Abstract
Free full text

Surface morphology and function of human pulmonary alveolar macrophages from smokers and non-smokers.
Abstract
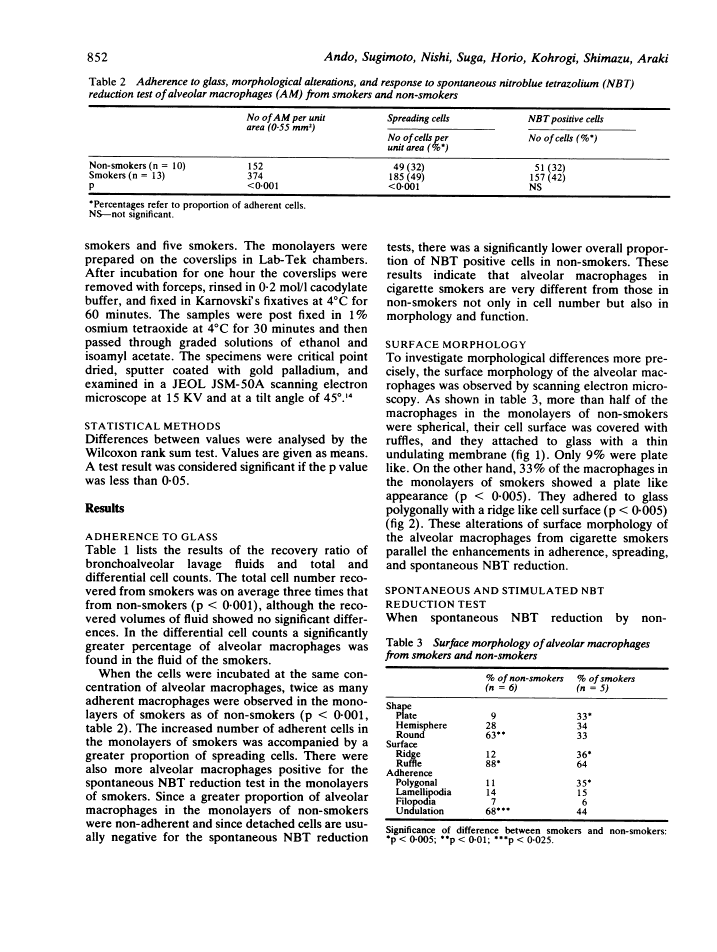
Pulmonary alveolar macrophages were obtained by saline lavage from 23 healthy male volunteers--10 non-smokers and 13 cigarette smokers. Lavage produced three times as many alveolar macrophages in smokers than in non-smokers. When macrophages from smokers and from non-smokers were incubated in vitro, more cells from smokers adhered to glass, spread out, and showed enhanced nitroblue tetrazolium (NBT) reduction. The surface morphology of alveolar macrophages from smokers showed more with a plate like appearance and ridge like membrane surface, while the macrophages from non-smokers were predominantly spherical with ruffles. The proportions of cells which stained highly for beta galactosidase were 55% in smokers and 11% in non-smokers. Thus, in a resting state in vitro, alveolar macrophages from smokers were more active than those from non-smokers. When, however, macrophages from smokers and non-smokers were incubated with immunobeads and with opsonised or non-opsonised BCG, the phagocytic activity and stimulated NBT reduction of alveolar macrophages from smokers were similar to or somewhat less than those of non-smokers.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREEN GM, KASS EH. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. [Europe PMC free article] [Abstract] [Google Scholar]
- Rodriguez RJ, White RR, Senior RM, Levine EA. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. [Abstract] [Google Scholar]
- Drath DB, Karnovsky ML, Huber GL. The effects of experimental exposure to tobacco smoke on the oxidative metabolism of alveolar macrophages. J Reticuloendothel Soc. 1979 Jun;25(6):597–604. [Abstract] [Google Scholar]
- Finch GL, Fisher GL, Hayes TL, Golde DW. Surface morphology and functional studies of human alveolar macrophages from cigarette smokers and nonsmokers. J Reticuloendothel Soc. 1982 Jul;32(1):1–23. [Abstract] [Google Scholar]
- Baehner RL, Murrmann SK, Davis J, Johnston RB., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. [Europe PMC free article] [Abstract] [Google Scholar]
- Ando M, Suga M, Sugimoto M, Tokuomi H. Superoxide production in pulmonary alveolar macrophages and killing of BCG by the superoxide-generating system with or without catalase. Infect Immun. 1979 May;24(2):404–410. [Europe PMC free article] [Abstract] [Google Scholar]
- Ando M, Suga M, Shima K, Sugimoto M, Tokuomi H. Activation of alveolar macrophages exposed to lavage-procured immunoglobulin G obtained from normal rabbit lungs. Infect Immun. 1978 May;20(2):476–484. [Europe PMC free article] [Abstract] [Google Scholar]
- Park BH, Fikrig SM, Smithwick EM. Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid. Lancet. 1968 Sep 7;2(7567):532–534. [Abstract] [Google Scholar]
- Ando M, Suga M, Shima K, Sugimoto M, Higuchi S. Different effects of phytohemagglutinin-activated lymphocytes and their culture supernatants on macrophage function. Infect Immun. 1976 May;13(5):1442–1448. [Europe PMC free article] [Abstract] [Google Scholar]
