Abstract
Free full text

Fibrillogenesis in Alzheimer's disease of amyloid beta peptides and apolipoprotein E.
Abstract
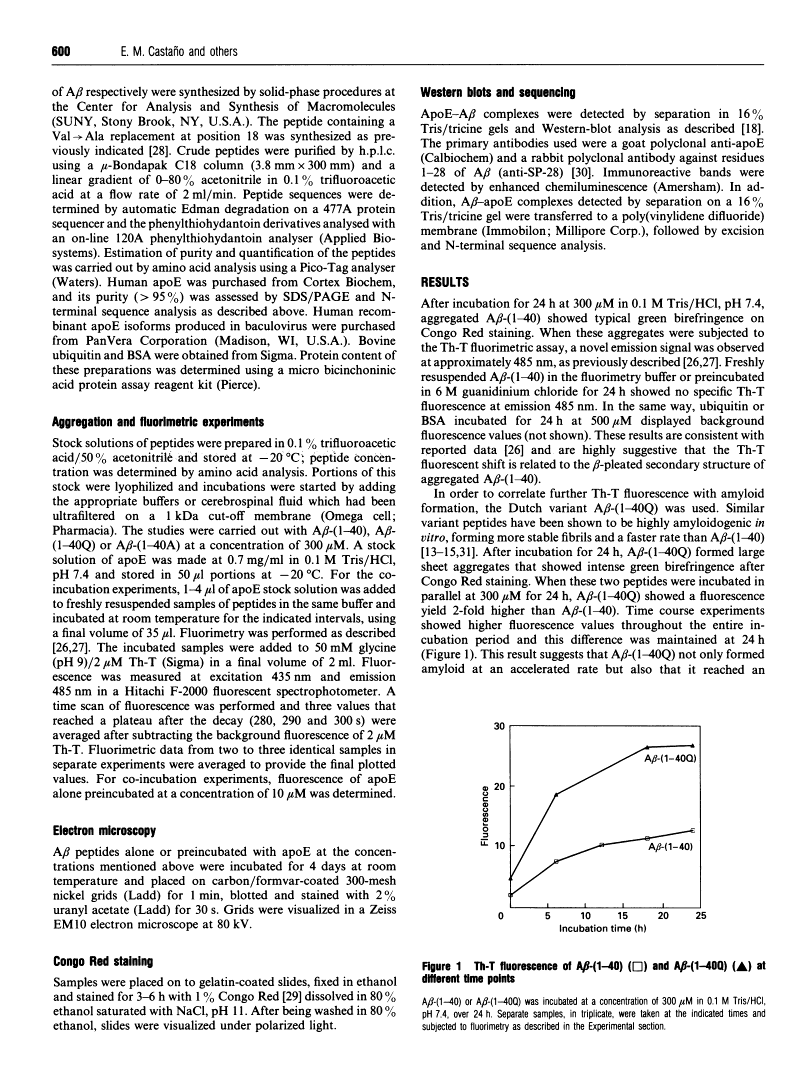
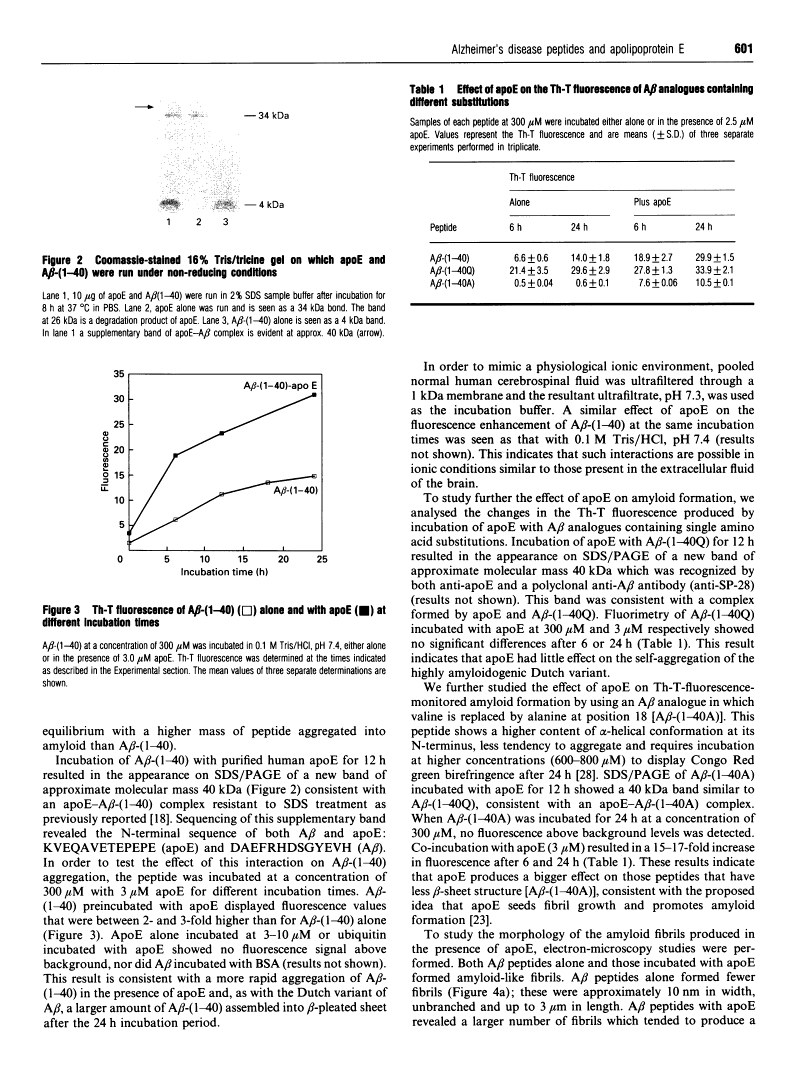
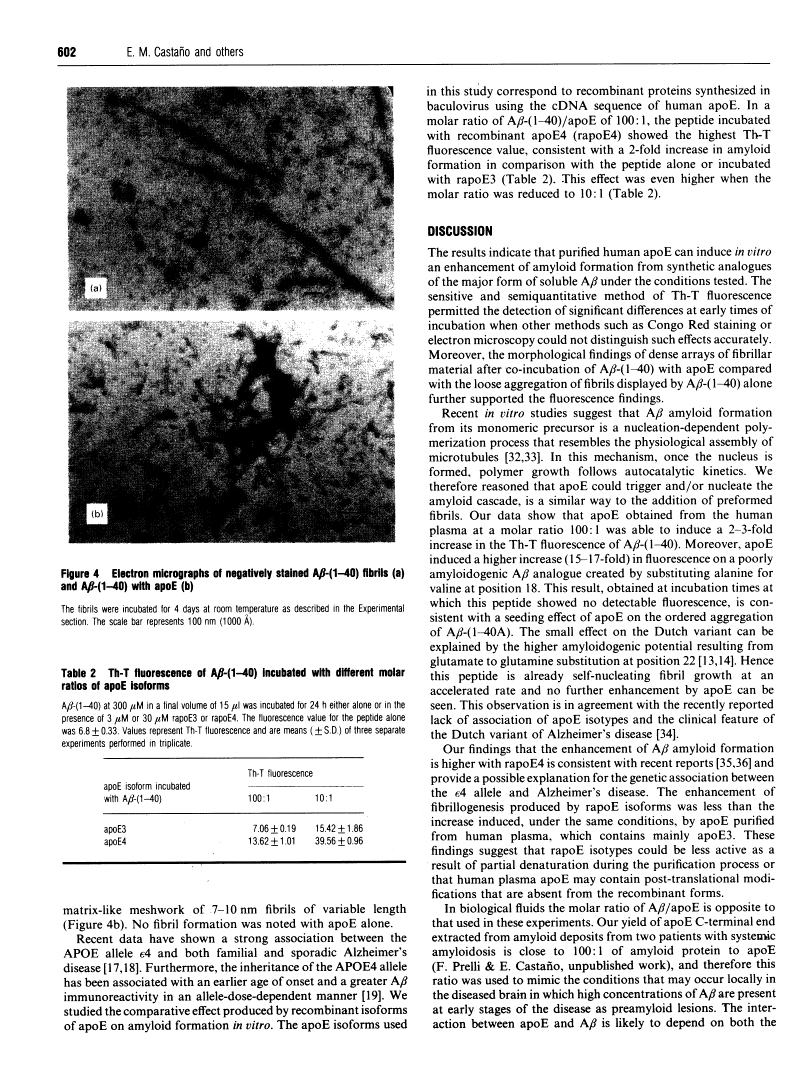
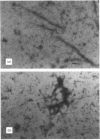
A central event in Alzheimer's disease is the conformational change from normally circulating soluble amyloid beta peptides (A beta) and tau proteins into amyloid fibrils, in the form of senile plaques and neurofibrillary tangles respectively. The apolipoprotein E (apoE) gene locus has recently been associated with late-onset Alzheimer's disease. It is not know whether apoE plays a direct role in the pathogenesis of the disease. In the present work we have investigated whether apoE can affect the known spontaneous in vitro formation of amyloid-like fibrils by synthetic A beta analogues using a thioflavine-T assay for fibril formation, electron microscopy and Congo Red staining. Our results show that, under the conditions used, apoE directly promotes amyloid fibril formation, increasing both the rate of fibrillogenesis and the total amount of amyloid formed. ApoE accelerated fibril formation of both wild-type A beta-(1-40) and A beta-(1-40A), an analogue created by the replacement of valine with alanine at residue 18, which alone produces few amyloid-like fibrils. However, apoE produced only a minimal effect on A beta-(1-40Q), found in the Dutch variant of Alzheimer's disease. When recombinant apoE isoforms were used, apoE4 was more efficient than apoE3 at enhancing amyloid formation. These in vitro observations support the hypothesis that apoE acts as a pathological chaperone, promoting the beta-pleated-sheet conformation of soluble A beta into amyloid fibres, and provide a possible explanation for the association of the apoE4 genetic isoform with Alzheimer's disease.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. [Abstract] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. [Europe PMC free article] [Abstract] [Google Scholar]
- Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller DL, Papayannopoulos IA, Styles J, Bobin SA, Lin YY, Biemann K, Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993 Feb 15;301(1):41–52. [Abstract] [Google Scholar]
- Selkoe DJ, Abraham CR, Podlisny MB, Duffy LK. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. [Abstract] [Google Scholar]
- Wisniewski T, Lalowski M, Levy E, Marques MR, Frangione B. The amino acid sequence of neuritic plaque amyloid from a familial Alzheimer's disease patient. Ann Neurol. 1994 Feb;35(2):245–246. [Abstract] [Google Scholar]
- Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. [Europe PMC free article] [Abstract] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. [Abstract] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. [Abstract] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. [Abstract] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. [Abstract] [Google Scholar]
- Cai XD, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. [Abstract] [Google Scholar]
- Wisniewski T, Ghiso J, Frangione B. Peptides homologous to the amyloid protein of Alzheimer's disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1247–1254. [Abstract] [Google Scholar]
- Clements A, Walsh DM, Williams CH, Allsop D. Effects of the mutations Glu22 to Gln and Ala21 to Gly on the aggregation of a synthetic fragment of the Alzheimer's amyloid beta/A4 peptide. Neurosci Lett. 1993 Oct 14;161(1):17–20. [Abstract] [Google Scholar]
- Fabian H, Szendrei GI, Mantsch HH, Otvos L., Jr Comparative analysis of human and Dutch-type Alzheimer beta-amyloid peptides by infrared spectroscopy and circular dichroism. Biochem Biophys Res Commun. 1993 Feb 26;191(1):232–239. [Abstract] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. [Abstract] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. [Abstract] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. [Europe PMC free article] [Abstract] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9649–9653. [Europe PMC free article] [Abstract] [Google Scholar]
- Wisniewski T, Golabek A, Matsubara E, Ghiso J, Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993 Apr 30;192(2):359–365. [Abstract] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Goedert M, Saunders AM, Huang D, Corder EH, Dong LM, Jakes R, Alberts MJ, Gilbert JR, et al. Hypothesis: microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994 Feb;125(2):163–174. [Abstract] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [Abstract] [Google Scholar]
- Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. [Abstract] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991 Feb 8;541(1):163–166. [Abstract] [Google Scholar]
- Prelli F, Pras M, Frangione B. Degradation and deposition of amyloid AA fibrils are tissue specific. Biochemistry. 1987 Dec 15;26(25):8251–8256. [Abstract] [Google Scholar]
- Naiki H, Higuchi K, Nakakuki K, Takeda T. Kinetic analysis of amyloid fibril polymerization in vitro. Lab Invest. 1991 Jul;65(1):104–110. [Abstract] [Google Scholar]
