Abstract
Free full text

Periplasmic protein associated with the oligopeptide permeases of Salmonella typhimurium and Escherichia coli.
Abstract
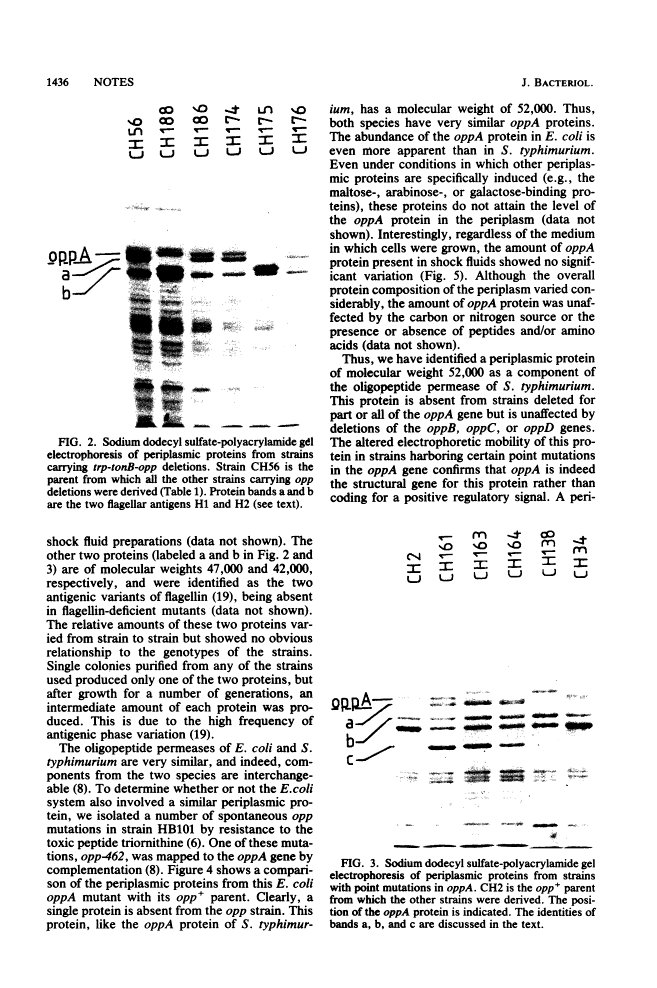
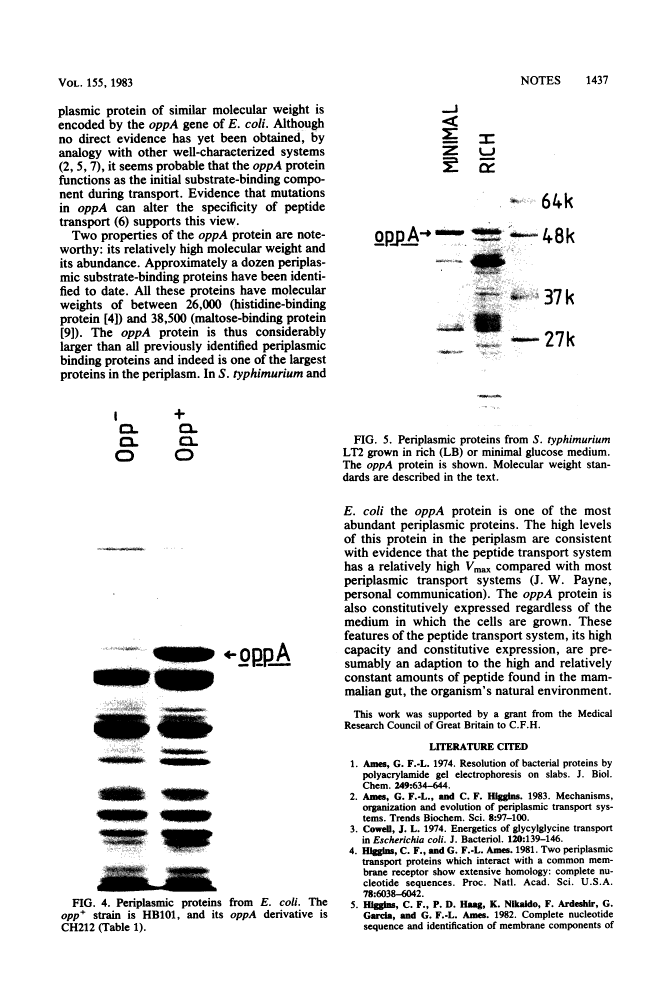
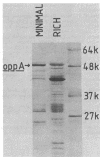
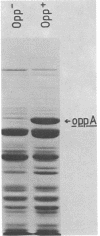
A periplasmic protein essential for the function of the oligopeptide transport system of Salmonella typhimurium was identified. This protein, encoded by the oppA gene, is one of the most abundant proteins in the periplasm and, with an apparent molecular weight of 52,000, is considerably larger than any other known periplasmic transport component. A similarly abundant periplasmic protein forms part of the oligopeptide transport system of Escherichia coli.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames GF. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [Abstract] [Google Scholar]
- Cowell JL. Energetics of glycylglycine transport in Escherichia coli. J Bacteriol. 1974 Oct;120(1):139–146. [Europe PMC free article] [Abstract] [Google Scholar]
- Higgins CF, Ames GF. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6038–6042. [Europe PMC free article] [Abstract] [Google Scholar]
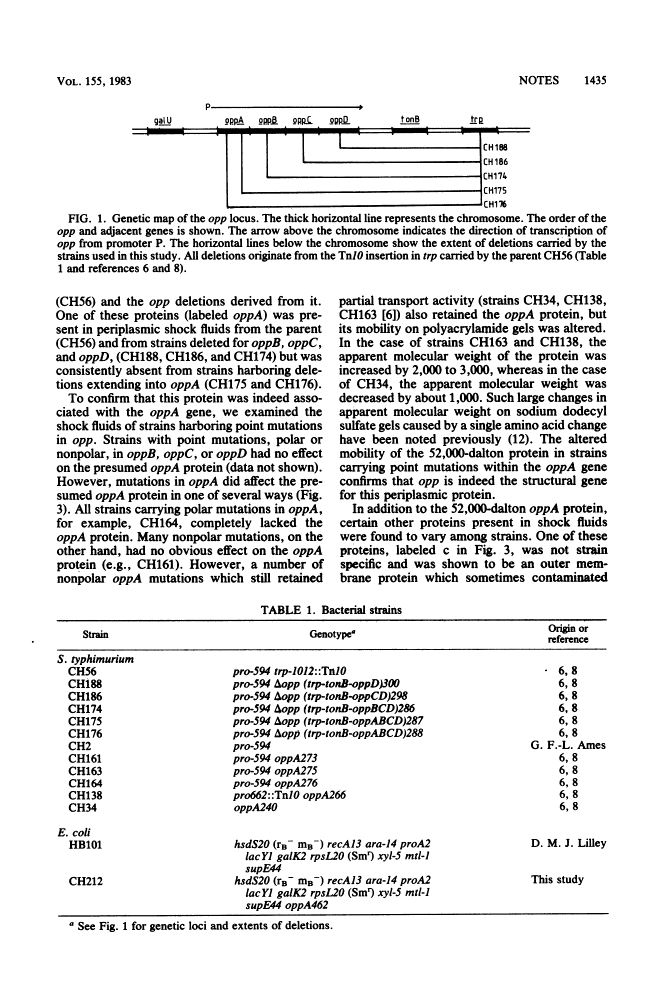
- Higgins CF, Hardie MM, Jamieson D, Powell LM. Genetic map of the opp (Oligopeptide permease) locus of Salmonella typhimurium. J Bacteriol. 1983 Feb;153(2):830–836. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Noel D, Nikaido K, Ames GF. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. [Abstract] [Google Scholar]
- Nossal NG, Heppel LA. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [Abstract] [Google Scholar]
- Payne JW, Bell G. Direct determination of the properties of peptide transport systems in Escherichia coli, using a fluorescent-labeling procedure. J Bacteriol. 1979 Jan;137(1):447–455. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.155.3.1434-1438.1983
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/jb/155/3/1434.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/155/3/1434
