Abstract
Free full text

Distribution of T-lymphocyte subsets in Hodgkin's disease characterized by monoclonal antibodies.
Abstract
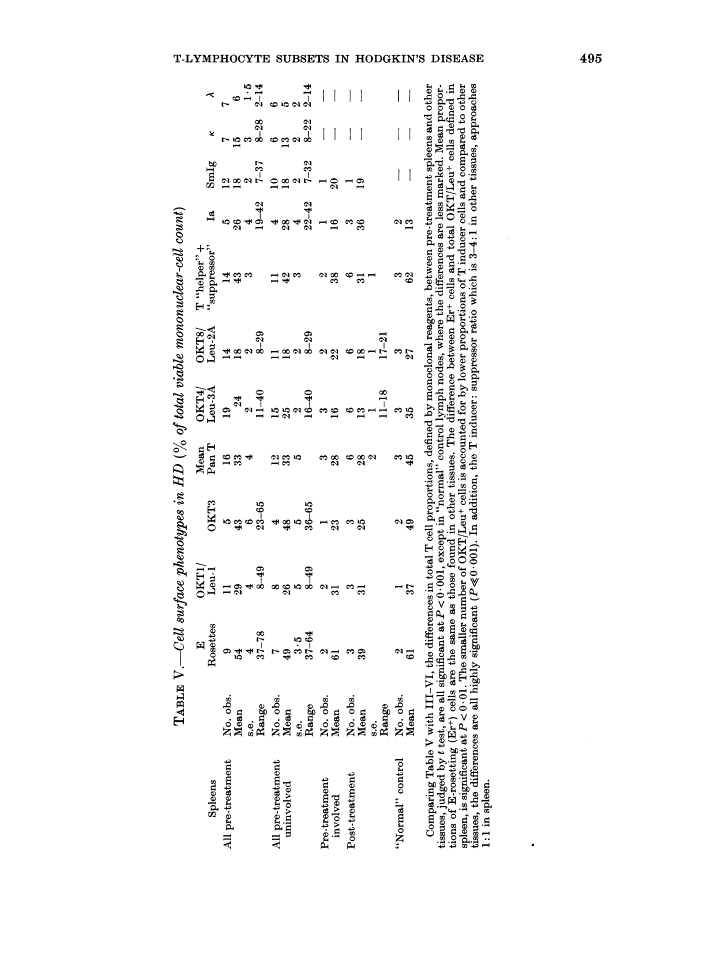
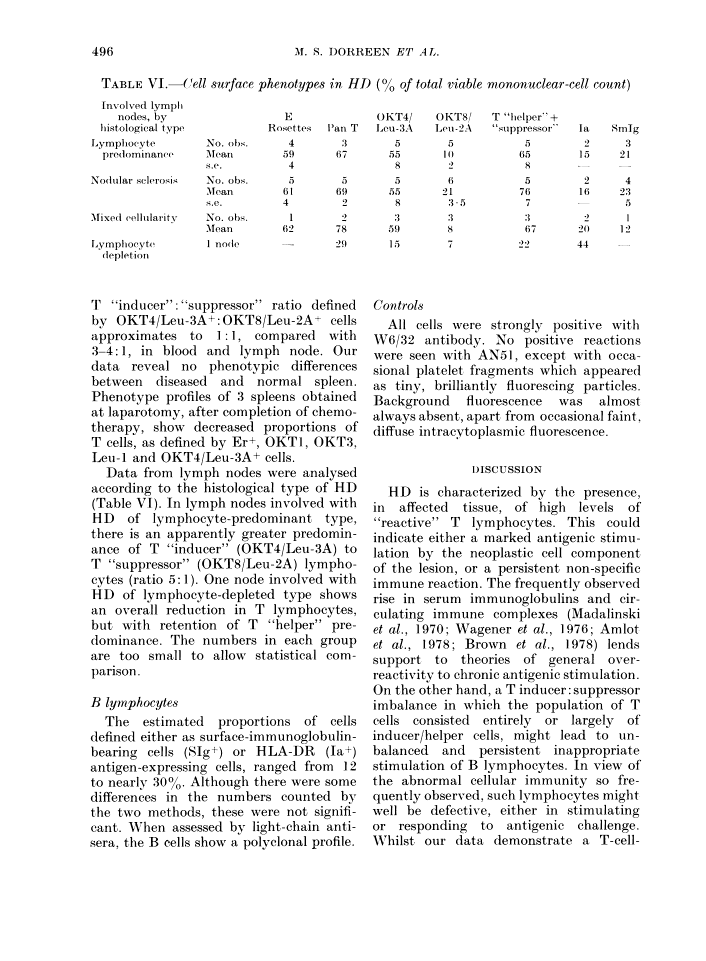
Mononuclear-cell suspensions of lymph nodes, spleen and blood from 24 patients with active Hodgkin's disease (HD) were studied for possible imbalance of T and B lymphocytes, and T-lymphocyte subsets, using monospecific anti-T antibodies and other reagents. A profile showing T-cell predominance was demonstrated in lymph nodes and blood, with total T-cells ranging from 50-70% of the cell count. As defined by monoclonal antibodies, 70-85 of the latter comprised the "inducer" subclass, the remainder being "suppressor" cells. There were no essential differences between histologically involved and uninvolved lymph nodes from HD patients, though total T-cell proportions were lower in "normal lymph node" controls. The profiles of spleens electively removed, as part of pre-treatment staging procedures, showed reduced total T-cell numbers, whether these were involved with HD or not. These differences are accounted for principally by fewer T "inducer" cells (24%, in spleen, v. 54% in involved lymph nodes and 47% in "normal" control nodes). Possible explanations for these findings are discussed. Our results demonstrate similar profiles in histologically diseased and normal tissue, rather than any clear imbalance of T-cell proportions which might explain the profound disturbances of T-cell function frequently demonstrated in vivo and in vitro.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amlot PL, Pussell B, Slaney JM, Williams BD. Correlation between immune complexes and prognostic factors in Hodgkin's disease. Clin Exp Immunol. 1978 Feb;31(2):166–173. [Abstract] [Google Scholar]
- Bobrove AM, Fuks Z, Strober S, Kaplan HS. Quantitation of T and B lymphocytes and cellular immune function in Hodgkin's disease. Cancer. 1975 Jul;36(1):169–179. [Abstract] [Google Scholar]
- Brodsky FM, Parham P, Barnstable CJ, Crumpton MJ, Bodmer WF. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. [Abstract] [Google Scholar]
- Brown CA, Hall CL, Long JC, Carey K, Weitzman SA, Aisenberg AC. Circulating immune complexes in Hodgkin's disease. Am J Med. 1978 Feb;64(2):289–294. [Abstract] [Google Scholar]
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [Abstract] [Google Scholar]
- Sousa MD, Yang M, Lopes-Corrales E, Tan C, Hansen JA, Dupont B, Good RA. Ecotaxis: the principle and its application to the study of Hodgkin's disease. Clin Exp Immunol. 1977 Jan;27(1):143–151. [Abstract] [Google Scholar]
- Dutton RW. Suppressor T cells. Transplant Rev. 1975;26:39–55. [Abstract] [Google Scholar]
- Eltringham JR, Kaplan HS. Impaired delayed-hypersensitivity responses in 154 patients with untreated Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:107–115. [Abstract] [Google Scholar]
