Abstract
Free full text

Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man.
Abstract
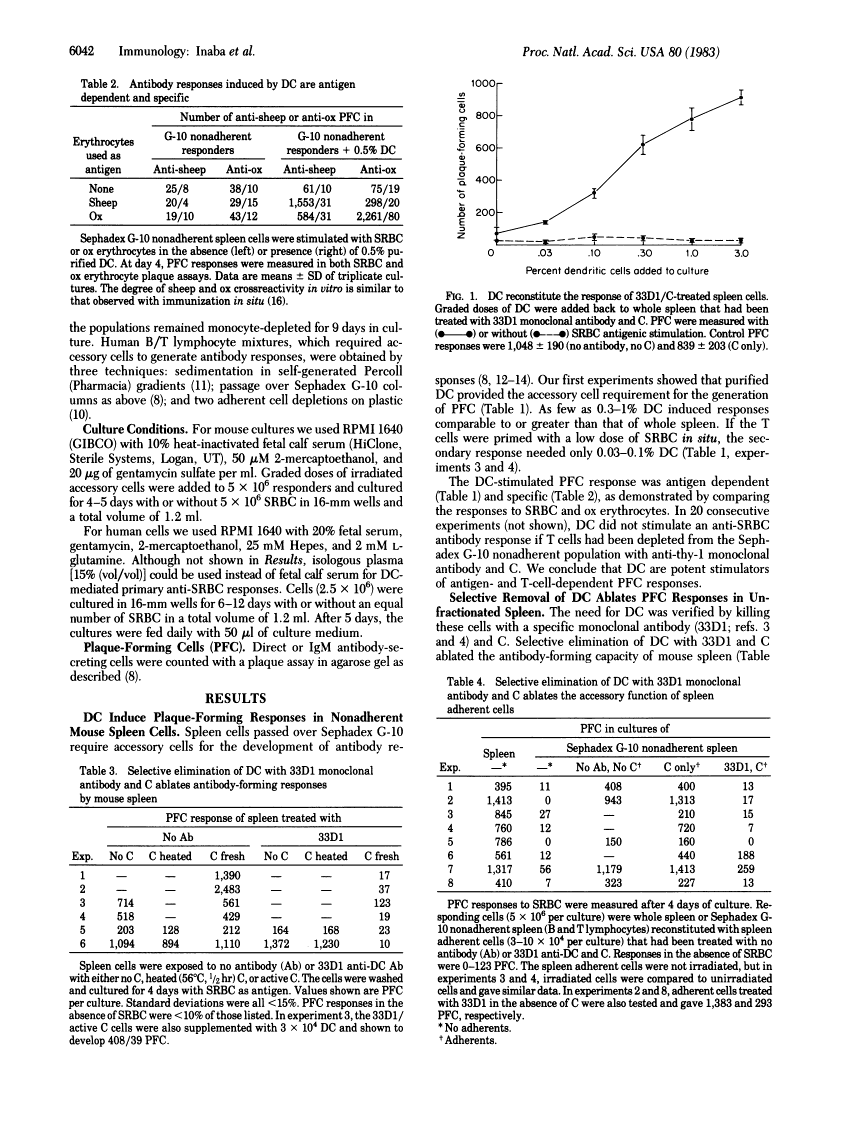
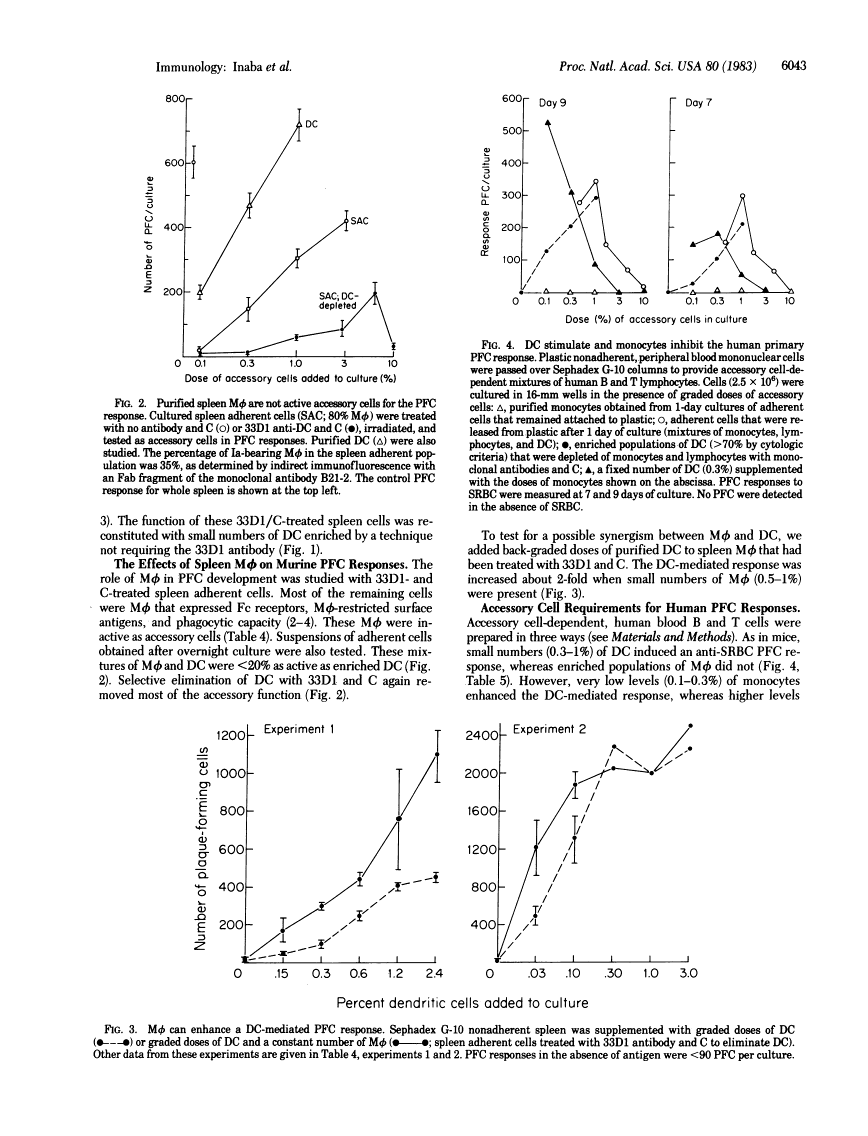
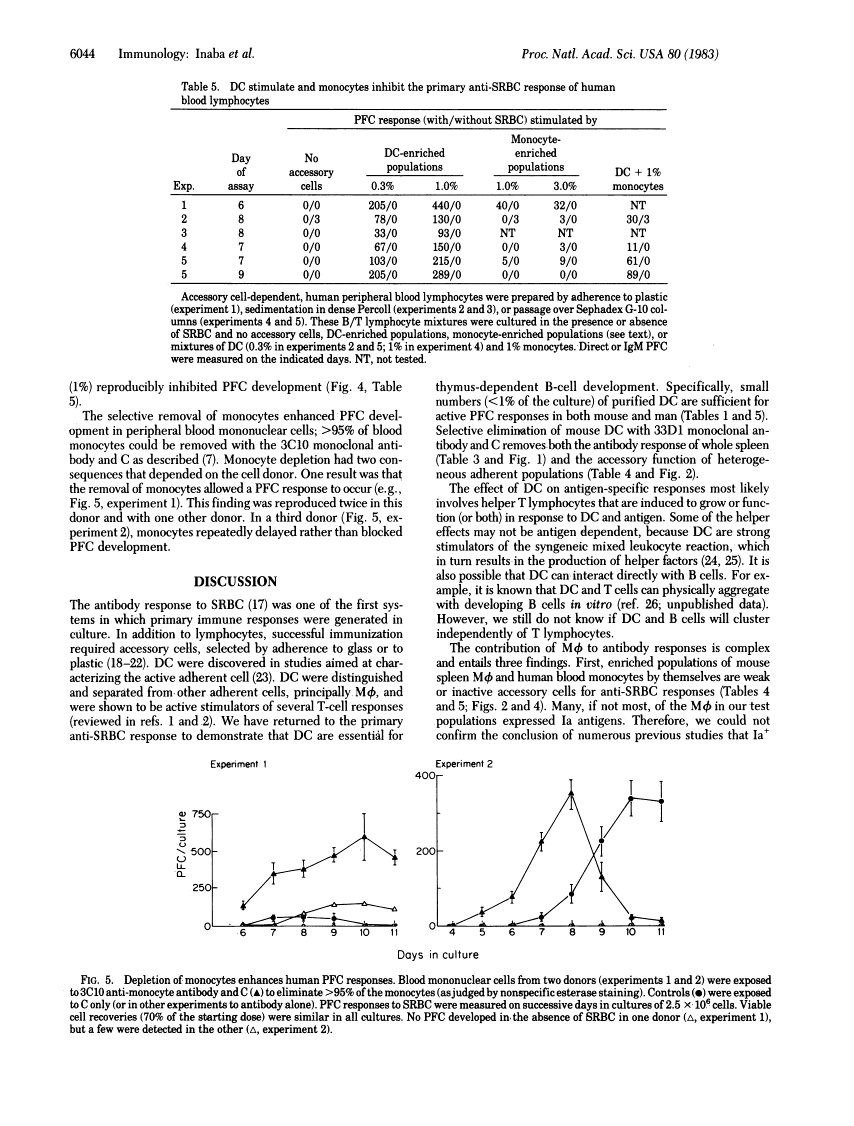
We report that dendritic cells (DC) are necessary and potent accessory cells for anti-sheep erythrocyte responses in both mouse and man. In mice, a small number of DC (0.3-1% of the culture) restores the response of B/T-lymphocyte mixtures to that observed in unfractionated spleen. An even lower dose (0.03-0.1% DC) is needed if the T cells have been primed to antigen. Responses are both antigen and T cell dependent. Selective depletion of DC from unfractionated spleen with the monoclonal antibody 33D1 and complement ablates the antibody response. In contrast to DC, purified spleen macrophages are weak or inactive stimulators. However, when mixed with DC, macrophages can increase the yield of antibody-secreting cells about 2-fold. In man, small numbers (0.3-1%) of blood DC stimulate antibody formation in vitro. Purified human monocytes do not stimulate but in low doses (1% of the culture) inhibit the antibody response. Likewise, selective removal of human monocytes with antibody and complement enhances or accelerates the development of antibody-secreting cells. We conclude that DC are required for the development of T-dependent antibody responses by mouse and human lymphocytes in vitro.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (881K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Steinman RM, Nussenzweig MC. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. [Abstract] [Google Scholar]
- Nussenzweig MC, Steinman RM, Witmer MD, Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):161–165. [Europe PMC free article] [Abstract] [Google Scholar]
- Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. [Europe PMC free article] [Abstract] [Google Scholar]
- Steinman RM, Nogueira N, Witmer MD, Tydings JD, Mellman IS. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. [Europe PMC free article] [Abstract] [Google Scholar]
- Marshak-Rothstein A, Fink P, Gridley T, Raulet DH, Bevan MJ, Gefter ML. Properties and applications of monoclonal antibodies directed against determinants of they Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [Abstract] [Google Scholar]
