Abstract
Free full text

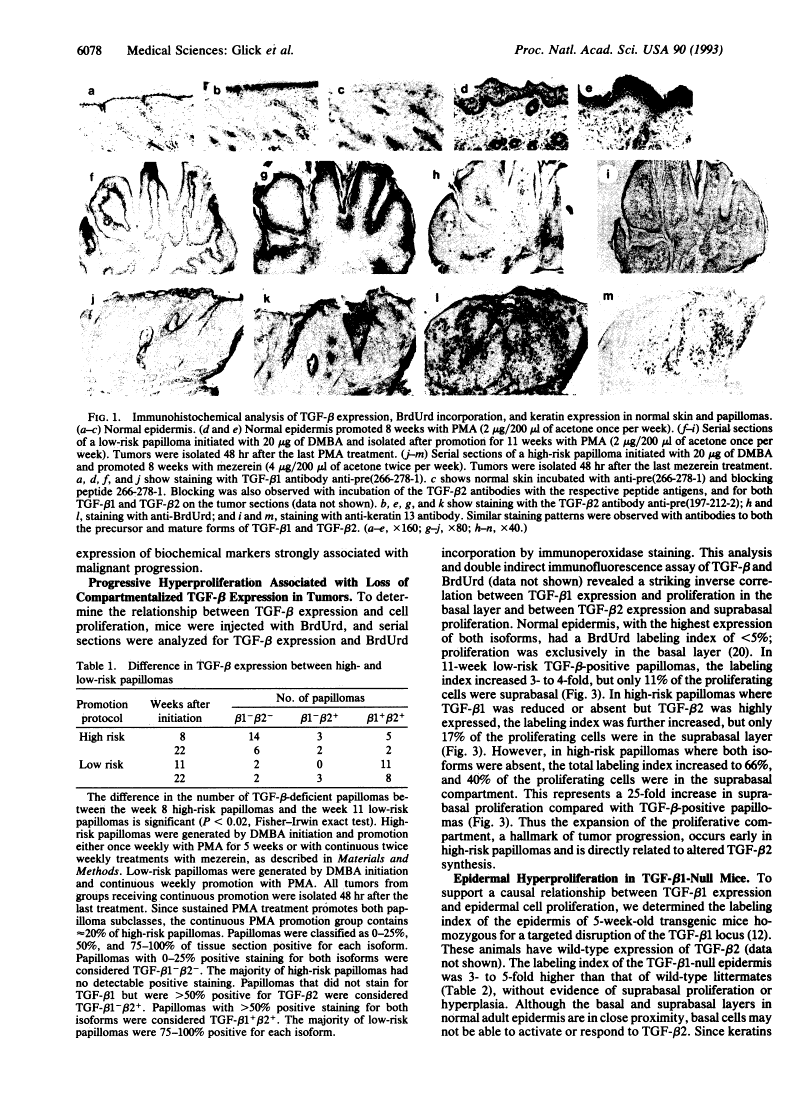
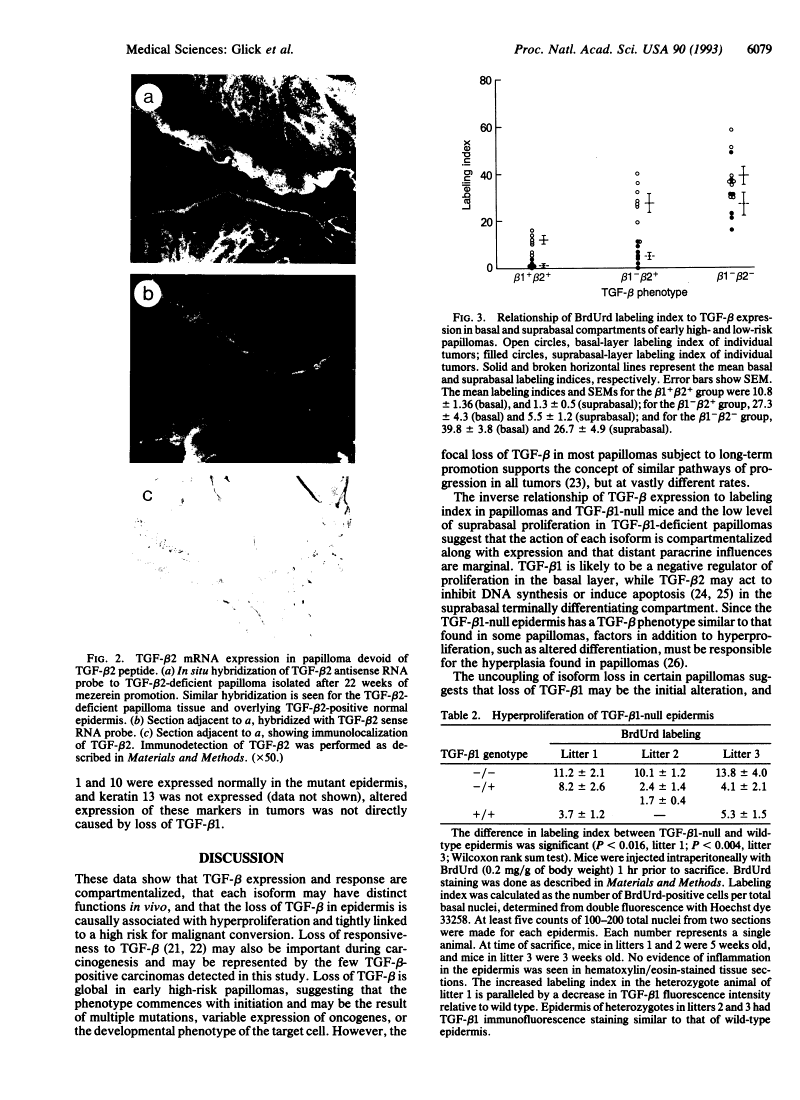
Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion.
Abstract
Mouse skin carcinomas arise from a small subpopulation of benign papillomas with an increased risk of malignant conversion. These papillomas arise with limited stimulation by tumor promoters, appear rapidly, and do not regress, suggesting that they differ in growth properties from the majority of benign tumors. The transforming growth factor beta (TGF-beta) proteins are expressed in the epidermis and are growth inhibitors for mouse keratinocytes in vitro; altered TGF-beta expression could influence the growth properties of high-risk papillomas. Normal epidermis, tumor promoter-treated epidermis, and skin papillomas at low risk for malignant conversion express TGF-beta 1 in the basal cell compartment and TGF-beta 2 in the suprabasal strata. In low-risk tumors, 90% of the proliferating cells are confined to the basal compartment. In contrast, the majority of high-risk papillomas are devoid of both TGF-beta 1 and TGF-beta 2 as soon as they arise; these tumors have up to 40% of the proliferating cells in the suprabasal layers. Squamous cell carcinomas are also devoid of TGF-beta, suggesting that they arise from the TGF-beta-deficient high-risk papillomas. In some high-risk papillomas, TGF-beta 1 loss can occur first and correlates with basal cell hyperproliferation, while TGF-beta 2 loss correlates with suprabasal hyperproliferation. Similarly, TGF-beta 1-null transgenic mice, which express wild-type levels of TGF-beta 2 in epidermis but no TGF-beta 1 in the basal layer, have a hyperproliferative basal cell layer without suprabasal proliferation. In tumors, loss of TGF-beta is controlled at the posttranscriptional level and is associated with expression of keratin 13, a documented marker of malignant progression. These results show that TGF-beta expression and function are compartmentalized in epidermis and epidermal tumors and that loss of TGF-beta is an early, biologically relevant risk factor for malignant progression.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Pai SB, Steele VE, Nettesheim P. Neoplastic transformation of primary tracheal epithelial cell cultures. Carcinogenesis. 1983;4(4):369–374. [Abstract] [Google Scholar]
- Balmain A, Pragnell IB. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. [Abstract] [Google Scholar]
- Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. [Abstract] [Google Scholar]
- Hennings H, Shores R, Mitchell P, Spangler EF, Yuspa SH. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis. 1985 Nov;6(11):1607–1610. [Abstract] [Google Scholar]
- Hennings H, Spangler EF, Shores R, Mitchell P, Devor D, Shamsuddin AK, Elgjo KM, Yuspa SH. Malignant conversion and metastasis of mouse skin tumors: a comparison of SENCAR and CD-1 mice. Environ Health Perspect. 1986 Sep;68:69–74. [Europe PMC free article] [Abstract] [Google Scholar]
- Glick AB, Sporn MB, Yuspa SH. Altered regulation of TGF-beta 1 and TGF-alpha in primary keratinocytes and papillomas expressing v-Ha-ras. Mol Carcinog. 1991;4(3):210–219. [Abstract] [Google Scholar]
- Akhurst RJ, Fee F, Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988 Jan 28;331(6154):363–365. [Abstract] [Google Scholar]
- Fowlis DJ, Flanders KC, Duffie E, Balmain A, Akhurst RJ. Discordant transforming growth factor beta 1 RNA and protein localization during chemical carcinogenesis of the skin. Cell Growth Differ. 1992 Feb;3(2):81–91. [Abstract] [Google Scholar]
- Kane CJ, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991 Jul;148(1):157–173. [Abstract] [Google Scholar]
- Glick AB, Danielpour D, Morgan D, Sporn MB, Yuspa SH. Induction and autocrine receptor binding of transforming growth factor-beta 2 during terminal differentiation of primary mouse keratinocytes. Mol Endocrinol. 1990 Jan;4(1):46–52. [Abstract] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. [Europe PMC free article] [Abstract] [Google Scholar]
- Heine U, Munoz EF, Flanders KC, Ellingsworth LR, Lam HY, Thompson NL, Roberts AB, Sporn MB. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. [Europe PMC free article] [Abstract] [Google Scholar]
- Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. [Europe PMC free article] [Abstract] [Google Scholar]
- Flanders KC, Cissel DS, Mullen LT, Danielpour D, Sporn MB, Roberts AB. Antibodies to transforming growth factor-beta 2 peptides: specific detection of TGF-beta 2 in immunoassays. Growth Factors. 1990;3(1):45–52. [Abstract] [Google Scholar]
- Nischt R, Roop DR, Mehrel T, Yuspa SH, Rentrop M, Winter H, Schweizer J. Aberrant expression during two-stage mouse skin carcinogenesis of a type I 47-kDa keratin, K13, normally associated with terminal differentiation of internal stratified epithelia. Mol Carcinog. 1988;1(2):96–108. [Abstract] [Google Scholar]