- Wisniewski T, Castano E, Ghiso J, Frangione B. Cerebrospinal fluid inhibits Alzheimer beta-amyloid fibril formation in vitro. Ann Neurol. 1993 Oct;34(4):631–633. [Abstract] [Google Scholar]
- Castaño EM, Ghiso J, Prelli F, Gorevic PD, Migheli A, Frangione B. In vitro formation of amyloid fibrils from two synthetic peptides of different lengths homologous to Alzheimer's disease beta-protein. Biochem Biophys Res Commun. 1986 Dec 15;141(2):782–789. [Abstract] [Google Scholar]
- Ghiso J, Wisniewski T, Vidal R, Rostagno A, Frangione B. Epitope map of two polyclonal antibodies that recognize amyloid lesions in patients with Alzheimer's disease. Biochem J. 1992 Mar 1;282(Pt 2):517–522. [Europe PMC free article] [Abstract] [Google Scholar]
- Fraser PE, Nguyen JT, Inouye H, Surewicz WK, Selkoe DJ, Podlisny MB, Kirschner DA. Fibril formation by primate, rodent, and Dutch-hemorrhagic analogues of Alzheimer amyloid beta-protein. Biochemistry. 1992 Nov 10;31(44):10716–10723. [Abstract] [Google Scholar]
- Jarrett JT, Lansbury PT., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. [Abstract] [Google Scholar]
- Wisniewski T, Ghiso J, Frangione B. Alzheimer's disease and soluble A beta. Neurobiol Aging. 1994 Mar-Apr;15(2):143–152. [Abstract] [Google Scholar]
- Haan J, Van Broeckhoven C, van Duijn CM, Voorhoeve E, van Harskamp F, van Swieten JC, Maat-Schieman ML, Roos RA, Bakker E. The apolipoprotein E epsilon 4 allele does not influence the clinical expression of the amyloid precursor protein gene codon 693 or 692 mutations. Ann Neurol. 1994 Sep;36(3):434–437. [Abstract] [Google Scholar]
- Smyrk TC. Colon cancer connections. Cancer syndrome meets molecular biology meets histopathology. Am J Pathol. 1994 Jul;145(1):1–6. [Europe PMC free article] [Abstract] [Google Scholar]
- Ma J, Yee A, Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994 Nov 3;372(6501):92–94. [Abstract] [Google Scholar]
- Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK, et al. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8368–8372. [Europe PMC free article] [Abstract] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. [Abstract] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. [Europe PMC free article] [Abstract] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. Aggregation-related toxicity of synthetic beta-amyloid protein in hippocampal cultures. Eur J Pharmacol. 1991 Aug 14;207(4):367–368. [Abstract] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994 May 6;264(5160):850–852. [Abstract] [Google Scholar]