- Van Voorhis WC, Steinman RM, Hair LS, Luban J, Witmer MD, Koide S, Cohn ZA. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. [Europe PMC free article] [Abstract] [Google Scholar]
- Inaba K, Muramatsu S. Participation of Ia antigen-bearing nonmacrophage cells in the manifestation of accessory cell activity for in vitro antibody response. Microbiol Immunol. 1980;24(7):683–689. [Abstract] [Google Scholar]
- Nussenzweig MC, Steinman RM. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980 May 1;151(5):1196–1212. [Europe PMC free article] [Abstract] [Google Scholar]
- Van Voorhis WC, Hair LS, Steinman RM, Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. [Europe PMC free article] [Abstract] [Google Scholar]
- Gmelig-Meyling F, Waldmann TA. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. [Abstract] [Google Scholar]
- Inaba K, Nakano K, Muramatsu S. Cellular synergy in the manifestation of accessory cell activity for in vitro antibody response. J Immunol. 1981 Aug;127(2):452–461. [Abstract] [Google Scholar]
- Ly IA, Mishell RI. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. [Abstract] [Google Scholar]
- Hodes RJ, Singer A. Cellular and genetic control of antibody responses in vitro. I. Cellular requirements for the generation of genetically controlled primary IgM responses to soluble antigens. Eur J Immunol. 1977 Dec;7(12):892–897. [Abstract] [Google Scholar]
- Falkoff R, Kettman J. Differential stimulation of precursor cells and carrier-specific thymus-derived cell activity in the in vivo reponse to heterologous erythrocytes in mice. J Immunol. 1972 Jan;108(1):54–58. [Abstract] [Google Scholar]
- Radovich J, Talmage DW. Antigenic competition: cellular or humoral. Science. 1967 Oct 27;158(3800):512–514. [Abstract] [Google Scholar]
- Mishell RI, Dutton RW. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. [Europe PMC free article] [Abstract] [Google Scholar]
- Mosier DE. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. [Abstract] [Google Scholar]
- Mosier DE, Coppleson LW. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. [Europe PMC free article] [Abstract] [Google Scholar]