- Roop DR, Krieg TM, Mehrel T, Cheng CK, Yuspa SH. Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 1988 Jun 1;48(11):3245–3252. [Abstract] [Google Scholar]
- Shivers BD, Schachter BS, Pfaff DW. In situ hybridization for the study of gene expression in the brain. Methods Enzymol. 1986;124:497–510. [Abstract] [Google Scholar]
- Fitzpatrick DR, Denhez F, Kondaiah P, Akhurst RJ. Differential expression of TGF beta isoforms in murine palatogenesis. Development. 1990 Jul;109(3):585–595. [Abstract] [Google Scholar]
- Huitfeldt HS, Heyden A, Clausen OP, Thrane EV, Roop D, Yuspa SH. Altered regulation of growth and expression of differentiation-associated keratins in benign mouse skin tumors. Carcinogenesis. 1991 Nov;12(11):2063–2067. [Abstract] [Google Scholar]
- Missero C, Ramon y Cajal S, Dotto GP. Escape from transforming growth factor beta control and oncogene cooperation in skin tumor development. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9613–9617. [Europe PMC free article] [Abstract] [Google Scholar]
- Shipley GD, Pittelkow MR, Wille JJ, Jr, Scott RE, Moses HL. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986 Apr;46(4 Pt 2):2068–2071. [Abstract] [Google Scholar]
- Aldaz CM, Conti CJ, Klein-Szanto AJ, Slaga TJ. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2029–2032. [Europe PMC free article] [Abstract] [Google Scholar]
- Yanagihara K, Tsumuraya M. Transforming growth factor beta 1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res. 1992 Jul 15;52(14):4042–4045. [Abstract] [Google Scholar]
- Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee E, Punnonen K, Cheng C, Glick A, Dlugosz A, Yuspa SH. Analysis of phospholipid metabolism in murine keratinocytes transformed by the v-ras oncogene: relationship of phosphatidylinositol turnover and cytokine stimulation to the transformed phenotype. Carcinogenesis. 1992 Dec;13(12):2367–2373. [Abstract] [Google Scholar]
- Kim SJ, Park K, Koeller D, Kim KY, Wakefield LM, Sporn MB, Roberts AB. Post-transcriptional regulation of the human transforming growth factor-beta 1 gene. J Biol Chem. 1992 Jul 5;267(19):13702–13707. [Abstract] [Google Scholar]
- Hennings H, Shores R, Balaschak M, Yuspa SH. Sensitivity of subpopulations of mouse skin papillomas to malignant conversion by urethane or 4-nitroquinoline N-oxide. Cancer Res. 1990 Feb 1;50(3):653–657. [Abstract] [Google Scholar]
