Abstract
Free full text

Deletion mapping a eukaryotic promoter.
Abstract
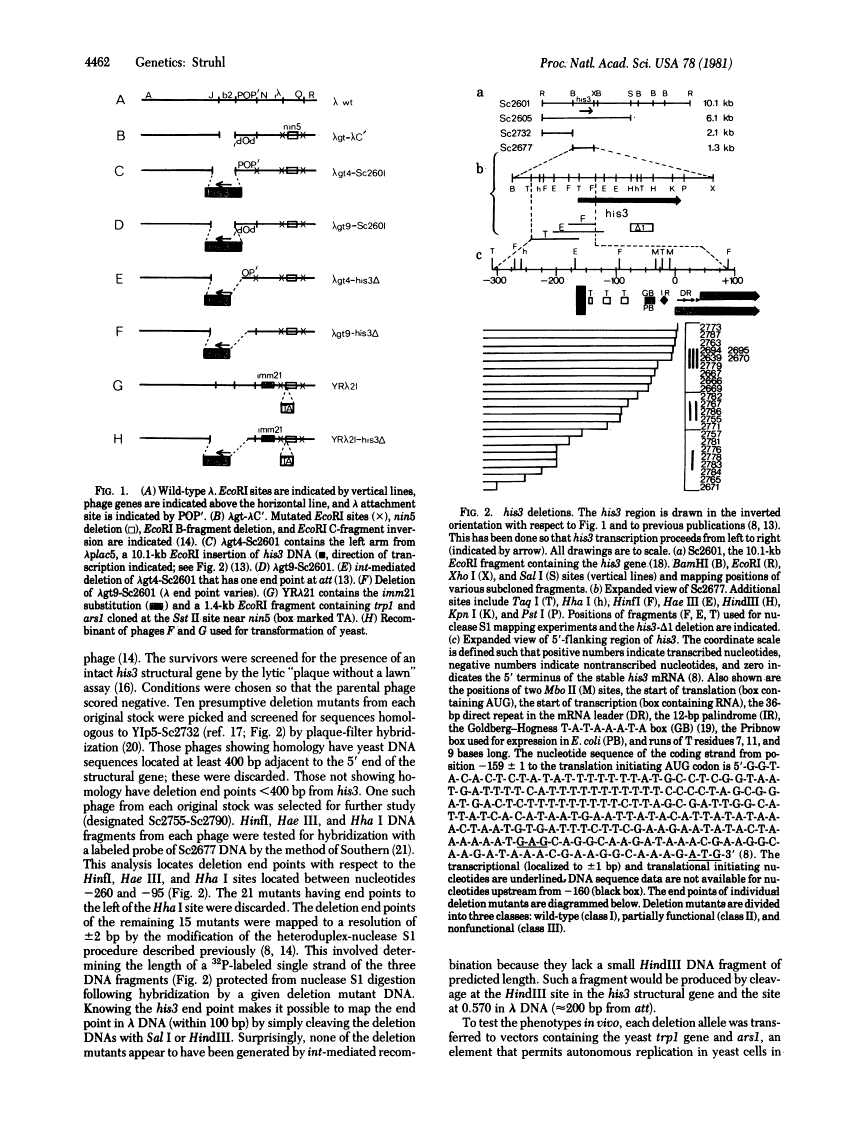
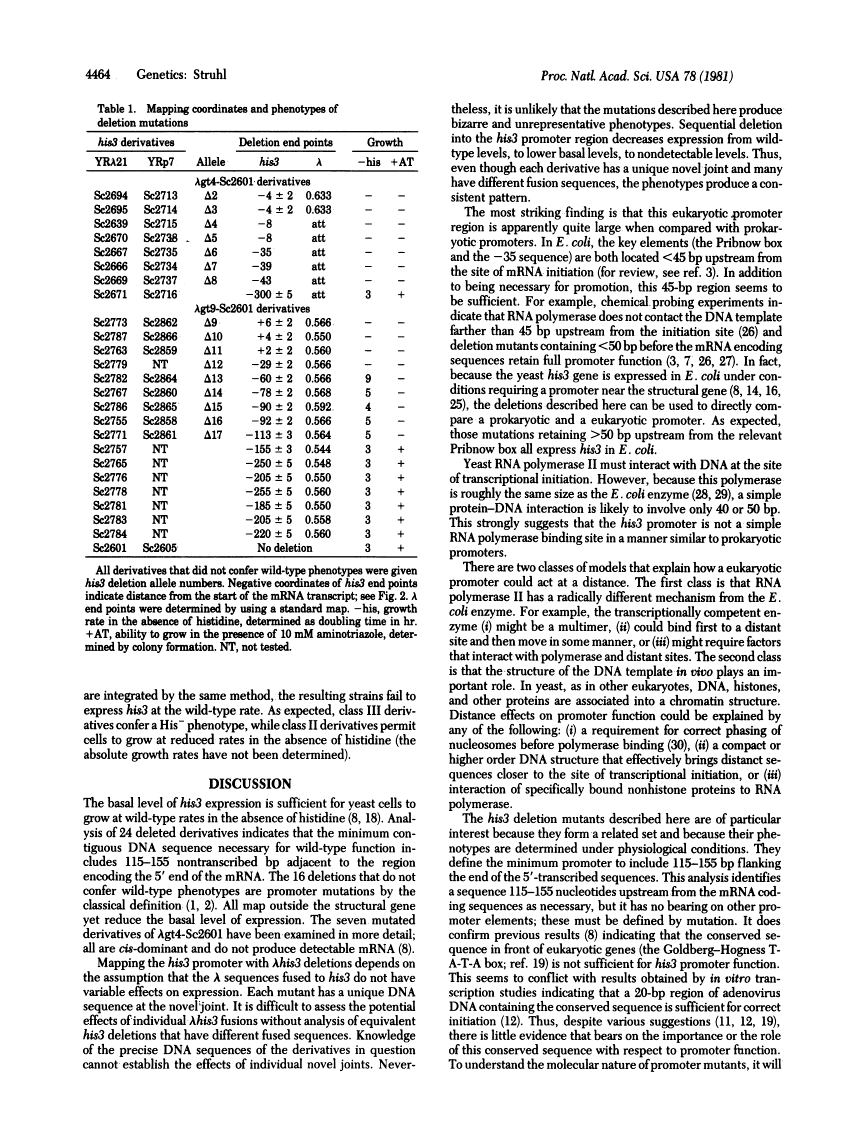
The phenotypes of 24 mutants that successively delete DNA sequences adjacent to the 5' end of the Saccharomyces cerevisiae (yeast) his3 structural gene are described. Deletions retaining greater than 155 base pairs before the mRNA coding sequences are phenotypically indistinguishable from the wild-type his3 allele. Deletions having end points between 113 and 65 base pairs before the transcription initiation site express his3 at reduced levels. Mutations retaining less than 45 base pairs are indistinguishable from null alleles of the his3 locus. These results indicate (i) that a sequence(s) located 113--155 base pairs upstream from the transcribed region is necessary for wild-type expression and (ii) that the T-A-T-A box (a sequence in front of most eukaryotic genes) is not sufficient for wild-type promoter function. Thus, the yeast his3 promoter region appears large when compared with prokaryotic promoters, suggesting that it may be more complex than a simple site of interaction between RNA polymerase and DNA.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- JACOB F, ULLMAN A, MONOD J. LE PROMOTEUR, 'EL'EMENT G'EN'ETIQUE N'ECESSAIRE 'A L'EXPRESSION D'UN OP'ERON. C R Hebd Seances Acad Sci. 1964 Mar 16;258:3125–3128. [Abstract] [Google Scholar]
- Scaife J, Beckwith JR. Mutational alteration of the maximal level of Lac operon expression. Cold Spring Harb Symp Quant Biol. 1966;31:403–408. [Abstract] [Google Scholar]
- Rosenberg M, Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. [Abstract] [Google Scholar]
- Roberts JW. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. [Abstract] [Google Scholar]
- Eron L, Block R. Mechanism of initiation and repression of in vitro transcription of the lac operon of Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1828–1832. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. [Europe PMC free article] [Abstract] [Google Scholar]
- Siebenlist U, Simpson RB, Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. [Abstract] [Google Scholar]
- Benoist C, Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. [Europe PMC free article] [Abstract] [Google Scholar]
