Abstract
Free full text

Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation.
Abstract
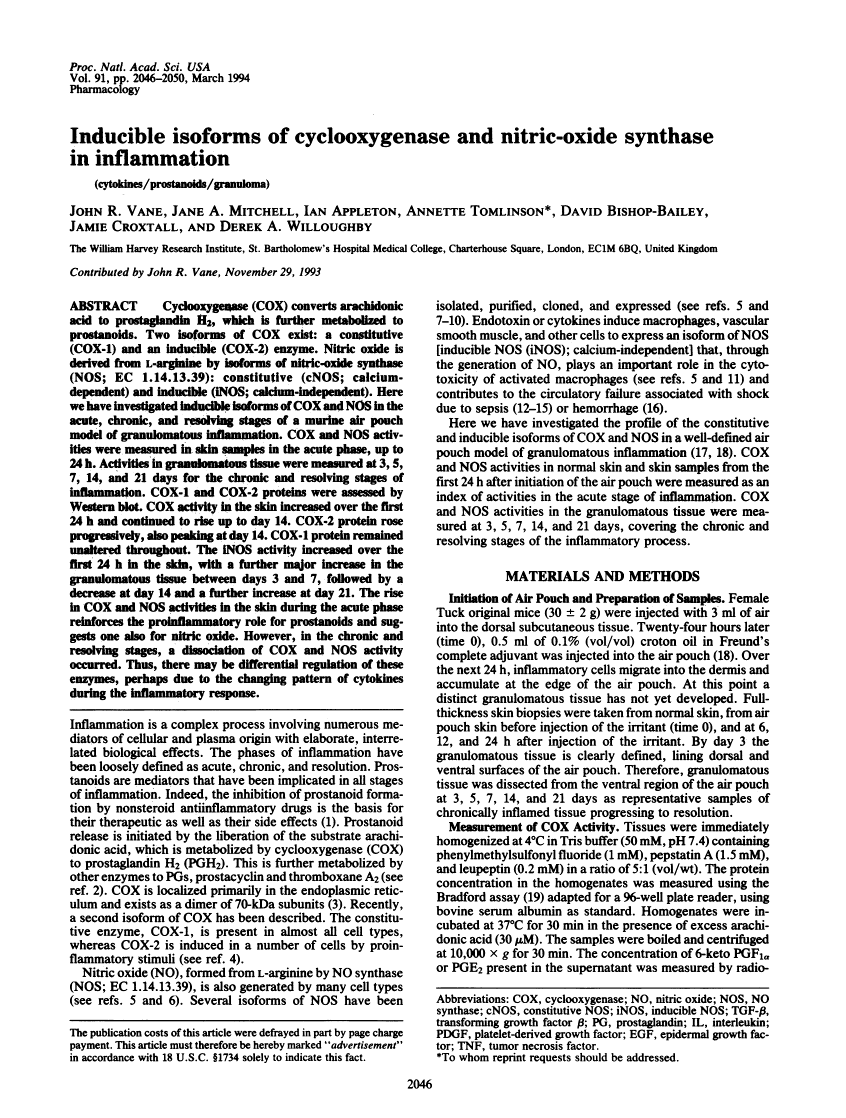
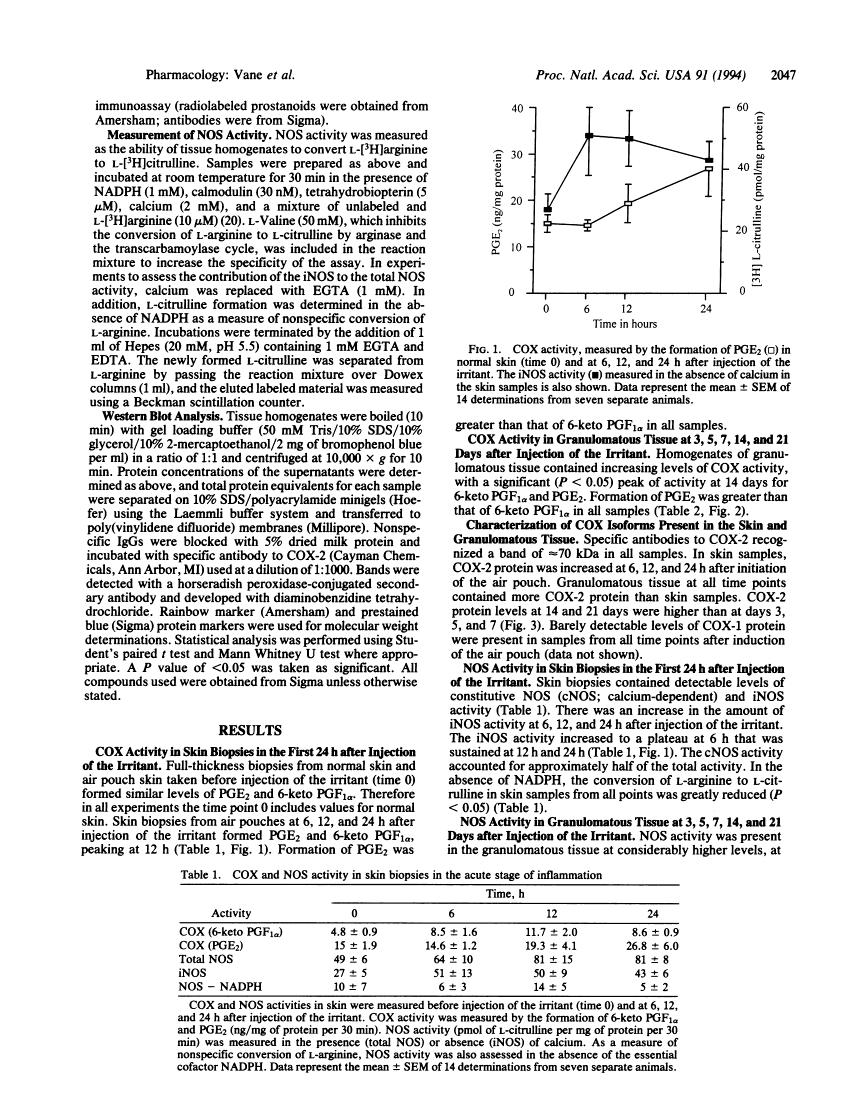
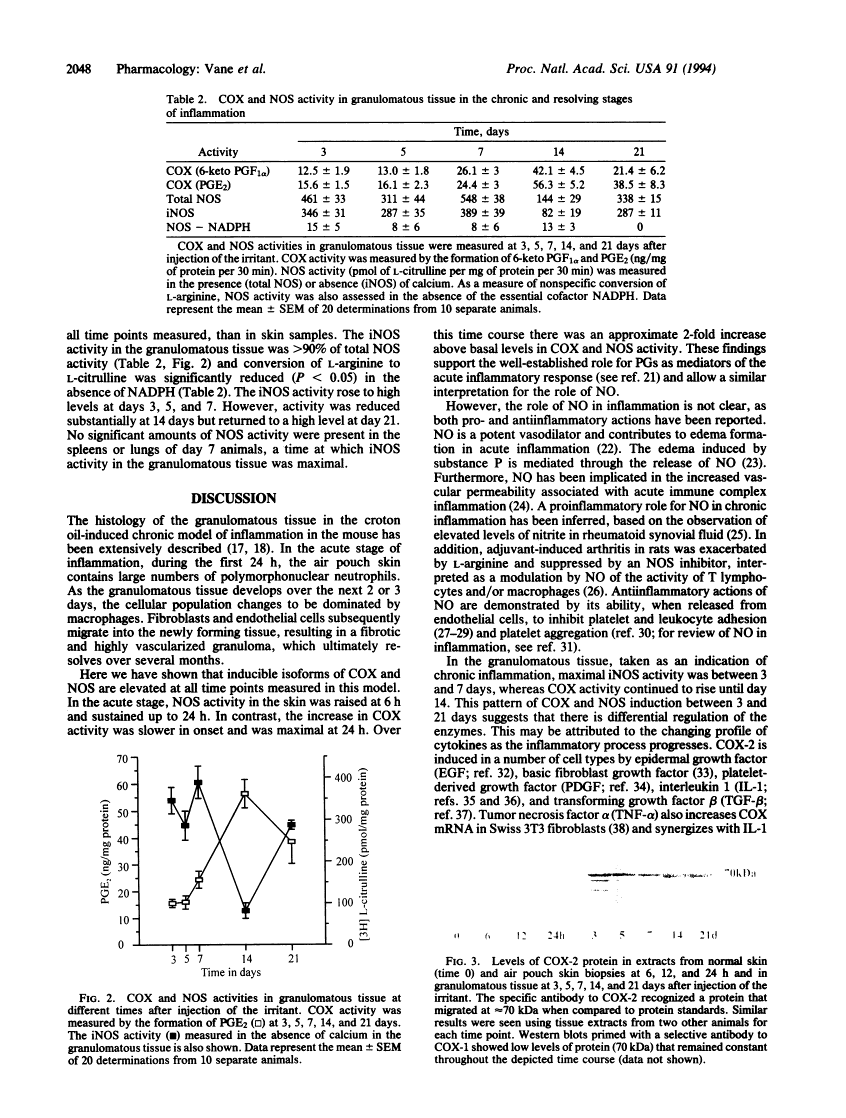
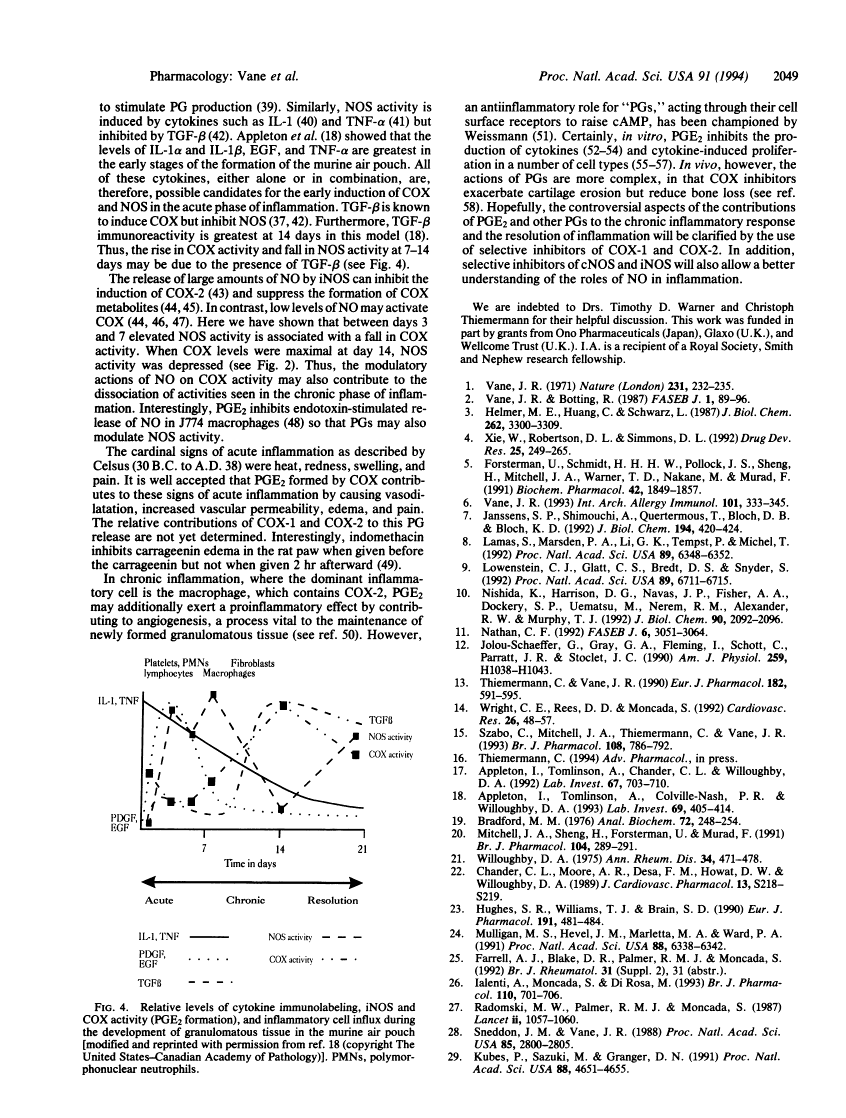
Cyclooxygenase (COX) converts arachidonic acid to prostaglandin H2, which is further metabolized to prostanoids. Two isoforms of COX exist: a constitutive (COX-1) and an inducible (COX-2) enzyme. Nitric oxide is derived from L-arginine by isoforms of nitric-oxide synthase (NOS; EC 1.14.13.39): constitutive (cNOS; calcium-dependent) and inducible (iNOS; calcium-independent). Here we have investigated inducible isoforms of COX and NOS in the acute, chronic, and resolving stages of a murine air pouch model of granulomatous inflammation. COX and NOS activities were measured in skin samples in the acute phase, up to 24 h. Activities in granulomatous tissue were measured at 3, 5, 7, 14, and 21 days for the chronic and resolving stages of inflammation. COX-1 and COX-2 proteins were assessed by Western blot. COX activity in the skin increased over the first 24 h and continued to rise up to day 14. COX-2 protein rose progressively, also peaking at day 14. COX-1 protein remained unaltered throughout. The iNOS activity increased over the first 24 h in the skin, with a further major increase in the granulomatous tissue between days 3 and 7, followed by a decrease at day 14 and a further increase at day 21. The rise in COX and NOS activities in the skin during the acute phase reinforces the proinflammatory role for prostanoids and suggests one also for nitric oxide. However, in the chronic and resolving stages, a dissociation of COX and NOS activity occurred. Thus, there may be differential regulation of these enzymes, perhaps due to the changing pattern of cytokines during the inflammatory response.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1007K), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. [Abstract] [Google Scholar]
- Vane J, Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987 Aug;1(2):89–96. [Abstract] [Google Scholar]
