Abstract
Free full text

Ultrastructure of the membrane attack complex of complement: detection of the tetramolecular C9-polymerizing complex C5b-8.
Abstract
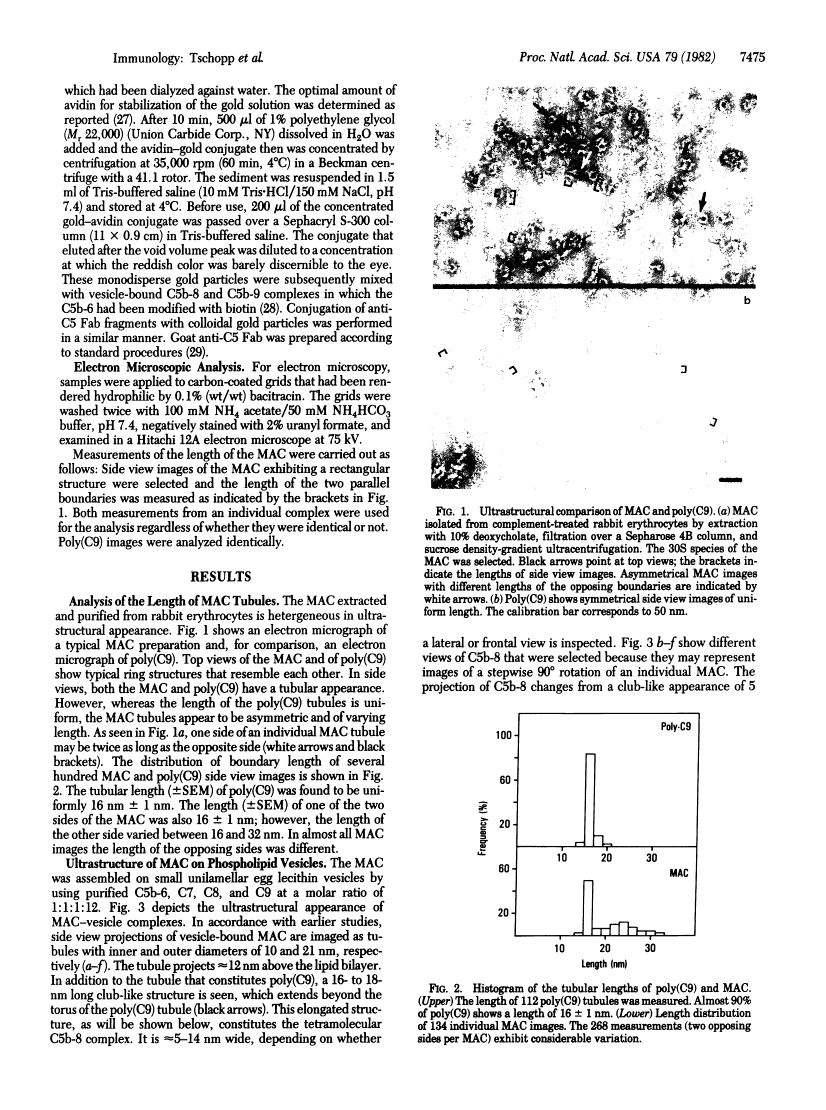
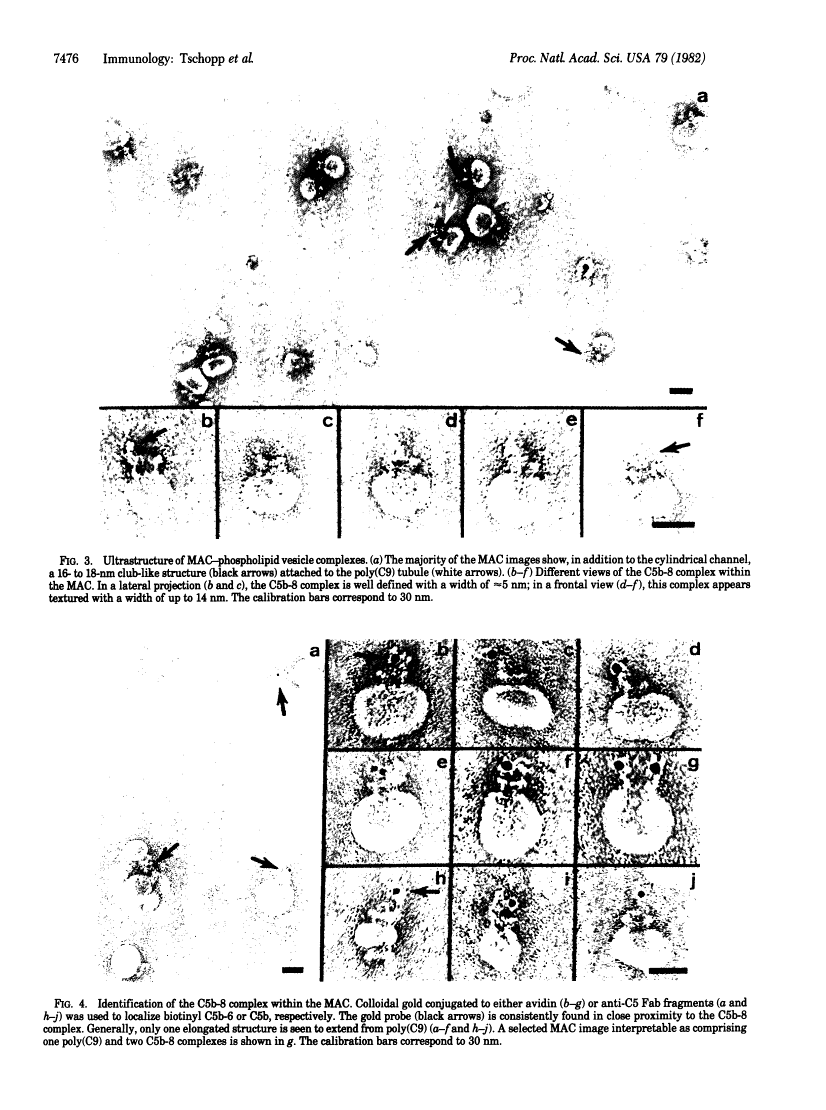
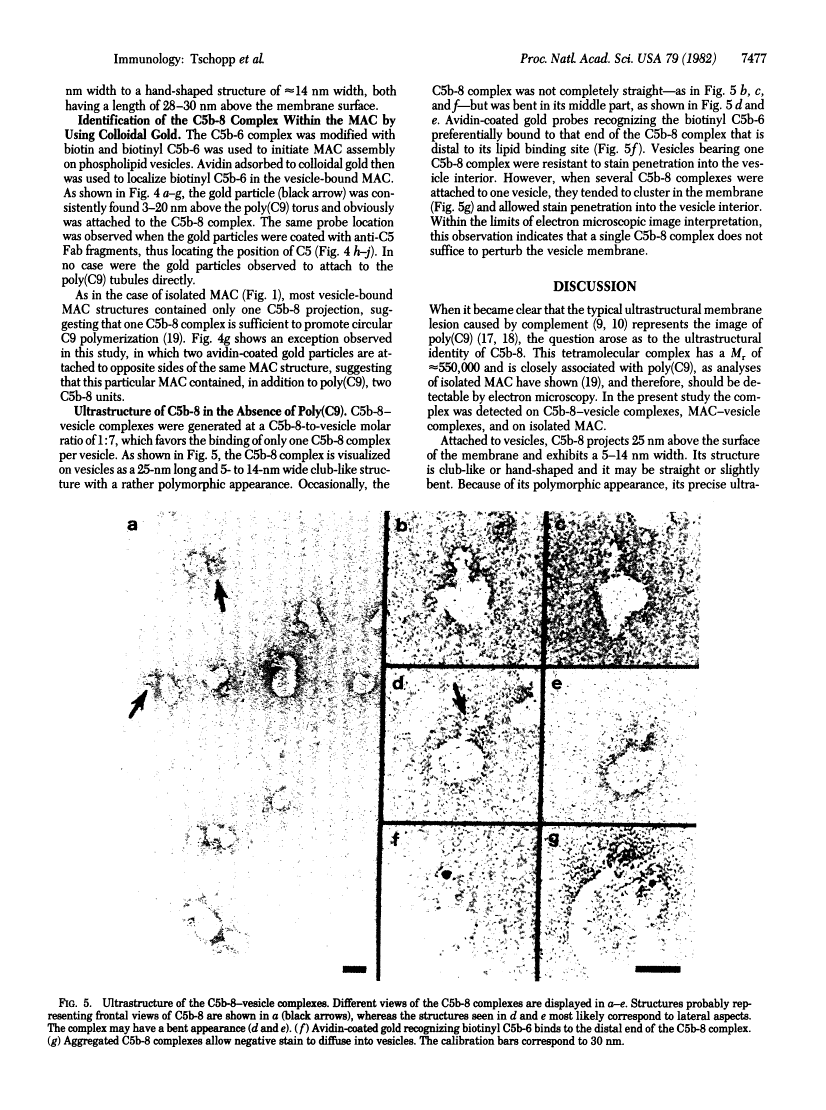
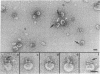
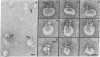
The ultrastructure of the membrane attack complex (MAC) of complement had been described as representing a hollow cylinder of defined dimensions that is composed of the proteins C5b, C6, C7, C8, and C9. After the characteristic cylindrical structure was identified as polymerized C9 [poly(C9)], the question arose as to the ultrastructural identity and topology of the C9-polymerizing complex C5b-8. An electron microscopic analysis of isolated MAC revealed an asymmetry of individual complexes with respect to their length. Whereas the length of one boundary (+/- SEM) was always 16 +/- 1 nm, the length of the other varied between 16 and 32 nm. In contrast, poly(C9), formed spontaneously from isolated C9, had a uniform tubule length (+/- SEM) of 16 +/- 1 nm. On examination of MAC-phospholipid vesicle complexes, an elongated structure was detected that was closely associated with the poly(C9) tubule and that extended 16-18 nm beyond the torus of the tubule and 28-30 nm above the membrane surface. The width of this structure varied depending on its two-dimensional projection in the electron microscope. By using biotinyl C5b-6 in the formation of the MAC and avidin-coated colloidal gold particles for the ultrastructural analysis, this heretofore unrecognized subunit of the MAC could be identified as the tetramolecular C5b-8 complex. Identification also was achieved by using anti-C5 Fab-coated colloidal gold particles. A similar elongated structure of 25 nm length (above the surface of the membrane) was observed on single C5b-8-vesicle complexes. It is concluded that the C5b-8 complex, which catalyzes poly(C9) formation, constitutes a structure of discrete morphology that remains as such identifiable in the fully assembled MAC, in which it is closely associated with the poly(C9) tubule.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kolb WP, Haxby JA, Arroyave CM, Müller-Eberhard HJ. Molecular analysis of the membrane attack mechanism of complement. J Exp Med. 1972 Mar 1;135(3):549–566. [Europe PMC free article] [Abstract] [Google Scholar]