- Bendig MM, Thomas T, Folk WR. Regulatory mutants of polyoma virus defective in DNA replication and the synthesis of early proteins. Cell. 1980 Jun;20(2):401–409. [Abstract] [Google Scholar]
- Grosschedl R, Birnstiel ML. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. [Europe PMC free article] [Abstract] [Google Scholar]
- Corden J, Wasylyk B, Buchwalder A, Sassone-Corsi P, Kedinger C, Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. [Abstract] [Google Scholar]
- Weil PA, Luse DS, Segall J, Roeder RG. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. [Abstract] [Google Scholar]
- Struhl K, Davis RW. A physical, genetic and transcriptional map of the cloned his3 gene region of Saccharomyces cerevisiae. J Mol Biol. 1980 Jan 25;136(3):309–332. [Abstract] [Google Scholar]
- Thomas M, Cameron JR, Davis RW. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. [Europe PMC free article] [Abstract] [Google Scholar]
- Struhl K, Stinchcomb DT, Davis RW. A physiological study of functional expression in Escherichia coli of the cloned yeast imidazoleglycerolphosphate dehydratase gene. J Mol Biol. 1980 Jan 25;136(3):291–307. [Abstract] [Google Scholar]
- Struhl K, Stinchcomb DT, Scherer S, Davis RW. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. [Europe PMC free article] [Abstract] [Google Scholar]
- Struhl K, Cameron JR, Davis RW. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. [Europe PMC free article] [Abstract] [Google Scholar]
- Benton WD, Davis RW. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Stinchcomb DT, Struhl K, Davis RW. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. [Abstract] [Google Scholar]
- Scherer S, Davis RW. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolfner M, Yep D, Messenguy F, Fink GR. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. [Abstract] [Google Scholar]
- FINK GR. GENE-ENZYME RELATIONS IN HISTIDINE BIOSYNTHESIS IN YEAST. Science. 1964 Oct 23;146(3643):525–527. [Abstract] [Google Scholar]
- Beckwith J, Grodzicker T, Arditti R. Evidence for two sites in the lac promoter region. J Mol Biol. 1972 Aug 14;69(1):155–160. [Abstract] [Google Scholar]
- Meyer BJ, Ptashne M. Gene regulation at the right operator (OR) of bacteriophage lambda. III. lambda repressor directly activates gene transcription. J Mol Biol. 1980 May 15;139(2):195–205. [Abstract] [Google Scholar]