- DANNENBERG AM, Jr, BURSTONE MS, WALTER PC, KINSLEY JW. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. [Europe PMC free article] [Abstract] [Google Scholar]
- Ando M. Macrophage activation in tuberculin reactions of rabbits with primary BCG infection and reinfection. J Reticuloendothel Soc. 1973 Aug;14(2):132–145. [Abstract] [Google Scholar]
- Imanishi K, Ando M, Ideta T, Tokuomi H. Histochemical studies of lysosomal enzyme and nitroblue tetrazolium reduction of phagocytes in the cerebrospinal fluid of patients with infectious diseases of the central nervous system. Acta Neurol Scand. 1981 Jul;64(1):54–65. [Abstract] [Google Scholar]
- Horio S, Ando M, Ukeshima A, Sugimoto M, Tokuomi H. Surface morphology and function of alveolar macrophages exposed to lung lavage fluids and serum from normal rabbits. J Reticuloendothel Soc. 1981 Feb;29(2):137–152. [Abstract] [Google Scholar]
- Sugimoto M, Higuchi S, Ando M, Horio S, Tokuomi H. The effect of cytochalasin B on the superoxide production by alveolar macrophages obtained from normal rabbit lungs. J Reticuloendothel Soc. 1982 Feb;31(2):117–130. [Abstract] [Google Scholar]
- Warr GA, Martin RR. Histochemical staining and in vitro spreading of human pulmonary alveolar macrophages: variability with cigarette smoking status. J Reticuloendothel Soc. 1978 Jan;23(1):53–62. [Abstract] [Google Scholar]
- Johnston RB, Jr, Keele BB, Jr, Misra HP, Lehmeyer JE, Webb LS, Baehner RL, RaJagopalan KV. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. [Europe PMC free article] [Abstract] [Google Scholar]
- Mann PE, Cohen AB, Finley TN, Ladman AJ. Alveolar macrophages. Structural and functional differences between nonsmokers and smokers of marijuana and tobacco. Lab Invest. 1971 Aug;25(2):111–120. [Abstract] [Google Scholar]
- Rasp FL, Clawson CC, Hoidal JR, Repine JE. Reversible impairment of the adherence of alveolar macrophages from cigarette smokers. Am Rev Respir Dis. 1978 Dec;118(6):979–986. [Abstract] [Google Scholar]
- Fisher GL, McNeill KL, Finch GL, Wilson FD, Golde DW. Functional evaluation of lung macrophages from cigarette smokers and nonsmokers. J Reticuloendothel Soc. 1982 Oct;32(4):311–321. [Abstract] [Google Scholar]
- Martin RR. Altered morphology and increased acid hydrolase content of pulmonary macrophages from cigarette smokers. Am Rev Respir Dis. 1973 Apr;107(4):596–601. [Abstract] [Google Scholar]
- Harris JO, Swenson EW, Johnson JE., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen AB, Cline MJ. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. [Europe PMC free article] [Abstract] [Google Scholar]
- Reynolds HY, Kazmierowski JA, Newball HH. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. [Europe PMC free article] [Abstract] [Google Scholar]
- Territo MC, Golde DW. The function of human alveolar macrophages. J Reticuloendothel Soc. 1979 Jan;25(1):111–120. [Abstract] [Google Scholar]