- Kolb WP, Müller-Eberhard HJ. The membrane attack mechanism of complement. Verification of a stable C5-9 complex in free solution. J Exp Med. 1973 Aug 1;138(2):438–451. [Europe PMC free article] [Abstract] [Google Scholar]
- Bhakdi S, Ey P, Bhakdi-Lehnen B. Isolation of the terminal complement complex from target sheep erythrocyte membranes. Biochim Biophys Acta. 1976 Feb 6;419(3):445–457. [Abstract] [Google Scholar]
- Kolb WP, Muller-Eberhard HJ. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975 Apr 1;141(4):724–735. [Europe PMC free article] [Abstract] [Google Scholar]
- Podack ER, Müller-Eberhard HJ. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [Abstract] [Google Scholar]
- Esser AF, Kolb WP, Podack ER, Müller-Eberhard HJ. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. [Europe PMC free article] [Abstract] [Google Scholar]
- Mayer MM. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. [Europe PMC free article] [Abstract] [Google Scholar]
- Podack ER, Müller-Eberhard HJ, Horst H, Hoppe W. Membrane attach complex of complement (MAC): three-dimensional analysis of MAC-phospholipid vesicle recombinants. J Immunol. 1982 May;128(5):2353–2357. [Abstract] [Google Scholar]
- BORSOS T, DOURMASHKIN RR, HUMPHREY JH. LESIONS IN ERYTHROCYTE MEMBRANES CAUSED BY IMMUNE HAEMOLYSIS. Nature. 1964 Apr 18;202:251–252. [Abstract] [Google Scholar]
- Humphrey JH, Dourmashkin RR. The lesions in cell membranes caused by complement. Adv Immunol. 1969;11:75–115. [Abstract] [Google Scholar]
- Tranum-Jensen J, Bhakdi S, Bhakdi-Lehnen B, Bjerrum OJ, Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. [Abstract] [Google Scholar]
- Biesecker G, Podack ER, Halverson CA, Müller-Eberhard HJ. C5b-9 dimer: isolation from complement lysed cells and ultrastructural identification with complement-dependent membrane lesions. J Exp Med. 1979 Feb 1;149(2):448–458. [Europe PMC free article] [Abstract] [Google Scholar]
- Podack ER, Esser AF, Biesecker G, Müller-Eberhard HJ. Membrane attack complex of complement: a structural analysis of its assembly. J Exp Med. 1980 Feb 1;151(2):301–313. [Europe PMC free article] [Abstract] [Google Scholar]
