Abstract
Free full text

Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein.
Abstract
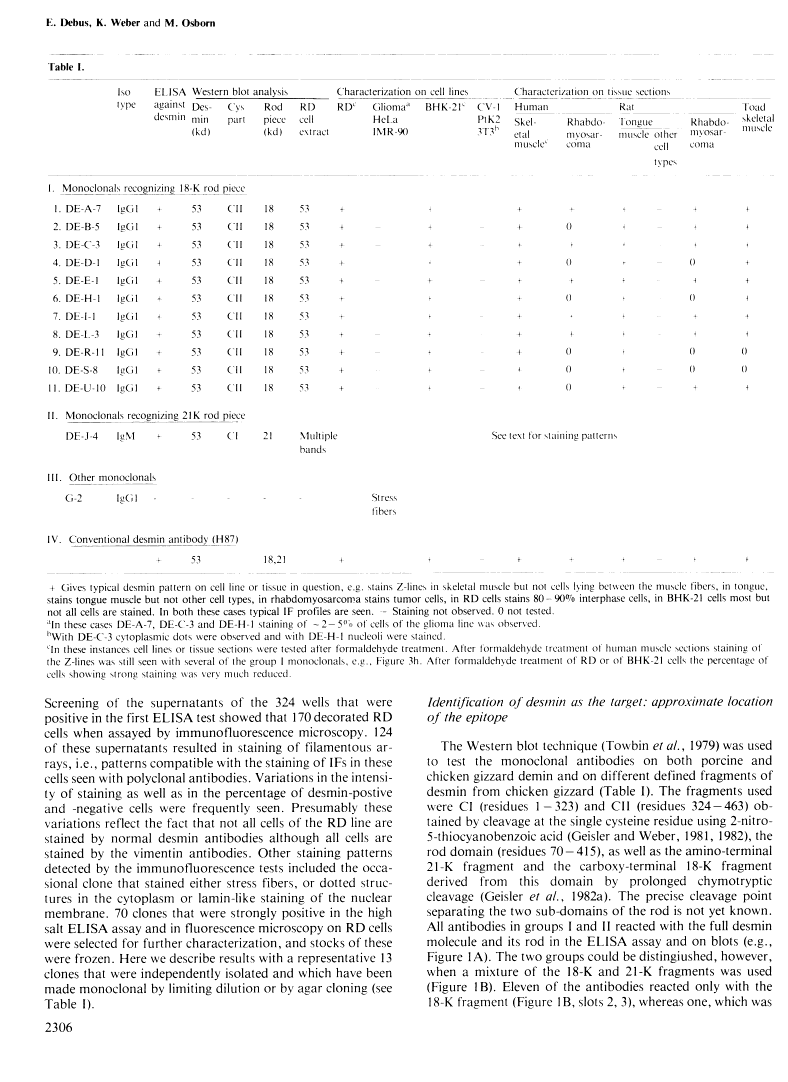
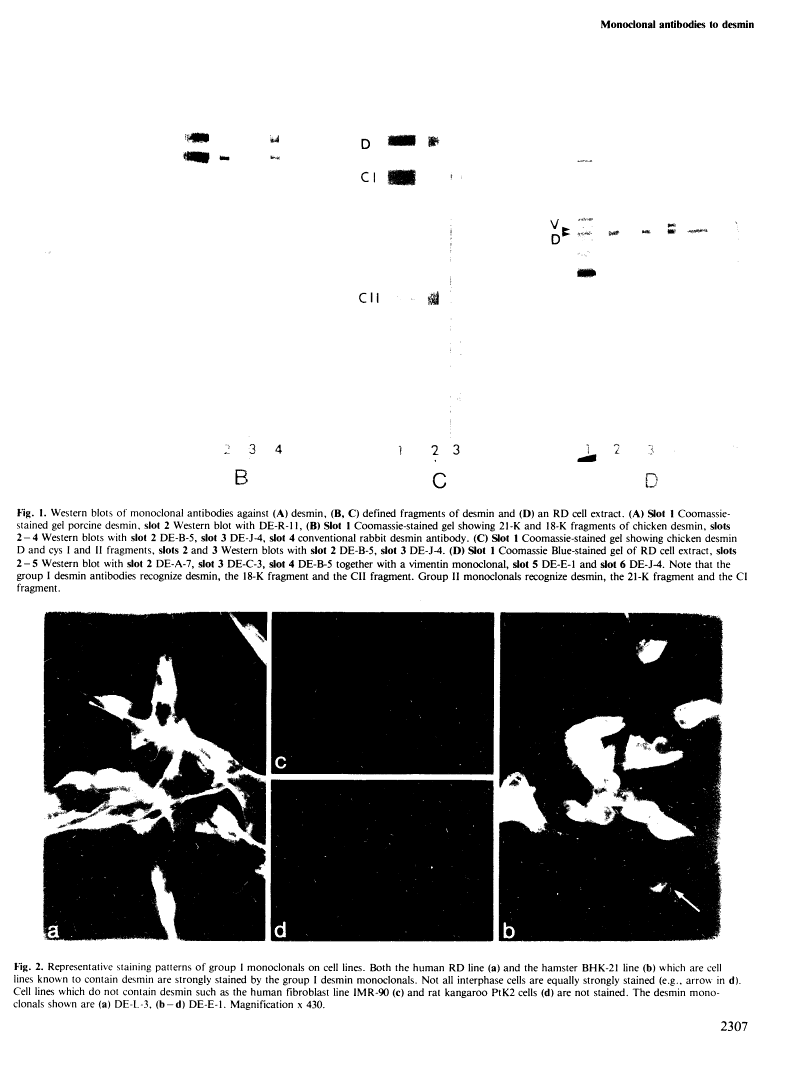
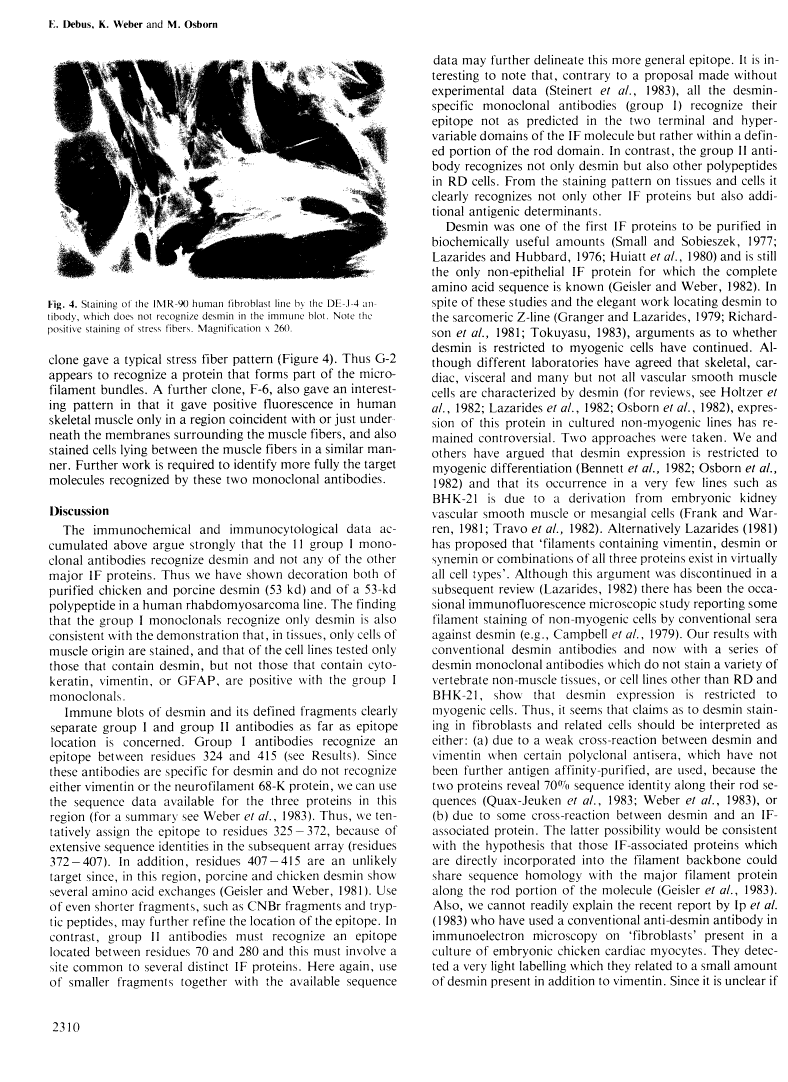
A set of monoclonal antibodies to desmin has been isolated from a fusion of mouse myeloma cells with spleen cells from mice immunized with purified porcine desmin. Eleven group I antibodies recognized desmin in the immune blot, and using defined desmin fragments the epitope has been tentatively assigned as lying between residues 325 and 372. When cell lines were tested in immunofluorescence only the human line RD and hamster BHK-21 were positive. When tissue sections were used, skeletal, cardiac, visceral and some vascular smooth muscle cells were positive. Thus, the group I antibodies appear specific for desmin and do not recognize other intermediate filament proteins. Group II monoclonals recognized not only desmin in the immune blot but also other polypeptides. The epitope of this class is located between residues 70 and 280. In immunofluorescence on cell lines and tissues, the staining patterns of group II antibodies were more complicated and demonstrate that not only other intermediate filament proteins but also additional antigenic determinants are being recognized. The group I antibodies stain, as expected from their desmin specificity, rat and human rhabdomyosarcomas and thus appear to be useful reagents in pathology.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M, Osborn M, Hölscher A, Schauer A, Weber K. The distribution of keratin type intermediate filaments in human breast cancer. An immunohistological study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(3):277–284. [Abstract] [Google Scholar]
- Altmannsberger M, Osborn M, Treuner J, Hölscher A, Weber K, Shauer A. Diagnosis of human childhood rhabdomyosarcoma of antibodies to desmin, the structural protein of muscle specific intermediate filaments. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39(2):203–215. [Abstract] [Google Scholar]
- Anderton BH, Breinburg D, Downes MJ, Green PJ, Tomlinson BE, Ulrich J, Wood JN, Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. [Abstract] [Google Scholar]
