Abstract
Free full text

Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron.
Abstract
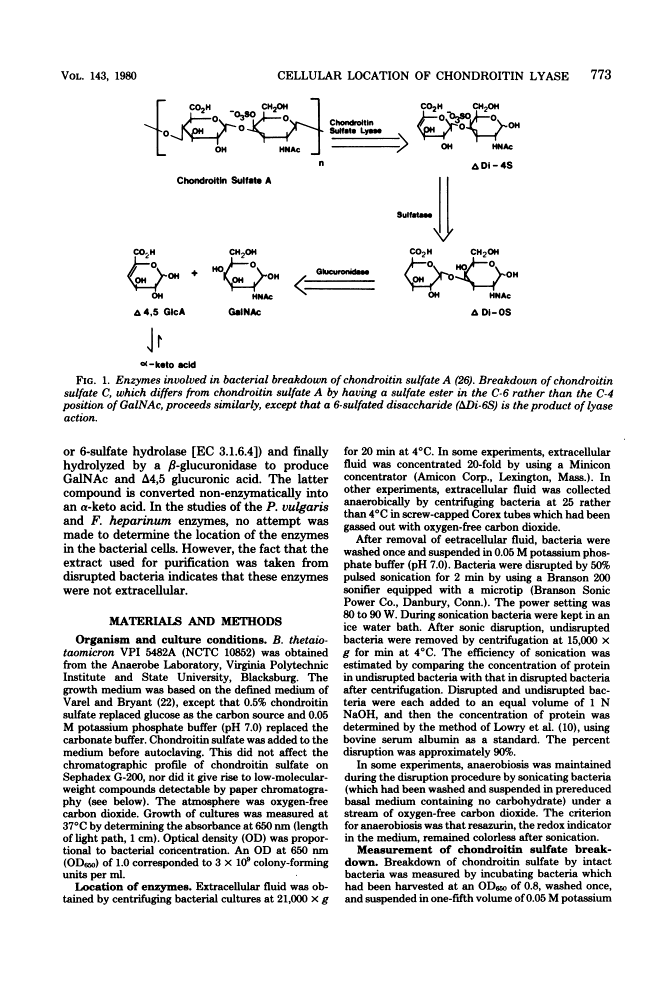
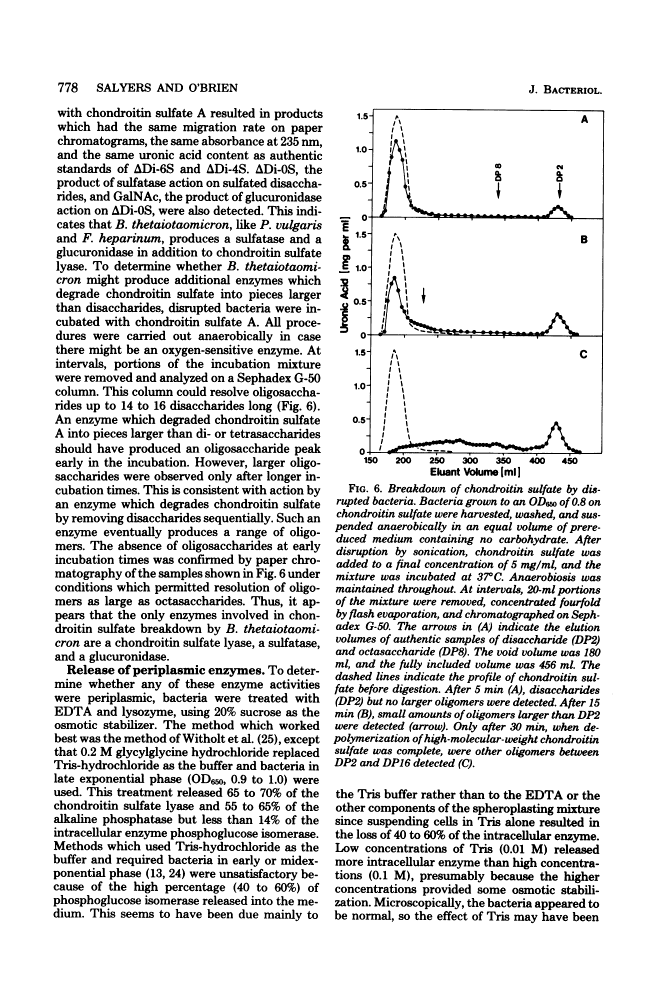
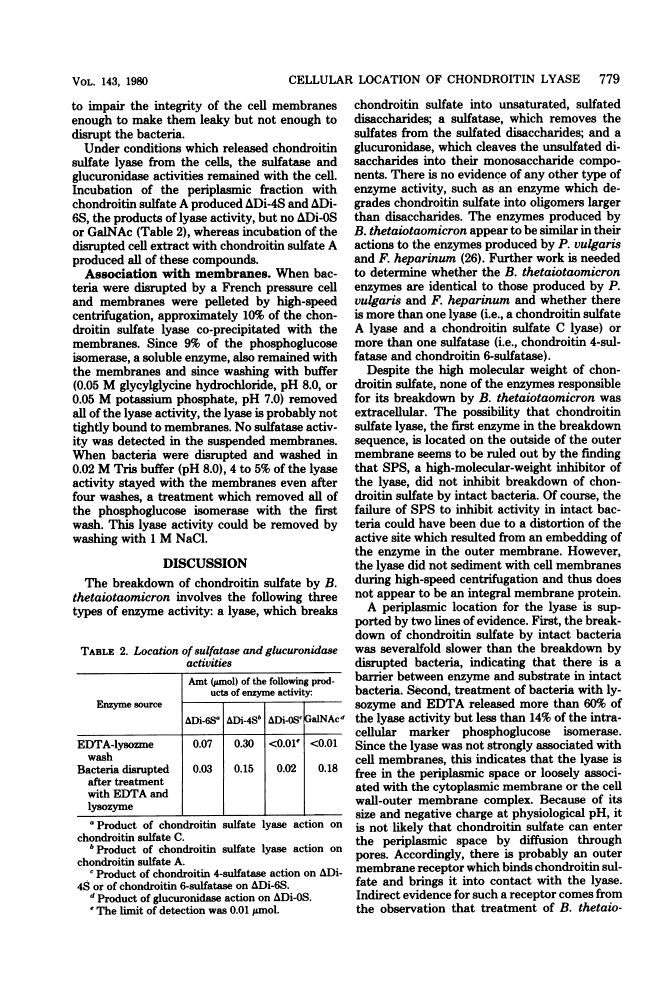
Bacteroides thetaiotaomicron, a gram-negative anaerobe found in human colons, could utilize chondroitin sulfate, a tissue mucopolysaccharide, as its sole source of carbohydrate. The enzymes responsible for the breakdown of chondroitin sulfate by B. thetaiotaomicron were similar to those produced by Proteus vulgaris and Flavobacterium heparinum and included a lyase (EC 4.2.2.4), which degraded chondroitin sulfate into sulfated disaccharides, sulfatases (EC 3.1.6.4), which removed the sulfate residues, and a glucuronidase, which broke the unsulfated disaccharides into monosaccharide components. Chondroitin sulfate lyase, the first enzyme in the breakdown sequence, was not extracellular. It appeared to be located in the periplasmic space since lyase activity was released by treatment with ethylenediaminetetraacetate and lysozyme. Moreover, sodium polyanethole sulfonate, a high-molecular-weight inhibitor of chondroitin lyase, did not inhibit breakdown of chondroitin sulfate by intact bacteria. The sulfatase and glucuronidase appeared to be intracellular. None of these enzymes was strongly bound to membranes, and none of the steps in the breakdown of chondroitin sulfate was sensitive to oxygen.
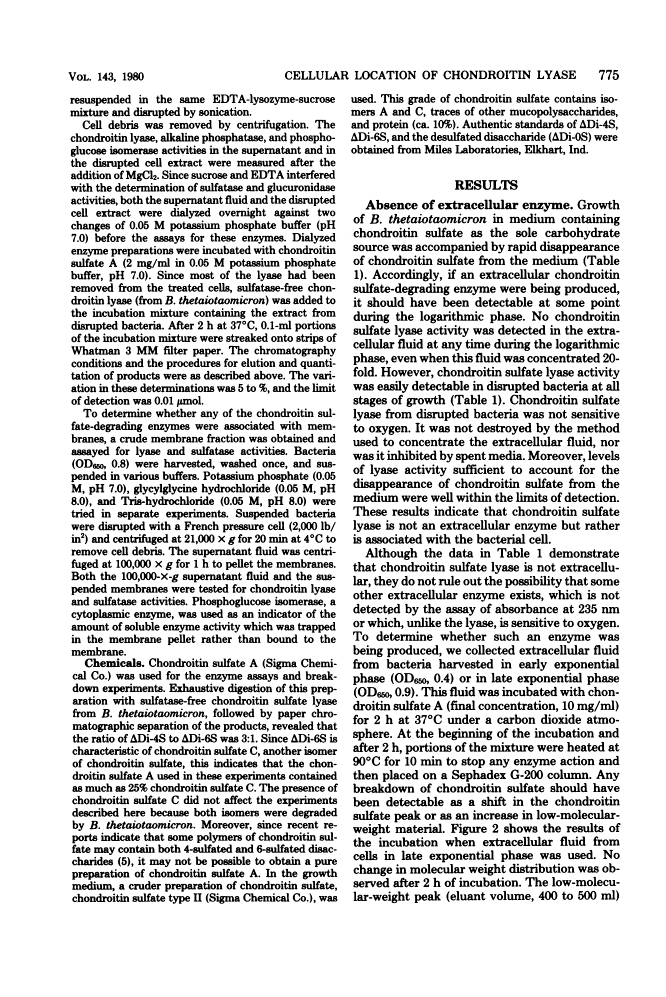
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. [Abstract] [Google Scholar]
