Abstract
Free full text

Complete nucleotide sequence of the gene encoding bacteriophage E endosialidase: implications for K1E endosialidase structure and function.
Abstract
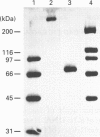
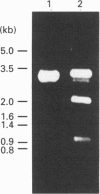
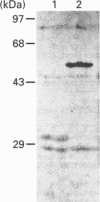
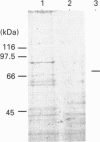
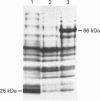
Bacteriophage E specifically recognizes and infects strains of Escherichia coli which display the alpha-2,8-linked polysialic acid K1 capsule. Bacteriophage E endosialidase, which is thought to be responsible for initial absorption of the phage to the host bacterium, was purified, and the N-terminal amino acid sequences of the polypeptide monomer and cyanogen bromide fragments were determined. Synthetic oligonucleotide probes were designed from the N-terminal amino acid sequences and used to identify restriction fragments of bacteriophage E DNA encoding the endosialidase. The primary nucleotide sequence of the bacteriophage E endosialidase gene contains an open reading frame encoding a 90 kDa polypeptide which is processed to give a mature 74 kDa protein. The native enzyme is probably a trimer of identical 74 kDa subunits. In the bacteriophage E genome the K1E endosialidase open reading frame is preceded by a putative upstream promoter region with homology to a bacteriophage SP6 promoter. A central region of 500 amino acids of the deduced protein sequence of the K1E endosialidase was found to have 84% identity to K1F endosialidase. Both endosialidases contain two copies of a sialidase sequence motif common to many bacterial and viral sialidases. These sequences flank the region of greatest identity between the two endosialidase forms, which suggests that this central domain is involved in binding and hydrolysis of the polysialic acid substrate.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gross RJ, Cheasty T, Rowe B. Isolation of bacteriophages specific for the K1 polysaccharide antigen of Escherichia coli. J Clin Microbiol. 1977 Dec;6(6):548–550. [Europe PMC free article] [Abstract] [Google Scholar]
- Robbins JB, McCracken GH, Jr, Gotschlich EC, Orskov F, Orskov I, Hanson LA. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. [Abstract] [Google Scholar]
- Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975 Mar 10;250(5):1926–1932. [Abstract] [Google Scholar]
- Orskov I, Orskov F, Jann B, Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. [Europe PMC free article] [Abstract] [Google Scholar]
- Tomlinson S, Taylor PW. Neuraminidase associated with coliphage E that specifically depolymerizes the Escherichia coli K1 capsular polysaccharide. J Virol. 1985 Aug;55(2):374–378. [Europe PMC free article] [Abstract] [Google Scholar]
- Livingston BD, Jacobs JL, Glick MC, Troy FA. Extended polysialic acid chains (n greater than 55) in glycoproteins from human neuroblastoma cells. J Biol Chem. 1988 Jul 5;263(19):9443–9448. [Abstract] [Google Scholar]
- Roth J, Zuber C, Wagner P, Taatjes DJ, Weisgerber C, Heitz PU, Goridis C, Bitter-Suermann D. Reexpression of poly(sialic acid) units of the neural cell adhesion molecule in Wilms tumor. Proc Natl Acad Sci U S A. 1988 May;85(9):2999–3003. [Europe PMC free article] [Abstract] [Google Scholar]
- Roth J, Zuber C, Wagner P, Blaha I, Bitter-Suermann D, Heitz PU. Presence of the long chain form of polysialic acid of the neural cell adhesion molecule in Wilms' tumor. Identification of a cell adhesion molecule as an oncodevelopmental antigen and implications for tumor histogenesis. Am J Pathol. 1988 Nov;133(2):227–240. [Europe PMC free article] [Abstract] [Google Scholar]
- Figarella-Branger DF, Durbec PL, Rougon GN. Differential spectrum of expression of neural cell adhesion molecule isoforms and L1 adhesion molecules on human neuroectodermal tumors. Cancer Res. 1990 Oct 1;50(19):6364–6370. [Abstract] [Google Scholar]
- Heitz PU, Komminoth P, Lackie PM, Zuber C, Roth J. Nachweis von Polysialinsäure und N-CAM in neuroendokrinen Tumoren. Verh Dtsch Ges Pathol. 1990;74:376–377. [Abstract] [Google Scholar]
- Moolenaar CE, Muller EJ, Schol DJ, Figdor CG, Bock E, Bitter-Suermann D, Michalides RJ. Expression of neural cell adhesion molecule-related sialoglycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res. 1990 Feb 15;50(4):1102–1106. [Abstract] [Google Scholar]
- Roth J, Zuber C. Immunoelectron microscopic investigation of surface coat of Wilms tumor cells. Dense lamina is composed of highly sialylated neural cell adhesion molecule. Lab Invest. 1990 Jan;62(1):55–60. [Abstract] [Google Scholar]
- Kwiatkowski B, Boschek B, Thiele H, Stirm S. Endo-N-acetylneuraminidase associated with bacteriophage particles. J Virol. 1982 Aug;43(2):697–704. [Europe PMC free article] [Abstract] [Google Scholar]
- Finne J, Mäkelä PH. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [Abstract] [Google Scholar]
- Hallenbeck PC, Vimr ER, Yu F, Bassler B, Troy FA. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J Biol Chem. 1987 Mar 15;262(8):3553–3561. [Abstract] [Google Scholar]
- Pelkonen S, Pelkonen J, Finne J. Common cleavage pattern of polysialic acid by bacteriophage endosialidases of different properties and origins. J Virol. 1989 Oct;63(10):4409–4416. [Europe PMC free article] [Abstract] [Google Scholar]