- Podack ER, Stoffel W, Esser AF, Müller-Eberhard HJ. Membrane attack complex of complement: distribution of subunits between the hydrocarbon phase of target membranes and water. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4544–4548. [Europe PMC free article] [Abstract] [Google Scholar]
- Hu VW, Esser AF, Podack ER, Wisnieski BJ. The membrane attack mechanism of complement: photolabeling reveals insertion of terminal proteins into target membrane. J Immunol. 1981 Jul;127(1):380–386. [Abstract] [Google Scholar]
- Tschopp J, Podack ER. Membranolysis by the ninth component of human complement. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1409–1414. [Abstract] [Google Scholar]
- Podack ER, Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. [Europe PMC free article] [Abstract] [Google Scholar]
- Tschopp J, Müller-Eberhard HJ, Podack ER. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. [Abstract] [Google Scholar]
- Podack ER, Tschoop J, Müller-Eberhard HJ. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. [Europe PMC free article] [Abstract] [Google Scholar]
- Podack ER, Müller-Eberhard HJ. Limited proteolysis of C5b-6: functional stability of the degraded complex. J Immunol. 1980 Jan;124(1):332–336. [Abstract] [Google Scholar]
- Podack ER, Kolb WP, Esser AF, Müller-Eberhard HJ. Structural similarities between C6 and C7 of human complement. J Immunol. 1979 Sep;123(3):1071–1077. [Abstract] [Google Scholar]
- Klob WP, Müller-Eberhard HJ. The membrane attack mechanism of complement: the three polypeptide chain structure of the eigth component (C8). J Exp Med. 1976 May 1;143(5):1131–1139. [Europe PMC free article] [Abstract] [Google Scholar]
- Biesecker G, Müller-Eberhard HJ. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [Abstract] [Google Scholar]
- Horisberger M, Rosset J, Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. [Abstract] [Google Scholar]
- Podack ER, Müller-Eberhard HJ. Membrane attack complex of complement. Evidence for its dimeric structure based on hybrid formation. J Biol Chem. 1981 Apr 10;256(7):3145–3148. [Abstract] [Google Scholar]
- Sundsmo JS, Müller-Eberhard HJ. Neoantigen of the complement membrane attack complex of cytotoxic human peripheral blood lymphocytes. J Immunol. 1979 Jun;122(6):2371–2378. [Abstract] [Google Scholar]
- Dourmashkin RR. The structural events associated with the attachment of complement components to cell membranes in reactive lysis. Immunology. 1978 Aug;35(2):205–212. [Abstract] [Google Scholar]