- Hemler ME, Huang C, Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [Abstract] [Google Scholar]
- Förstermann U, Schmidt HH, Pollock JS, Sheng H, Mitchell JA, Warner TD, Nakane M, Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991 Oct 24;42(10):1849–1857. [Abstract] [Google Scholar]
- Vane J. Control of the circulation by endothelial mediators. Inaugural G.B. West Memorial Lecture. Int Arch Allergy Immunol. 1993;101(4):333–345. [Abstract] [Google Scholar]
- Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. [Europe PMC free article] [Abstract] [Google Scholar]
- Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. [Europe PMC free article] [Abstract] [Google Scholar]
- Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992 Nov;90(5):2092–2096. [Europe PMC free article] [Abstract] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [Abstract] [Google Scholar]
- Julou-Schaeffer G, Gray GA, Fleming I, Schott C, Parratt JR, Stoclet JC. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. [Abstract] [Google Scholar]
- Thiemermann C, Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. [Abstract] [Google Scholar]
- Wright CE, Rees DD, Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. [Abstract] [Google Scholar]
- Szabó C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. [Europe PMC free article] [Abstract] [Google Scholar]
- Appleton I, Tomlinson A, Chander CL, Willoughby DA. Effect of endothelin-1 on croton oil-induced granulation tissue in the rat. A pharmacologic and immunohistochemical study. Lab Invest. 1992 Dec;67(6):703–710. [Abstract] [Google Scholar]
- Appleton I, Tomlinson A, Colville-Nash PR, Willoughby DA. Temporal and spatial immunolocalization of cytokines in murine chronic granulomatous tissue. Implications for their role in tissue development and repair processes. Lab Invest. 1993 Oct;69(4):405–414. [Abstract] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. [Abstract] [Google Scholar]
- Mitchell JA, Sheng H, Förstermann U, Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. [Europe PMC free article] [Abstract] [Google Scholar]