- Bennett GS, Fellini SA, Toyama Y, Holtzer H. Redistribution of intermediate filament subunits during skeletal myogenesis and maturation in vitro. J Cell Biol. 1979 Aug;82(2):577–584. [Europe PMC free article] [Abstract] [Google Scholar]
- Campbell GR, Chamley-Campbell J, Gröschel-Stewart U, Small JV, Anderson P. Antibody staining of 10-nm (100-A) filaments in cultured smooth, cardiac and skeletal muscle cells. J Cell Sci. 1979 Jun;37:303–322. [Abstract] [Google Scholar]
- Dahl D, Bignami A. Immunohistological localization of desmin, the muscle-type 100 A filament protein, in rat astrocytes and Müller glia. J Histochem Cytochem. 1982 Mar;30(3):207–213. [Abstract] [Google Scholar]
- Debus E, Weber K, Osborn M. Monoclonal cytokeratin antibodies that distinguish simple from stratified squamous epithelia: characterization on human tissues. EMBO J. 1982;1(12):1641–1647. [Europe PMC free article] [Abstract] [Google Scholar]
- Edström L, Thornell LE, Eriksson A. A new type of hereditary distal myopathy with characteristic sarcoplasmic bodies and intermediate (skeletin) filaments. J Neurol Sci. 1980 Aug;47(2):171–190. [Abstract] [Google Scholar]
- Fidzianska A, Goebel HH, Osborn M, Lenard HG, Osse G, Langenbeck U. Mallory body-like inclusions in a hereditary congenital neuromuscular disease. Muscle Nerve. 1983 Mar-Apr;6(3):195–200. [Abstract] [Google Scholar]
- Frank ED, Warren L. Aortic smooth muscle cells contain vimentin instead of desmin. Proc Natl Acad Sci U S A. 1981 May;78(5):3020–3024. [Europe PMC free article] [Abstract] [Google Scholar]
- Osborn M, Geisler N, Shaw G, Sharp G, Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. [Abstract] [Google Scholar]
- Fuchs E, Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. [Abstract] [Google Scholar]
- Gabbiani G, Kapanci Y, Barazzone P, Franke WW. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [Europe PMC free article] [Abstract] [Google Scholar]
- Geisler N, Weber K. Comparison of the proteins of two immunologically distinct intermediate-sized filaments by amino acid sequence analysis: desmin and vimentin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4120–4123. [Europe PMC free article] [Abstract] [Google Scholar]
- Geisler N, Weber K. The amino acid sequence of chicken muscle desmin provides a common structural model for intermediate filament proteins. EMBO J. 1982;1(12):1649–1656. [Europe PMC free article] [Abstract] [Google Scholar]