- Mayer MM. Complement, past and present. Harvey Lect. 1978;72:139–193. [Abstract] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.79.23.7474
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc347362?pdf=render
Citations & impact
Impact metrics
Article citations
Complement C7 and clusterin form a complex in circulation.
Front Immunol, 15:1330095, 25 Jan 2024
Cited by: 0 articles | PMID: 38333209 | PMCID: PMC10850381
Bacterial killing by complement requires direct anchoring of membrane attack complex precursor C5b-7.
PLoS Pathog, 16(6):e1008606, 22 Jun 2020
Cited by: 23 articles | PMID: 32569291 | PMCID: PMC7351214
Complement Membrane Attack Complex: New Roles, Mechanisms of Action, and Therapeutic Targets.
Am J Pathol, 190(6):1138-1150, 16 Mar 2020
Cited by: 72 articles | PMID: 32194049 | PMCID: PMC7280757
Review Free full text in Europe PMC
Complement C5b-9 and Cancer: Mechanisms of Cell Damage, Cancer Counteractions, and Approaches for Intervention.
Front Immunol, 10:752, 10 Apr 2019
Cited by: 35 articles | PMID: 31024572 | PMCID: PMC6467965
Review Free full text in Europe PMC
The mystery behind membrane insertion: a review of the complement membrane attack complex.
Philos Trans R Soc Lond B Biol Sci, 372(1726):20160221, 01 Aug 2017
Cited by: 68 articles | PMID: 28630159 | PMCID: PMC5483522
Review Free full text in Europe PMC
Go to all (46) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ultrastructure of the membrane attack complex of complement. Heterogeneity of the complex caused by different degree of C9 polymerization.
J Biol Chem, 259(12):7857-7863, 01 Jun 1984
Cited by: 43 articles | PMID: 6736027
Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly.
J Exp Med, 156(1):268-282, 01 Jul 1982
Cited by: 85 articles | PMID: 6177822 | PMCID: PMC2186720
Molecular composition of the tubular structure of the membrane attack complex of complement.
J Biol Chem, 259(13):8641-8647, 01 Jul 1984
Cited by: 35 articles | PMID: 6736043
Transmembrane channel-formation by five complement proteins.
Biochem Soc Symp, 50:235-246, 01 Jan 1985
Cited by: 11 articles | PMID: 2428370
Review
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: CA 27489
NIAID NIH HHS (2)
Grant ID: AI 17354
Grant ID: AI 18525