- Whitson JS, Mims MP, Strittmatter WJ, Yamaki T, Morrisett JD, Appel SH. Attenuation of the neurotoxic effect of A beta amyloid peptide by apolipoprotein E. Biochem Biophys Res Commun. 1994 Feb 28;199(1):163–170. [Abstract] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993 Oct;11(4):575–580. [Abstract] [Google Scholar]
- Vigushin DM, Gough J, Allan D, Alguacil A, Penner B, Pettigrew NM, Quinonez G, Bernstein K, Booth SE, Booth DR, et al. Familial nephropathic systemic amyloidosis caused by apolipoprotein AI variant Arg26. Q J Med. 1994 Mar;87(3):149–154. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj3060599
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1136559?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1042/bj3060599
Article citations
APOE from astrocytes restores Alzheimer's Aβ-pathology and DAM-like responses in APOE deficient microglia.
EMBO Mol Med, 11 Nov 2024
Cited by: 0 articles | PMID: 39528861
Apolipoprotein ε in Brain and Retinal Neurodegenerative Diseases.
Aging Dis, 14(4):1311-1330, 01 Aug 2023
Cited by: 4 articles | PMID: 37199411 | PMCID: PMC10389820
Review Free full text in Europe PMC
Neuronal ApoE4 in Alzheimer's disease and potential therapeutic targets.
Front Aging Neurosci, 15:1199434, 02 Jun 2023
Cited by: 8 articles | PMID: 37333457 | PMCID: PMC10272394
Review Free full text in Europe PMC
Astrocytic APOE4 removal confers cerebrovascular protection despite increased cerebral amyloid angiopathy.
Mol Neurodegener, 18(1):17, 16 Mar 2023
Cited by: 11 articles | PMID: 36922879 | PMCID: PMC10018855
APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer's disease pathology and brain diseases.
Mol Neurodegener, 17(1):62, 24 Sep 2022
Cited by: 61 articles | PMID: 36153580 | PMCID: PMC9509584
Review Free full text in Europe PMC
Go to all (144) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Acceleration of Alzheimer's fibril formation by apolipoprotein E in vitro.
Am J Pathol, 145(5):1030-1035, 01 Nov 1994
Cited by: 290 articles | PMID: 7977635 | PMCID: PMC1887417
Relationship between apolipoprotein E and the amyloid deposits and dystrophic neurites of Alzheimer's disease.
Neuropathol Appl Neurobiol, 23(6):483-491, 01 Dec 1997
Cited by: 20 articles | PMID: 9460714
Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3.
J Clin Invest, 94(2):860-869, 01 Aug 1994
Cited by: 245 articles | PMID: 8040342 | PMCID: PMC296168
Cerebrovascular transport of Alzheimer's amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier.
Life Sci, 59(18):1483-1497, 01 Jan 1996
Cited by: 88 articles | PMID: 8890929
Review
Funding
Funders who supported this work.
NIA NIH HHS (3)
Grant ID: AG00542
Grant ID: AG05891
Grant ID: AG 10953