- Dezélée S, Sentenac A. Role of DNA-RNA hybrids in eukaryotes. Purification and properties of yeast RNA polymerase B. Eur J Biochem. 1973 Apr 2;34(1):41–52. [Abstract] [Google Scholar]
- Wittig B, Wittig S. A phase relationship associates tRNA structural gene sequences with nucleosome cores. Cell. 1979 Dec;18(4):1173–1183. [Abstract] [Google Scholar]
- Benoist C, Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.78.7.4461
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/78/7/4461.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
What history tells us XXXV. Enhancers: their existence and characteristics have raised puzzling issues since their discovery.
J Biosci, 39(5):741-745, 01 Dec 2014
Cited by: 0 articles | PMID: 25431403
Review
Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors.
Cell, 116(2):247-257, 01 Jan 2004
Cited by: 225 articles | PMID: 14744435
Review
Characterisation of sequences required for RNA initiation from the PGK promoter of Saccharomyces cerevisiae.
Nucleic Acids Res, 18(11):3219-3225, 01 Jun 1990
Cited by: 15 articles | PMID: 2192358 | PMCID: PMC330926
Expression of a proteolipid gene from a high-copy-number plasmid confers trifluoperazine resistance to Saccharomyces cerevisiae.
Mol Cell Biol, 10(7):3397-3404, 01 Jul 1990
Cited by: 9 articles | PMID: 2192255 | PMCID: PMC360771
Some of the signals for 3'-end formation in transcription of the Saccharomyces cerevisiae Ty-D15 element are immediately downstream of the initiation site.
Mol Cell Biol, 9(6):2431-2444, 01 Jun 1989
Cited by: 22 articles | PMID: 2548082 | PMCID: PMC362316
Go to all (41) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The yeast his3 promoter contains at least two distinct elements.
Proc Natl Acad Sci U S A, 79(23):7385-7389, 01 Dec 1982
Cited by: 51 articles | PMID: 6760196 | PMCID: PMC347344
Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast.
Proc Natl Acad Sci U S A, 82(24):8419-8423, 01 Dec 1985
Cited by: 295 articles | PMID: 3909145 | PMCID: PMC390927
Genetic properties and chromatin structure of the yeast gal regulatory element: an enhancer-like sequence.
Proc Natl Acad Sci U S A, 81(24):7865-7869, 01 Dec 1984
Cited by: 88 articles | PMID: 6096864 | PMCID: PMC392253
Nucleotide sequence and transcriptional mapping of the yeast pet56-his3-ded1 gene region.
Nucleic Acids Res, 13(23):8587-8601, 01 Dec 1985
Cited by: 188 articles | PMID: 3001645 | PMCID: PMC322154