- Pierce CW. Immune responses in vitro. I. Cellular requirements for the immune response by nonprimed and primed spleen cells in vitro. J Exp Med. 1969 Aug 1;130(2):345–364. [Europe PMC free article] [Abstract] [Google Scholar]
- Shortman K, Diener E, Russell P, Armstrong WD. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoffmann M. Peritoneal macrophages in the immune response to SRBC in vitro. Immunology. 1970 Jun;18(6):791–797. [Abstract] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. [Europe PMC free article] [Abstract] [Google Scholar]
- Hausman PB, Stobo JD. Specificity and function of a human autologous reactive T cell. J Exp Med. 1979 Jun 1;149(6):1537–1542. [Europe PMC free article] [Abstract] [Google Scholar]
- Chiorazzi N, Fu SM, Kunkel HG. Induction of polyclonal antibody synthesis by human allogeneic and autologous helper factors. J Exp Med. 1979 Jun 1;149(6):1543–1548. [Europe PMC free article] [Abstract] [Google Scholar]
- Janossy G, Gomez de la Concha E, Luquetti A, Snajdr MJ, Waxdal MJ, Platts-Mills TA. T-cell regulation of immunoglobulin synthesis and proliferation in pokeweed (Pa-1)-stimulated human lymphocyte cultures. Scand J Immunol. 1977;6(1-2):109–123. [Abstract] [Google Scholar]
- Möller G, Lemke H, Opitz HG. The role of adherent cells in the immune response. Fibroblasts and products released by fibroblasts and peritoneal cells can substitute for adherent cells. Scand J Immunol. 1976;5(3):269–280. [Abstract] [Google Scholar]
- Wood DD, Gaul SL. Enhancement of the humoral response of T cell-depleted murine spleens by a factor derived from human monocytes in vitro. J Immunol. 1974 Sep;113(3):925–933. [Abstract] [Google Scholar]
- Chen C, Hirsch JG. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. [Europe PMC free article] [Abstract] [Google Scholar]
- Delfraissy JF, Galanaud P, Dormont J, Wallon C. Primary in vitro antibody response of human peripheral blood lymphocytes: role of phagocytic mononuclear cells. J Immunol. 1978 Apr;120(4):1283–1288. [Abstract] [Google Scholar]
- Morimoto C, Todd RF, 3rd, Distaso JA, Schlossman SF. The role of the macrophage in in vitro primary anti-DNP antibody production in man. J Immunol. 1981 Sep;127(3):1137–1141. [Abstract] [Google Scholar]