- Faltynek CR, Silbert JE. Copolymers of chondroitin 4-sulfate and chondroitin 6-sulfate in chick embryo epiphyses and other cartilage. J Biol Chem. 1978 Nov 10;253(21):7646–7649. [Abstract] [Google Scholar]
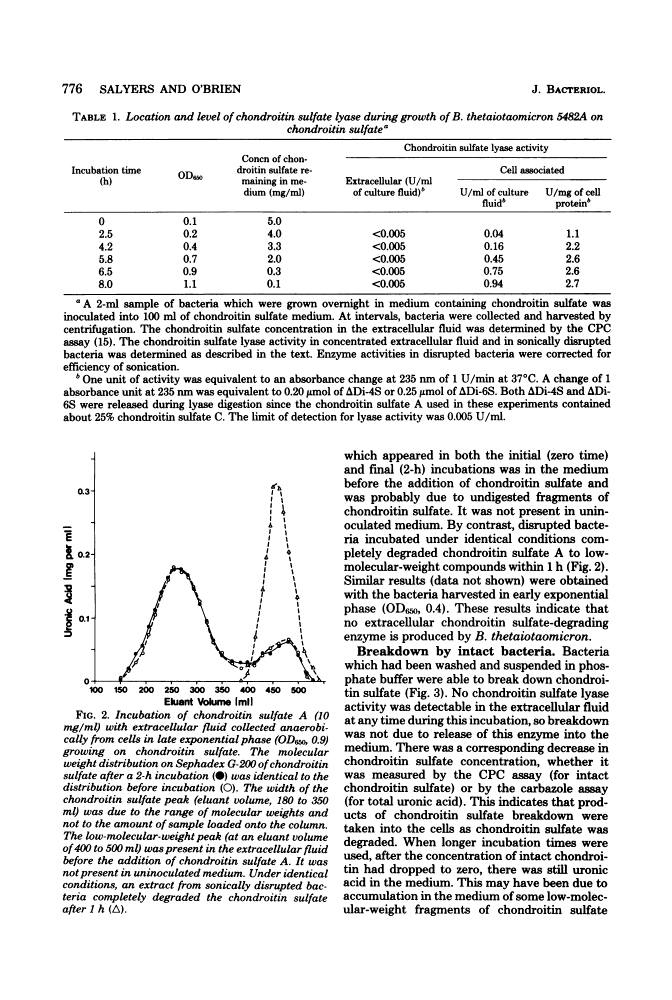
- GAREN A, LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. [Abstract] [Google Scholar]
- Lindahl U, Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
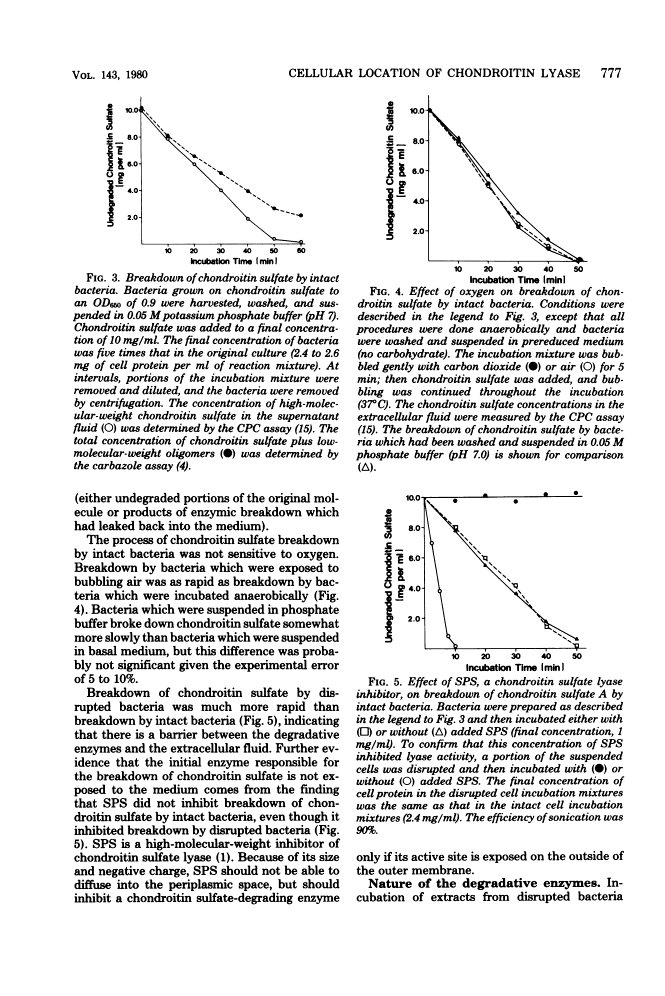
- Moore WE, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. [Europe PMC free article] [Abstract] [Google Scholar]
- Osborn MJ, Gander JE, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [Abstract] [Google Scholar]
- Saito H, Yamagata T, Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [Abstract] [Google Scholar]
- Salyers AA. Energy sources of major intestinal fermentative anaerobes. Am J Clin Nutr. 1979 Jan;32(1):158–163. [Abstract] [Google Scholar]
- Salyers AA, Kotarski SF. Induction of chondroitin sulfate lyase activity in Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):781–788. [Europe PMC free article] [Abstract] [Google Scholar]
- Salyers AA, Palmer JK, Wilkins TD. Laminarinase (beta-glucanase) activity in Bacteroides from the human colon. Appl Environ Microbiol. 1977 May;33(5):1118–1124. [Europe PMC free article] [Abstract] [Google Scholar]
- Salyers AA, Vercellotti JR, West SE, Wilkins TD. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. [Europe PMC free article] [Abstract] [Google Scholar]