- Willoughby DA. Heberden Oration, 1974. Human arthritis applied to animal models. Towards a better therapy. Ann Rheum Dis. 1975 Dec;34(6):471–478. [Europe PMC free article] [Abstract] [Google Scholar]
- Chander CL, Moore AR, Desa FM, Howat DW, Willoughby DA. Anti-inflammatory effects of endothelin-1. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S218–S219. [Abstract] [Google Scholar]
- Hughes SR, Williams TJ, Brain SD. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990 Dec 4;191(3):481–484. [Abstract] [Google Scholar]
- Mulligan MS, Hevel JM, Marletta MA, Ward PA. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. [Europe PMC free article] [Abstract] [Google Scholar]
- Ialenti A, Moncada S, Di Rosa M. Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol. 1993 Oct;110(2):701–706. [Europe PMC free article] [Abstract] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. [Abstract] [Google Scholar]
- Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2800–2804. [Europe PMC free article] [Abstract] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. [Europe PMC free article] [Abstract] [Google Scholar]
- Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. [Europe PMC free article] [Abstract] [Google Scholar]
- Bailey JM, Muza B, Hla T, Salata K. Restoration of prostacyclin synthase in vascular smooth muscle cells after aspirin treatment: regulation by epidermal growth factor. J Lipid Res. 1985 Jan;26(1):54–61. [Abstract] [Google Scholar]
- Goddard DH, Grossman SL, Newton R, Clark MA, Bomalaski JS. Regulation of synovial cell growth: basic fibroblast growth factor synergizes with interleukin 1 beta stimulating phospholipase A2 enzyme activity, phospholipase A2 activating protein production and release of prostaglandin E2 by rheumatoid arthritis synovial cells in culture. Cytokine. 1992 Sep;4(5):377–384. [Abstract] [Google Scholar]
- Lin AH, Bienkowski MJ, Gorman RR. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989 Oct 15;264(29):17379–17383. [Abstract] [Google Scholar]
