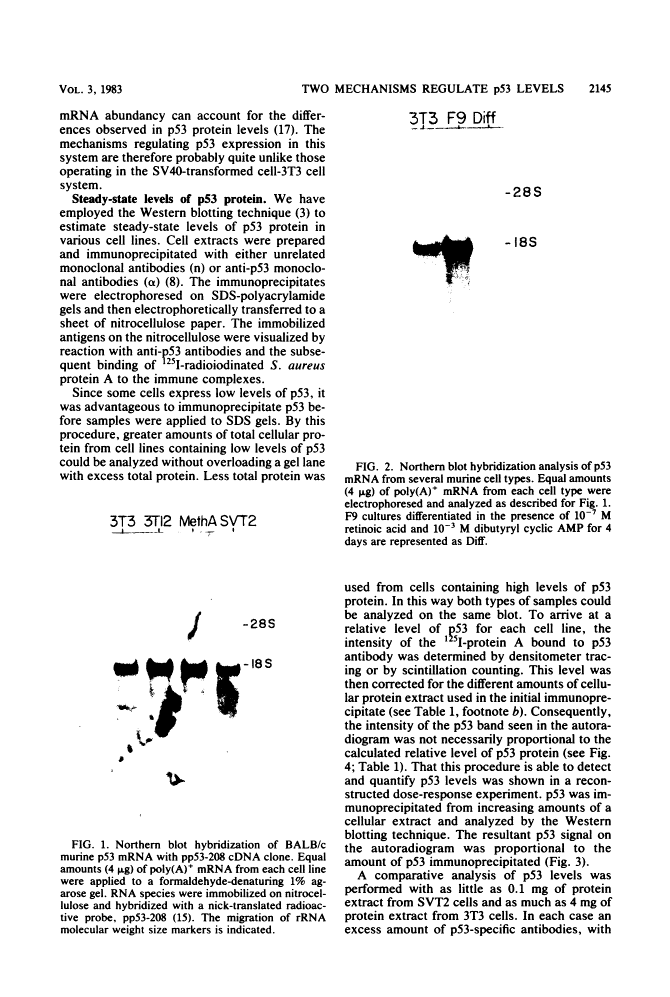
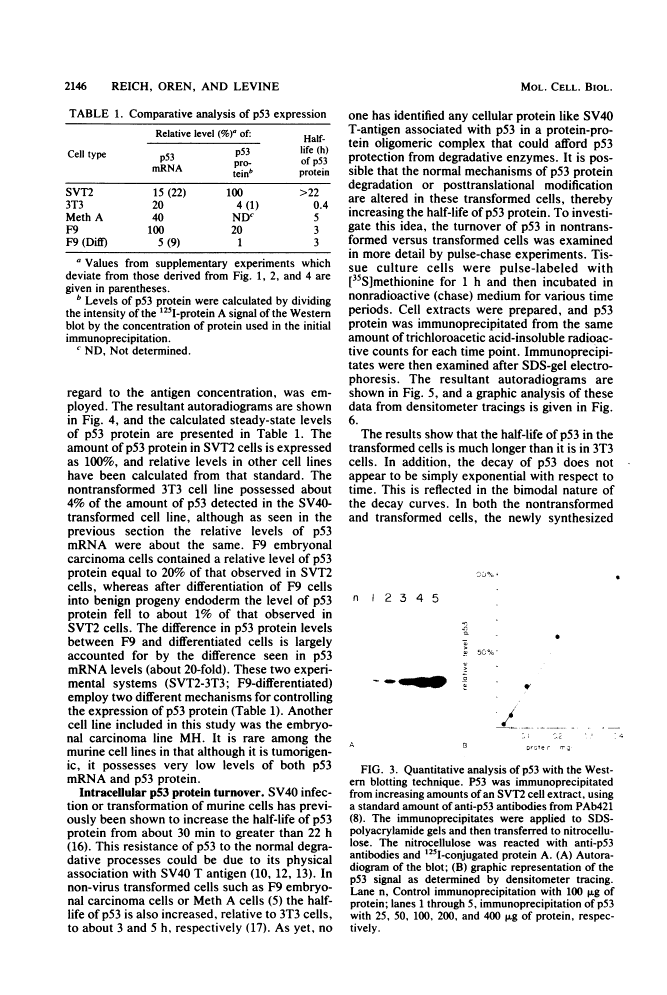
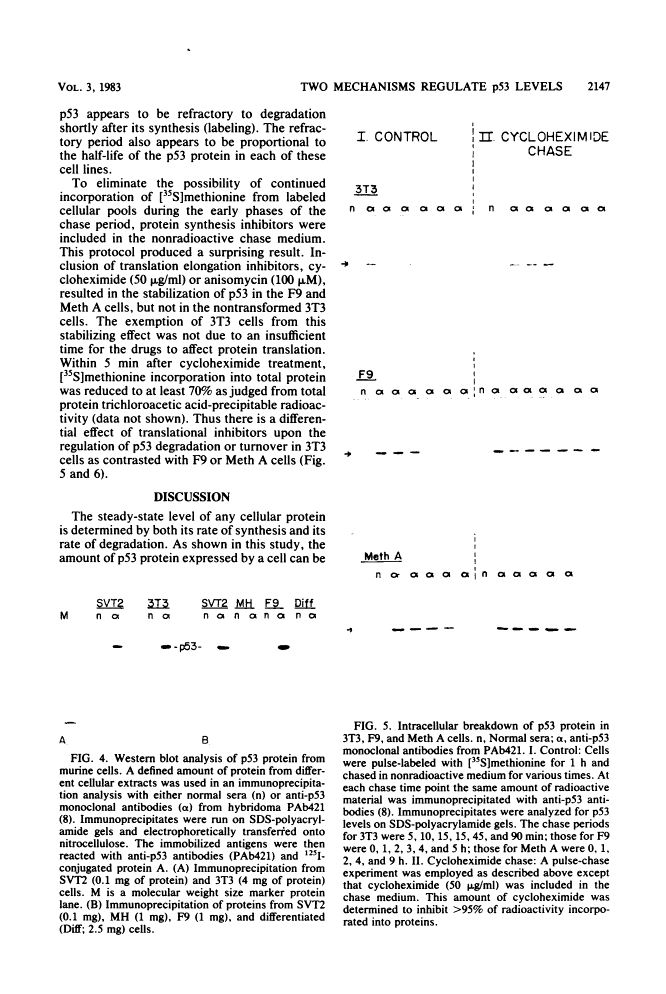
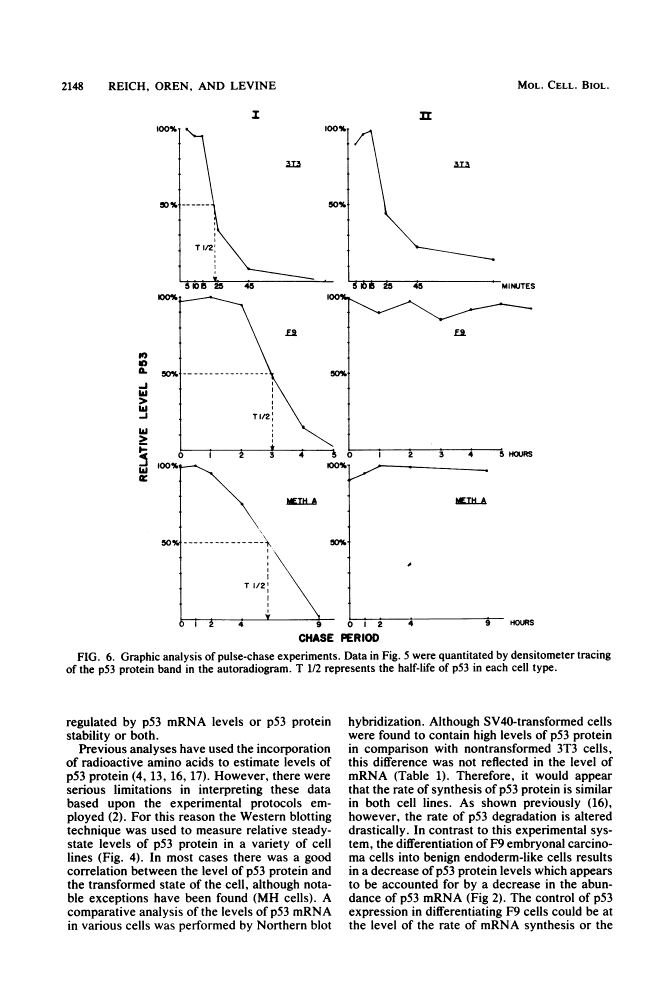
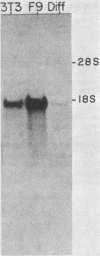
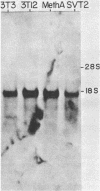
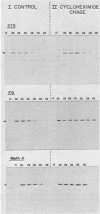
The steady-state levels of p53 protein and p53 mRNA in transformed and nontransformed cells were examined to elucidate the mechanisms controlling expression of p53. mRNA levels were determined by Northern blot hybridization analysis, employing a p53-specific cDNA clone (M. Oren and A.J. Levine, Proc. Natl. Acad. Sci. U.S.A. 80:56-59, 1983), and protein levels were determined by the Western blotting technique. Analysis of p53 mRNA revealed a single polyadenylated mRNA species migrating at ca. 18S. Levels of p53 mRNA in simian virus 40-transformed cell line (SVT2) and in an homologous nontransformed cell line (3T3) were equivalent, although the steady-state levels of p53 protein were 25- to 100-fold higher in the SVT2 cells than in the 3T3 cells. A study with a non-virus-transformed cell system revealed a different result. Embryonal carcinoma cells (F9) were found to have nearly 20-fold higher levels of p53 mRNA in comparison with differentiated benign progeny cells. In this system the difference in p53 mRNA levels corresponded to the difference in p53 protein levels. Pulse-chase experiments were performed to study the half-life of p53 protein in these four types of cells. The turnover of p53 protein occurred with biphasic kinetics. In addition, it was found that protein synthesis inhibitors placed in the medium during the chase period prevented the turnover of p53 protein in transformed cells, but not in nontransformed (3T3) cells. These results provide evidence that the regulation of p53 expression in cells can occur at the level of p53 mRNA abundancy or p53 protein stability depending upon the experimental system under study, and that a regulated degradation process controls the turnover of p53 protein.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson SA, Todaro GJ. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. [Abstract] [Google Scholar]
- Benchimol S, Pim D, Crawford L. Radioimmunoassay of the cellular protein p53 in mouse and human cell lines. EMBO J. 1982;1(9):1055–1062. [Europe PMC free article] [Abstract] [Google Scholar]