- Gupta S, Tan C. Subpopulations of human T lymphocytes. XIV. Abnormality of T-cell locomotion and of distribution of subpopulations of T and B lymphocytes in peripheral blood and spleen from children with untreated Hodgkin's disease. Clin Immunol Immunopathol. 1980 Feb;15(2):133–143. [Abstract] [Google Scholar]
- Habeshaw JA, Catley PF, Stansfeld AG, Brearley RL. Surface phenotyping, histology and the nature of non-Hodgkin lymphoma in 157 patients. Br J Cancer. 1979 Jul;40(1):11–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoffman RA, Kung PC, Hansen WP, Goldstein G. Simple and rapid measurement of human T lymphocytes and their subclasses in peripheral blood. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4914–4917. [Europe PMC free article] [Abstract] [Google Scholar]
- Hunter CP, Pinkus G, Woodward L, Moloney WC, Churchill WH. Increased T lymphocytes and IgMEA-receptor lymphocytes in Hodgkin's disease spleens. Cell Immunol. 1977 Jun 15;31(2):193–198. [Abstract] [Google Scholar]
- Kaur J, Spiers AS, Catovsky D, Galton DA. Increase of T lymphocytes in the spleen in Hodgkin's disease. Lancet. 1974 Oct 5;2(7884):800–802. [Abstract] [Google Scholar]
- Kung P, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. [Abstract] [Google Scholar]
- Levy R, Kaplan HS. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. [Abstract] [Google Scholar]
- Lukes RJ, Butler JJ. The pathology and nomenclature of Hodgkin's disease. Cancer Res. 1966 Jun;26(6):1063–1083. [Abstract] [Google Scholar]
- Moretta L, Webb SR, Grossi CE, Lydyard PM, Cooper MD. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. [Europe PMC free article] [Abstract] [Google Scholar]
- Payne SV, Jones DB, Haegert DG, Smith JL, Wright DH. T and B lymphocytes and Reed-Sternberg cells in Hodgkin's disease lymph nodes and spleens. Clin Exp Immunol. 1976 May;24(2):280–286. [Abstract] [Google Scholar]
- Poppema S, Bhan AK, Reinherz EL, McCluskey RT, Schlossman SF. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. [Europe PMC free article] [Abstract] [Google Scholar]
- Reinherz EL, Kung PC, Goldstein G, Schlossman SF. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [Abstract] [Google Scholar]
- Reinherz EL, Kung PC, Goldstein G, Schlossman SF. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [Abstract] [Google Scholar]
- Reinherz EL, Kung PC, Goldstein G, Schlossman SF. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [Abstract] [Google Scholar]
- Reinherz EL, Schlossman SF. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. [Abstract] [Google Scholar]
- Romagnani S, Maggi E, Biagiotti R, Giudizi MG, Amadori A, Ricci M. Altered proportion of T mu-and T gamma-cell subpopulations in patients with Hodgkin's disease. Scand J Immunol. 1978;7(6):511–514. [Abstract] [Google Scholar]
- Rowley DA, Fitch FW, Stuart FP, Köhler H, Cosenza H. Specific suppression of immune responses. Science. 1973 Sep 21;181(4105):1133–1141. [Abstract] [Google Scholar]
- SCHIER WW, ROTH A, OSTROFF G, SCHRIFT MH. Hodgkin's disease and immunity. Am J Med. 1956 Jan;20(1):94–99. [Abstract] [Google Scholar]