- Albrightson CR, Baenziger NL, Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [Abstract] [Google Scholar]
- Raz A, Wyche A, Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. [Europe PMC free article] [Abstract] [Google Scholar]
- Bailey JM, Verma M. Analytical procedures for a cryptic messenger RNA that mediates translational control of prostaglandin synthase by glucocorticoids. Anal Biochem. 1991 Jul;196(1):11–18. [Abstract] [Google Scholar]
- Burch RM, Tiffany CW. Tumor necrosis factor causes amplification of arachidonic acid metabolism in response to interleukin 1, bradykinin, and other agonists. J Cell Physiol. 1989 Oct;141(1):85–89. [Abstract] [Google Scholar]
- Goppelt-Struebe M, Wolter D, Resch K. Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase. Br J Pharmacol. 1989 Dec;98(4):1287–1295. [Europe PMC free article] [Abstract] [Google Scholar]
- Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [Abstract] [Google Scholar]
- Drapier JC, Wietzerbin J, Hibbs JB., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. [Abstract] [Google Scholar]
- Schini VB, Durante W, Elizondo E, Scott-Burden T, Junquero DC, Schafer AI, Vanhoutte PM. The induction of nitric oxide synthase activity is inhibited by TGF-beta 1, PDGFAB and PDGFBB in vascular smooth muscle cells. Eur J Pharmacol. 1992 Jun 17;216(3):379–383. [Abstract] [Google Scholar]
- Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, Simmons RL, Evans CH. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [Abstract] [Google Scholar]
- Stadler J, Harbrecht BG, Di Silvio M, Curran RD, Jordan ML, Simmons RL, Billiar TR. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993 Feb;53(2):165–172. [Abstract] [Google Scholar]
- Inoue T, Fukuo K, Morimoto S, Koh E, Ogihara T. Nitric oxide mediates interleukin-1-induced prostaglandin E2 production by vascular smooth muscle cells. Biochem Biophys Res Commun. 1993 Jul 15;194(1):420–424. [Abstract] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7240–7244. [Europe PMC free article] [Abstract] [Google Scholar]