- Misiti J, Waldmann TA. In vitro generation of antigen-specific hemolytic plaque-forming cells from human peripheral blood mononuclear cells. J Exp Med. 1981 Oct 1;154(4):1069–1084. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.80.19.6041
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc534356?pdf=render
Citations & impact
Impact metrics
Article citations
The discovery of dendritic cells.
J Exp Med, 218(6):e20210830, 26 May 2021
Cited by: 2 articles | PMID: 34037675 | PMCID: PMC8160582
Dendritic cells: The first step.
J Exp Med, 218(3):e20202077, 01 Mar 2021
Cited by: 4 articles | PMID: 33600554 | PMCID: PMC7888350
A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention.
Cancers (Basel), 11(12):E1909, 01 Dec 2019
Cited by: 9 articles | PMID: 31805690 | PMCID: PMC6966557
Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells.
Nanomedicine, 12(3):677-687, 01 Dec 2015
Cited by: 62 articles | PMID: 26656533 | PMCID: PMC4839472
Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization.
Proc Natl Acad Sci U S A, 112(2):488-493, 29 Dec 2014
Cited by: 69 articles | PMID: 25548169 | PMCID: PMC4299250
Go to all (93) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice.
J Exp Med, 157(2):613-627, 01 Feb 1983
Cited by: 217 articles | PMID: 6185614 | PMCID: PMC2186925
Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction.
J Exp Med, 151(5):1196-1212, 01 May 1980
Cited by: 236 articles | PMID: 6445399 | PMCID: PMC2185855
The role of dendritic cells as stimulators of minor lymphocyte-stimulating locus-specific T cell responses in the mouse. I. Differential capacity of dendritic cells to stimulate minor lymphocyte-stimulating locus-reactive T cell hybridomas and the primary anti-minor lymphocyte-stimulating locus mixed lymphocyte reaction.
J Immunol, 142(4):1069-1078, 01 Feb 1989
Cited by: 4 articles | PMID: 2464636
Activation of T and B lymphocytes in vitro. IV. Regulatory influence on specific T cell functions by a thymus extract factor.
J Immunol, 114(4):1248-1254, 01 Apr 1975
Cited by: 7 articles | PMID: 1090671
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: CA 30198
NIAID NIH HHS (1)
Grant ID: AI 13013