- SCHWEIGER A. [Separation of simple sugars on cellulose lavers]. J Chromatogr. 1962 Nov;9:374–376. [Abstract] [Google Scholar]
- Varel VH, Bryant MP. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. [Europe PMC free article] [Abstract] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. [Abstract] [Google Scholar]
- Weiss RL. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. [Europe PMC free article] [Abstract] [Google Scholar]
- Witholt B, Boekhout M, Brock M, Kingma J, Heerikhuizen HV, Leij LD. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. [Abstract] [Google Scholar]
- Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.143.2.772-780.1980
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/jb/143/2/772.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/143/2/772
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/143/2/772
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/107073794
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jb.143.2.772-780.1980
Article citations
Mucin glycans and their degradation by gut microbiota.
Glycoconj J, 40(4):493-512, 15 Jun 2023
Cited by: 6 articles | PMID: 37318672
Review
Construction and characterization of a genome-scale ordered mutant collection of Bacteroides thetaiotaomicron.
BMC Biol, 20(1):285, 17 Dec 2022
Cited by: 5 articles | PMID: 36527020 | PMCID: PMC9758874
Hungatella hathewayi, an Efficient Glycosaminoglycan-Degrading Firmicutes from Human Gut and Its Chondroitin ABC Exolyase with High Activity and Broad Substrate Specificity.