- Geisler N, Kaufmann E, Weber K. Proteinchemical characterization of three structurally distinct domains along the protofilament unit of desmin 10 nm filaments. Cell. 1982 Aug;30(1):277–286. [Abstract] [Google Scholar]
- Geisler N, Kaufmann E, Fischer S, Plessmann U, Weber K. Neurofilament architecture combines structural principles of intermediate filaments with carboxy-terminal extensions increasing in size between triplet proteins. EMBO J. 1983;2(8):1295–1302. [Europe PMC free article] [Abstract] [Google Scholar]
- Gigi O, Geiger B, Eshhar Z, Moll R, Schmid E, Winter S, Schiller DL, Franke WW. Detection of a cytokeratin determinant common to diverse epithelial cells by a broadly cross-reacting monoclonal antibody. EMBO J. 1982;1(11):1429–1437. [Europe PMC free article] [Abstract] [Google Scholar]
- Granger BL, Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. [Abstract] [Google Scholar]
- Hanukoglu I, Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. [Abstract] [Google Scholar]
- Holtzer H, Bennett GS, Tapscott SJ, Croop JM, Toyama Y. Intermediate-size filaments: changes in synthesis and distribution in cells of the myogenic and neurogenic lineages. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):317–329. [Abstract] [Google Scholar]
- Huiatt TW, Robson RM, Arakawa N, Stromer MH. Desmin from avian smooth muscle. Purification and partial characterization. J Biol Chem. 1980 Jul 25;255(14):6981–6989. [Abstract] [Google Scholar]
- Ip W, Danto SI, Fischman DA. Detection of desmin-containing intermediate filaments in cultured muscle and nonmuscle cells by immunoelectron microscopy. J Cell Biol. 1983 Feb;96(2):401–408. [Europe PMC free article] [Abstract] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. [Abstract] [Google Scholar]
- Lane EB. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. [Europe PMC free article] [Abstract] [Google Scholar]
- Lazarides E. Intermediate filaments--chemical heterogeneity in differentiation. Cell. 1981 Mar;23(3):649–650. [Abstract] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. [Abstract] [Google Scholar]
- Lazarides E, Hubbard BD. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. [Europe PMC free article] [Abstract] [Google Scholar]