- Marotta P, Sautebin L, Di Rosa M. Modulation of the induction of nitric oxide synthase by eicosanoids in the murine macrophage cell line J774. Br J Pharmacol. 1992 Nov;107(3):640–641. [Europe PMC free article] [Abstract] [Google Scholar]
- Boughton-Smith NK, Deakin AM, Follenfant RL, Whittle BJ, Garland LG. Role of oxygen radicals and arachidonic acid metabolites in the reverse passive Arthus reaction and carrageenin paw oedema in the rat. Br J Pharmacol. 1993 Oct;110(2):896–902. [Europe PMC free article] [Abstract] [Google Scholar]
- Weissmann G. Prostaglandins as modulators rather than mediators of inflammation. J Lipid Mediat. 1993 Mar-Apr;6(1-3):275–286. [Abstract] [Google Scholar]
- Gordon D, Bray MA, Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. [Abstract] [Google Scholar]
- Quill H, Gaur A, Phipps RP. Prostaglandin E2-dependent induction of granulocyte-macrophage colony-stimulating factor secretion by cloned murine helper T cells. J Immunol. 1989 Feb 1;142(3):813–818. [Abstract] [Google Scholar]
- Ferreri NR, Sarr T, Askenase PW, Ruddle NH. Molecular regulation of tumor necrosis factor-alpha and lymphotoxin production in T cells. Inhibition by prostaglandin E2. J Biol Chem. 1992 May 5;267(13):9443–9449. [Abstract] [Google Scholar]
- Stahl RA, Thaiss F, Haberstroh U, Kahf S, Shaw A, Schoeppe W. Cyclooxygenase inhibition enhances rat interleukin 1 beta-induced growth of rat mesangial cells in culture. Am J Physiol. 1990 Sep;259(3 Pt 2):F419–F424. [Abstract] [Google Scholar]
- Hori T, Yamanaka Y, Hayakawa M, Shibamoto S, Tsujimoto M, Oku N, Ito F. Prostaglandins antagonize fibroblast proliferation stimulated by tumor necrosis factor. Biochem Biophys Res Commun. 1991 Jan 31;174(2):758–766. [Abstract] [Google Scholar]
- Albina JE, Henry WL., Jr Suppression of lymphocyte proliferation through the nitric oxide synthesizing pathway. J Surg Res. 1991 Apr;50(4):403–409. [Abstract] [Google Scholar]
- Willoughby DA, Colville-Nash PR, Seed MP. Inflammation, prostaglandins, and loss of function. J Lipid Mediat. 1993 Mar-Apr;6(1-3):287–293. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.91.6.2046