Appl Environ Microbiol, 88(22):e0154622, 07 Nov 2022
Cited by: 3 articles | PMID: 36342199 | PMCID: PMC9680638
Enhanced propagation of Granulicatella adiacens from human oral microbiota by hyaluronan.
Sci Rep, 12(1):10948, 29 Jun 2022
Cited by: 2 articles | PMID: 35768476 | PMCID: PMC9243090
Metagenomic, (bio)chemical, and microscopic analyses reveal the potential for the cycling of sulfated EPS in Shark Bay pustular mats.
ISME Commun, 2(1):43, 19 May 2022
Cited by: 5 articles | PMID: 37938726 | PMCID: PMC9723792
Go to all (75) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparison of proteins involved in chondroitin sulfate utilization by three colonic Bacteroides species.
Appl Environ Microbiol, 51(5):978-984, 01 May 1986
Cited by: 5 articles | PMID: 3089150 | PMCID: PMC238997
Evidence for differential regulation of genes in the chondroitin sulfate utilization pathway of Bacteroides thetaiotaomicron.
J Bacteriol, 174(1):342-344, 01 Jan 1992
Cited by: 9 articles | PMID: 1729221 | PMCID: PMC205717
Utilization of chondroitin sulfate by Bacteroides thetaiotaomicron growing in carbohydrate-limited continuous culture.
J Bacteriol, 150(3):1008-1015, 01 Jun 1982
Cited by: 13 articles | PMID: 6804433 | PMCID: PMC216316
Use of targeted insertional mutagenesis to determine whether chondroitin lyase II is essential for chondroitin sulfate utilization by Bacteroides thetaiotaomicron.
J Bacteriol, 166(3):966-971, 01 Jun 1986
Cited by: 21 articles | PMID: 3011755 | PMCID: PMC215219