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/155/3/1434
Citations & impact
Impact metrics
Article citations
Hfq protein and GcvB small RNA tailoring of oppA target mRNA to levels allowing translation activation by MicF small RNA in Escherichia coli.
RNA Biol, 20(1):59-76, 01 Jan 2023
Cited by: 0 articles | PMID: 36860088 | PMCID: PMC9988348
Peptidoglycan Sensing Prevents Quiescence and Promotes Quorum-Independent Colony Growth of Uropathogenic Escherichia coli.
J Bacteriol, 202(20):e00157-20, 23 Sep 2020
Cited by: 3 articles | PMID: 32778561 | PMCID: PMC7515244
A Recurrent Silent Mutation Implicates fecA in Ethanol Tolerance by Escherichia coli.
BMC Microbiol, 18(1):36, 18 Apr 2018
Cited by: 3 articles | PMID: 29669516 | PMCID: PMC5907409
A Chemical-Genomic Screen of Neglected Antibiotics Reveals Illicit Transport of Kasugamycin and Blasticidin S.
PLoS Genet, 12(6):e1006124, 29 Jun 2016
Cited by: 26 articles | PMID: 27355376 | PMCID: PMC4927156
Free-energy calculations of residue mutations in a tripeptide using various methods to overcome inefficient sampling.
J Comput Chem, 37(29):2597-2605, 16 Sep 2016
Cited by: 4 articles | PMID: 27634475 | PMCID: PMC5082540
Go to all (53) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Peptide uptake by Salmonella typhimurium. The periplasmic oligopeptide-binding protein.
Eur J Biochem, 158(3):561-567, 01 Aug 1986
Cited by: 43 articles | PMID: 3525163
Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli.
J Bacteriol, 153(3):1548-1551, 01 Mar 1983
Cited by: 29 articles | PMID: 6338001 | PMCID: PMC221809
MppA, a periplasmic binding protein essential for import of the bacterial cell wall peptide L-alanyl-gamma-D-glutamyl-meso-diaminopimelate.
J Bacteriol, 180(5):1215-1223, 01 Mar 1998
Cited by: 65 articles | PMID: 9495761 | PMCID: PMC107010
Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli.
J Bacteriol, 169(8):3861-3865, 01 Aug 1987
Cited by: 108 articles | PMID: 3301822 | PMCID: PMC212484