- Sokal JE. Immunologic deficiency in Hodgkin's disease. Tumori. 1973 Sep-Oct;59(5):343–350. [Abstract] [Google Scholar]
- Wagener DJ, Van Munster PJ, Haanen C. The immunoglobulins in Hodgkin's disease. Eur J Cancer. 1976 Sep;12(9):683–688. [Abstract] [Google Scholar]
- Webb DR, Jr, Jamieson T. Control of mitogen-induced transformation: characterization of a splenic suppressor cell and its mode of action. Cell Immunol. 1976 Jun 1;24(1):45–57. [Abstract] [Google Scholar]
- Young RC, Corder MP, Berard CW, DeVita VT. Immune alterations in Hodgkin's disease. Effect of delayed hypersensitivity and lymphocyte transformation on course and survival. Arch Intern Med. 1973 Mar;131(3):446–454. [Abstract] [Google Scholar]
Associated Data
Articles from British Journal of Cancer are provided here courtesy of Cancer Research UK
Full text links
Read article at publisher's site: https://doi.org/10.1038/bjc.1982.84
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2010995
Citations & impact
Impact metrics
Citations of article over time
Article citations
Cancer Immunotherapy and the Immune Response in Hodgkin Lymphoma.
Front Oncol, 8:193, 04 Jun 2018
Cited by: 7 articles | PMID: 29915720 | PMCID: PMC5994413
Review Free full text in Europe PMC
Expression of the signal transduction molecule zeta in peripheral and tumour-associated lymphocytes in Hodgkin's disease in relation to the Epstein-Barr virus status of the tumour cells.
Br J Haematol, 116(4):765-773, 01 Mar 2002
Cited by: 0 articles | PMID: 11886379
Therapeutic and prognostic implications of peripheral blood lymphopenia in patients with Hodgkin's disease.
Leuk Lymphoma, 34(5-6):519-527, 01 Aug 1999
Cited by: 19 articles | PMID: 10492075
Generation of human monoclonal antibodies recognising membranous antigens of the lung adenocarcinoma cell line A549 using an AMeX immunohistostaining method.
Br J Cancer, 74(3):359-367, 01 Aug 1996
Cited by: 8 articles | PMID: 8695349 | PMCID: PMC2074651
Epstein-Barr virus positivity in Hodgkin's disease does not correlate with an HLA A2-negative phenotype.
Cancer, 73(12):3059-3063, 01 Jun 1994
Cited by: 16 articles | PMID: 7515326
Go to all (24) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Displacement of T lymphocytes with the 'Helper/Inducer' phenotype from peripheral blood to lymphoid organs in untreated patients with Hodgkin's disease.
Scand J Haematol, 31(4):305-314, 01 Oct 1983
Cited by: 22 articles | PMID: 6226085
T and B lymphocytes in lymph nodes and spleen of patients with Hodgkin's disease.
Neoplasma, 26(3):307-313, 01 Jan 1979
Cited by: 1 article | PMID: 394011
The T and B lymphocyte content of lymph nodes and spleen in Hodgkin's disease.
Br J Exp Pathol, 58(6):712-716, 01 Dec 1977
Cited by: 3 articles | PMID: 305255 | PMCID: PMC2041294
[Immunoregulatory subpopulations of T-lymphocytes and their distribution in the peripheral blood, lymph nodes and spleen of patients with lymphogranulomatosis].
Ter Arkh, 61(7):30-32, 01 Jan 1989
Cited by: 0 articles | PMID: 2686065