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc43306?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.91.6.2046
Article citations
Redox regulation of macrophages.
Redox Biol, 72:103123, 12 Mar 2024
Cited by: 3 articles | PMID: 38615489 | PMCID: PMC11026845
Review Free full text in Europe PMC
Analysis of Neutrophil Responses to Biological Exposures.
Curr Protoc, 3(6):e827, 01 Jun 2023
Cited by: 1 article | PMID: 37358215
Antioxidant and Antineuroinflammatory Mechanisms of Kaempferol-3-O-β-d-Glucuronate on Lipopolysaccharide-Stimulated BV2 Microglial Cells through the Nrf2/HO-1 Signaling Cascade and MAPK/NF-κB Pathway.
ACS Omega, 8(7):6538-6549, 10 Feb 2023
Cited by: 7 articles | PMID: 36844518 | PMCID: PMC9948190
Aloe Extracts Inhibit Skin Inflammatory Responses by Regulating NF-κB, ERK, and JNK Signaling Pathways in an LPS-Induced RAW264.7 Macrophages Model.
Clin Cosmet Investig Dermatol, 16:267-278, 28 Jan 2023
Cited by: 1 article | PMID: 36742263 | PMCID: PMC9891070
Anti-Inflammatory, Antioxidative, and Nitric Oxide-Scavenging Activities of a Quercetin Nanosuspension with Polyethylene Glycol in LPS-Induced RAW 264.7 Macrophages.
Molecules, 27(21):7432, 01 Nov 2022
Cited by: 6 articles | PMID: 36364256 | PMCID: PMC9659305
Go to all (577) article citations
Other citations
Wikipedia
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Characterization of the induction of nitric oxide synthase and cyclo-oxygenase in rat aorta in organ culture.
Br J Pharmacol, 121(1):125-133, 01 May 1997
Cited by: 72 articles | PMID: 9146896 | PMCID: PMC1564653
Co-regulation between cyclo-oxygenase-2 and inducible nitric oxide synthase expression in the time-course of murine inflammation.
Naunyn Schmiedebergs Arch Pharmacol, 361(1):98-106, 01 Jan 2000
Cited by: 93 articles | PMID: 10651154
Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy.
Br J Pharmacol, 113(3):693-698, 01 Nov 1994
Cited by: 131 articles | PMID: 7532080 | PMCID: PMC1510410
Cox-2, iNOS and p53 as play-makers of tumor angiogenesis (review).
Int J Mol Med, 2(6):715-719, 01 Dec 1998
Cited by: 93 articles | PMID: 9850741
Review
Funding
Funders who supported this work.