- Petter JG, Vimr ER. Complete nucleotide sequence of the bacteriophage K1F tail gene encoding endo-N-acylneuraminidase (endo-N) and comparison to an endo-N homolog in bacteriophage PK1E. J Bacteriol. 1993 Jul;175(14):4354–4363. [Europe PMC free article] [Abstract] [Google Scholar]
- Horgan IE. A modified spectrophotometric method for determination of nanogram quantities of sialic acid. Clin Chim Acta. 1981 Nov 11;116(3):409–415. [Abstract] [Google Scholar]
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. [Europe PMC free article] [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Holm L. Codon usage and gene expression. Nucleic Acids Res. 1986 Apr 11;14(7):3075–3087. [Europe PMC free article] [Abstract] [Google Scholar]
- Dower WJ, Miller JF, Ragsdale CW. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. [Europe PMC free article] [Abstract] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. [Abstract] [Google Scholar]
- Staden R, McLachlan AD. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. [Europe PMC free article] [Abstract] [Google Scholar]
- Staden R. Finding protein coding regions in genomic sequences. Methods Enzymol. 1990;183:163–180. [Abstract] [Google Scholar]
- Fricker LD, Adelman JP, Douglass J, Thompson RC, von Strandmann RP, Hutton J. Isolation and sequence analysis of cDNA for rat carboxypeptidase E [EC 3.4.17.10], a neuropeptide processing enzyme. Mol Endocrinol. 1989 Apr;3(4):666–673. [Abstract] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. [Abstract] [Google Scholar]
- Pearson WR. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. [Abstract] [Google Scholar]
- Staden R. Searching for patterns in protein and nucleic acid sequences. Methods Enzymol. 1990;183:193–211. [Abstract] [Google Scholar]
- Roggentin P, Rothe B, Kaper JB, Galen J, Lawrisuk L, Vimr ER, Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. [Abstract] [Google Scholar]
- Rothe B, Rothe B, Roggentin P, Schauer R. The sialidase gene from Clostridium septicum: cloning, sequencing, expression in Escherichia coli and identification of conserved sequences in sialidases and other proteins. Mol Gen Genet. 1991 Apr;226(1-2):190–197. [Abstract] [Google Scholar]
- Rieger D, Freund-Mölbert E, Stirm S. Escherichia coli capsule bacteriophages. III. Fragments of bacteriophage 29. J Virol. 1975 Apr;15(4):964–975. [Europe PMC free article] [Abstract] [Google Scholar]
- Saraste M, Sibbald PR, Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. [Abstract] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. [Europe PMC free article] [Abstract] [Google Scholar]
- Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. [Europe PMC free article] [Abstract] [Google Scholar]
- Pelkonen S, Aalto J, Finne J. Differential activities of bacteriophage depolymerase on bacterial polysaccharide: binding is essential but degradation is inhibitory in phage infection of K1-defective Escherichia coli. J Bacteriol. 1992 Dec;174(23):7757–7761. [Europe PMC free article] [Abstract] [Google Scholar]
- Crennell SJ, Garman EF, Laver WG, Vimr ER, Taylor GL. Crystal structure of a bacterial sialidase (from Salmonella typhimurium LT2) shows the same fold as an influenza virus neuraminidase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9852–9856. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj3090543
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1135765?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Therapeutic Application of Phage Capsule Depolymerases against K1, K5, and K30 Capsulated E. coli in Mice.
Front Microbiol, 8:2257, 16 Nov 2017
Cited by: 46 articles | PMID: 29201019 | PMCID: PMC5696595
Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process.
Appl Microbiol Biotechnol, 101(8):3103-3119, 23 Mar 2017
Cited by: 174 articles | PMID: 28337580 | PMCID: PMC5380687
Review Free full text in Europe PMC
Genomic analysis of Bacillus subtilis lytic bacteriophage ϕNIT1 capable of obstructing natto fermentation carrying genes for the capsule-lytic soluble enzymes poly-γ-glutamate hydrolase and levanase.
Biosci Biotechnol Biochem, 81(1):135-146, 22 Sep 2016
Cited by: 9 articles | PMID: 27885938
Bacteriophage-encoded depolymerases: their diversity and biotechnological applications.
Appl Microbiol Biotechnol, 100(5):2141-2151, 15 Jan 2016
Cited by: 207 articles | PMID: 26767986
Review
Bacteriophages and phage-derived proteins--application approaches.
Curr Med Chem, 22(14):1757-1773, 01 Jan 2015
Cited by: 104 articles | PMID: 25666799 | PMCID: PMC4468916
Review Free full text in Europe PMC
Go to all (30) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cloning, expression, and purification of the K5 capsular polysaccharide lyase (KflA) from coliphage K5A: evidence for two distinct K5 lyase enzymes.
J Bacteriol, 182(13):3761-3766, 01 Jul 2000
Cited by: 36 articles | PMID: 10850992 | PMCID: PMC94548
Complete nucleotide sequence of the bacteriophage K1F tail gene encoding endo-N-acylneuraminidase (endo-N) and comparison to an endo-N homolog in bacteriophage PK1E.
J Bacteriol, 175(14):4354-4363, 01 Jul 1993
Cited by: 56 articles | PMID: 8331067 | PMCID: PMC204875
Proteolytic processing and oligomerization of bacteriophage-derived endosialidases.
J Biol Chem, 278(15):12634-12644, 29 Jan 2003
Cited by: 43 articles | PMID: 12556457
The genome of bacteriophage K1F, a T7-like phage that has acquired the ability to replicate on K1 strains of Escherichia coli.
J Bacteriol, 187(24):8499-8503, 01 Dec 2005
Cited by: 59 articles | PMID: 16321955 | PMCID: PMC1317022