- Warr GA, Martin RR. Chemotactic responsiveness of human alveolar macrophages: effects of cigarette smoking. Infect Immun. 1974 Apr;9(4):769–771. [Europe PMC free article] [Abstract] [Google Scholar]
- Demarest GB, Hudson LD, Altman LC. Impaired alveolar macrophage chemotaxis in patients with acute smoke inhalation. Am Rev Respir Dis. 1979 Feb;119(2):279–286. [Abstract] [Google Scholar]
- Hoidal JR, Fox RB, LeMarbe PA, Perri R, Repine JE. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. [Abstract] [Google Scholar]
- Hoidal JR, Niewoehner DE. Lung phagocyte recruitment and metabolic alterations induced by cigarette smoke in humans and in hamsters. Am Rev Respir Dis. 1982 Sep;126(3):548–552. [Abstract] [Google Scholar]
- Finch GL, Fisher GL, Hayes TL, Golde DW. Morphological studies of cultured human pulmonary macrophages. Scan Electron Microsc. 1980;(3):315–326. [Abstract] [Google Scholar]
Associated Data
Articles from Thorax are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/thx.39.11.850
Read article for free, from open access legal sources, via Unpaywall:
https://thorax.bmj.com/content/thoraxjnl/39/11/850.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
On the path to predicting immune responses in the lung: Modeling the pulmonary innate immune system at the air-liquid interface (ALI).
Eur J Pharm Sci, 191:106596, 26 Sep 2023
Cited by: 3 articles | PMID: 37770004 | PMCID: PMC10658361
Review Free full text in Europe PMC
Abundance of Non-Polarized Lung Macrophages with Poor Phagocytic Function in Chronic Obstructive Pulmonary Disease (COPD).
Biomedicines, 8(10):E398, 08 Oct 2020
Cited by: 10 articles | PMID: 33050042 | PMCID: PMC7650830
Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease.
Int J Mol Sci, 21(3):E853, 28 Jan 2020
Cited by: 33 articles | PMID: 32013028 | PMCID: PMC7037150
Review Free full text in Europe PMC
Cigarette smoke extract may induce lysosomal storage disease-like adverse health effects.
J Appl Toxicol, 39(3):510-524, 28 Nov 2018
Cited by: 2 articles | PMID: 30485468
Epidemiologic Evidence of and Potential Mechanisms by Which Second-Hand Smoke Causes Predisposition to Latent and Active Tuberculosis.
Immune Netw, 18(3):e22, 26 Jun 2018
Cited by: 10 articles | PMID: 29984040 | PMCID: PMC6026693
Review Free full text in Europe PMC
Go to all (29) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[A study of surface morphology and function of human pulmonary alveolar macrophages].
Nihon Kyobu Shikkan Gakkai Zasshi, 23(7):757-766, 01 Jul 1985
Cited by: 0 articles | PMID: 3001395
Enhanced luminol-dependent chemiluminescence of stimulated rat alveolar macrophages by pretreatment with alveolar lining material.
J Leukoc Biol, 40(1):55-64, 01 Jul 1986
Cited by: 10 articles | PMID: 3458864
Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers.
J Clin Invest, 49(11):2086-2096, 01 Nov 1970
Cited by: 136 articles | PMID: 4319967 | PMCID: PMC535784
The pulmonary alveolar macrophage.
Aust N Z J Med, 14(5 suppl 3):721-730, 01 Oct 1984
Cited by: 1 article | PMID: 6398050
Review