- Miettinen M, Lehto VP, Badley RA, Virtanen I. Expression of intermediate filaments in soft-tissue sarcomas. Int J Cancer. 1982 Nov 15;30(5):541–546. [Abstract] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. [Abstract] [Google Scholar]
- Osborn M, Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [Abstract] [Google Scholar]
- Osborn M, Caselitz J, Weber K. Heterogeneity of intermediate filament expression in vascular smooth muscle: a gradient in desmin positive cells from the rat aortic arch to the level of the arteria iliaca communis. Differentiation. 1981;20(3):196–202. [Abstract] [Google Scholar]
- Osborn M, Geisler N, Shaw G, Sharp G, Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. [Abstract] [Google Scholar]
- Pruss RM, Mirsky R, Raff MC, Thorpe R, Dowding AJ, Anderton BH. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. [Abstract] [Google Scholar]
- Quax-Jeuken YE, Quax WJ, Bloemendal H. Primary and secondary structure of hamster vimentin predicted from the nucleotide sequence. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3548–3552. [Europe PMC free article] [Abstract] [Google Scholar]
- Richardson FL, Stromer MH, Huiatt TW, Robson RM. Immunoelectron and immunofluorescence localization of desmin in mature avian muscles. Eur J Cell Biol. 1981 Dec;26(1):91–101. [Abstract] [Google Scholar]
- Small JV, Sobieszek A. Studies on the function and composition of the 10-NM(100-A) filaments of vertebrate smooth muscle. J Cell Sci. 1977 Feb;23:243–268. [Abstract] [Google Scholar]
- Steinert PM, Rice RH, Roop DR, Trus BL, Steven AC. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. [Abstract] [Google Scholar]
- Stoeckel ME, Osborn M, Porte A, Sacrez A, Batzenschlager A, Weber K. An unusual familial cardiomyopathy characterized by aberrant accumulations of desmin-type intermediate filaments. Virchows Arch A Pathol Anat Histol. 1981;393(1):53–60. [Abstract] [Google Scholar]
- Tokuyasu KT. Visualization of longitudinally-oriented intermediate filaments in frozen sections of chicken cardiac muscle by a new staining method. J Cell Biol. 1983 Aug;97(2):562–565. [Europe PMC free article] [Abstract] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. [Europe PMC free article] [Abstract] [Google Scholar]