- Burnette WN. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. [Abstract] [Google Scholar]
- Crawford LV, Pim DC, Gurney EG, Goodfellow P, Taylor-Papadimitriou J. Detection of a common feature in several human tumor cell lines--a 53,000-dalton protein. Proc Natl Acad Sci U S A. 1981 Jan;78(1):41–45. [Europe PMC free article] [Abstract] [Google Scholar]
- DeLeo AB, Jay G, Appella E, Dubois GC, Law LW, Old LJ. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldberg DA. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. [Europe PMC free article] [Abstract] [Google Scholar]
- Gurney EG, Harrison RO, Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. [Europe PMC free article] [Abstract] [Google Scholar]
- Harlow E, Crawford LV, Pim DC, Williamson NM. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. [Europe PMC free article] [Abstract] [Google Scholar]
- Hershko A, Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. [Abstract] [Google Scholar]
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. [Abstract] [Google Scholar]
- Lehrach H, Diamond D, Wozney JM, Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. [Abstract] [Google Scholar]
- Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. [Abstract] [Google Scholar]
- Linzer DI, Maltzman W, Levine AJ. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. [Abstract] [Google Scholar]
- Milner J, Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. [Abstract] [Google Scholar]
- Oren M, Levine AJ. Molecular cloning of a cDNA specific for the murine p53 cellular tumor antigen. Proc Natl Acad Sci U S A. 1983 Jan;80(1):56–59. [Europe PMC free article] [Abstract] [Google Scholar]
- Oren M, Maltzman W, Levine AJ. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. [Europe PMC free article] [Abstract] [Google Scholar]
- Rotter V, Boss MA, Baltimore D. Increased concentration of an apparently identical cellular protein in cells transformed by either Abelson murine leukemia virus or other transforming agents. J Virol. 1981 Apr;38(1):336–346. [Europe PMC free article] [Abstract] [Google Scholar]
- Sarnow P, Ho YS, Williams J, Levine AJ. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. [Abstract] [Google Scholar]
- Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. [Abstract] [Google Scholar]
- Thomas PS. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.3.12.2143-2150.1983
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/3/12/2143
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/3/12/2143
Citations & impact
Impact metrics
Citations of article over time
Article citations
DNA-delivered monoclonal antibodies targeting the p53 R175H mutant epitope inhibit tumor development in mice.
Genes Dis, 11(4):100994, 23 Jun 2023
Cited by: 1 article | PMID: 38560504 | PMCID: PMC10980946
Identification of a small-molecule RPL11 mimetic that inhibits tumor growth by targeting MDM2-p53 pathway.
Mol Med, 28(1):109, 07 Sep 2022
Cited by: 4 articles | PMID: 36071402 | PMCID: PMC9450376
Pattern of nucleotide variants of TP53 and their correlation with the expression of p53 and its downstream proteins in a Sri Lankan cohort of breast and colorectal cancer patients.
BMC Cancer, 20(1):72, 30 Jan 2020
Cited by: 6 articles | PMID: 32000721 | PMCID: PMC6990524
Nucleotide variants and protein expression of TP53 in a Sri Lankan cohort of patients with head and neck cancer.
Mol Med Rep, 19(4):2781-2791, 11 Feb 2019
Cited by: 1 article | PMID: 30816478 | PMCID: PMC6423636
In-Tether Chiral Center Induced Helical Peptide Modulators Target p53-MDM2/MDMX and Inhibit Tumor Growth in Stem-Like Cancer Cell.
Theranostics, 7(18):4566-4576, 13 Oct 2017
Cited by: 12 articles | PMID: 29158845 | PMCID: PMC5695149
Go to all (196) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Regulation of the cellular p53 tumor antigen in teratocarcinoma cells and their differentiated progeny.
Mol Cell Biol, 2(4):443-449, 01 Apr 1982
Cited by: 72 articles | PMID: 6287239 | PMCID: PMC369808
Molecular cloning of a cDNA specific for the murine p53 cellular tumor antigen.
Proc Natl Acad Sci U S A, 80(1):56-59, 01 Jan 1983
Cited by: 56 articles | PMID: 6296874 | PMCID: PMC393308
UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells.
Mol Cell Biol, 4(9):1689-1694, 01 Sep 1984
Cited by: 498 articles | PMID: 6092932 | PMCID: PMC368974
[Isolation and characteristics of clones complementary to mRNA of the murine cellular tumor antigen p53].
Genetika, 24(4):602-612, 01 Apr 1988
Cited by: 1 article | PMID: 2840339