- Travo P, Weber K, Osborn M. Co-existence of vimentin and desmin type intermediate filaments in a subpopulation of adult rat vascular smooth muscle cells growing in primary culture. Exp Cell Res. 1982 May;139(1):87–94. [Abstract] [Google Scholar]
- Tseng SC, Jarvinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. [Abstract] [Google Scholar]
- Tuszynski GP, Frank ED, Damsky CH, Buck CA, Warren L. The detection of smooth muscle desmin-like protein in BHK21/C13 fibroblasts. J Biol Chem. 1979 Jul 10;254(13):6138–6143. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1983.tb01738.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc555449?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1983.tb01738.x
Article citations
ANT1 overexpression models: Some similarities with facioscapulohumeral muscular dystrophy.
Redox Biol, 56:102450, 22 Aug 2022
Cited by: 4 articles | PMID: 36030628 | PMCID: PMC9434167
Immunophenotyping of Sheep Paraffin-Embedded Peripheral Lymph Nodes.
Front Immunol, 9:2892, 11 Dec 2018
Cited by: 11 articles | PMID: 30619264 | PMCID: PMC6297804
Morphometric analysis of astrocytes in brainstem respiratory regions.
J Comp Neurol, 526(13):2032-2047, 22 Aug 2018
Cited by: 30 articles | PMID: 29888789 | PMCID: PMC6158060
Desmin-positive anaplastic plasmacytoma involving the nasopharynx.
Histopathology, 71(1):156-158, 31 Mar 2017
Cited by: 2 articles | PMID: 28168725
Essential roles of LEM-domain protein MAN1 during organogenesis in Xenopus laevis and overlapping functions of emerin.
Eur J Cell Biol, 92(8-9):280-294, 01 Aug 2013
Cited by: 9 articles | PMID: 24252515
Go to all (164) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Monoclonal antibodies specific for vimentin.
Eur J Cell Biol, 34(1):137-143, 01 May 1984
Cited by: 278 articles | PMID: 6428888
A common antigenic determinant of vimentin and desmin defined by monoclonal antibody.
Folia Biol (Praha), 32(5):295-303, 01 Jan 1986
Cited by: 10 articles | PMID: 2465190
Immunocytochemical analysis of intermediate filaments in embryonic heart cells with monoclonal antibodies to desmin.
J Cell Biol, 98(6):2179-2191, 01 Jun 1984
Cited by: 70 articles | PMID: 6373792 | PMCID: PMC2113046
Application of monoclonal antibodies to intermediate filament proteins in surgical pathology of head and neck tumours. An overview.
Appl Pathol, 6(1):35-48, 01 Jan 1988
Cited by: 2 articles | PMID: 2451926
Review