- Fürstenberger G, Rogers M, Schnapke R, Bauer G, Höfler P, Marks F. Stimulatory role of transforming growth factors in multistage skin carcinogenesis: possible explanation for the tumor-inducing effect of wounding in initiated NMRI mouse skin. Int J Cancer. 1989 May 15;43(5):915–921. [Abstract] [Google Scholar]
- Hennings H, Shores R, Wenk ML, Spangler EF, Tarone R, Yuspa SH. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature. 1983 Jul 7;304(5921):67–69. [Abstract] [Google Scholar]
- Bremner R, Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990 May 4;61(3):407–417. [Abstract] [Google Scholar]
- Greenhalgh DA, Welty DJ, Player A, Yuspa SH. Two oncogenes, v-fos and v-ras, cooperate to convert normal keratinocytes to squamous cell carcinoma. Proc Natl Acad Sci U S A. 1990 Jan;87(2):643–647. [Europe PMC free article] [Abstract] [Google Scholar]
- Dotto GP, O'Connell J, Patskan G, Conti C, Ariza A, Slaga TJ. Malignant progression of papilloma-derived keratinocytes: differential effects of the ras, neu, and p53 oncogenes. Mol Carcinog. 1988;1(3):171–179. [Abstract] [Google Scholar]
- Wu SP, Theodorescu D, Kerbel RS, Willson JK, Mulder KM, Humphrey LE, Brattain MG. TGF-beta 1 is an autocrine-negative growth regulator of human colon carcinoma FET cells in vivo as revealed by transfection of an antisense expression vector. J Cell Biol. 1992 Jan;116(1):187–196. [Europe PMC free article] [Abstract] [Google Scholar]
- Aldaz CM, Trono D, Larcher F, Slaga TJ, Conti CJ. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol Carcinog. 1989;2(1):22–26. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.90.13.6076
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc46870?pdf=render
Citations & impact
Impact metrics
Article citations
TGF-β signaling in health, disease, and therapeutics.
Signal Transduct Target Ther, 9(1):61, 22 Mar 2024
Cited by: 36 articles | PMID: 38514615 | PMCID: PMC10958066
Review Free full text in Europe PMC
Serum-Induced Proliferation of Human Cardiac Stem Cells Is Modulated via TGFβRI/II and SMAD2/3.
Int J Mol Sci, 25(2):959, 12 Jan 2024
Cited by: 2 articles | PMID: 38256034 | PMCID: PMC10815425
TGFβ1 regulates HRas-mediated activation of IRE1α through the PERK-RPAP2 axis in keratinocytes.
Mol Carcinog, 61(10):958-971, 17 Aug 2022
Cited by: 0 articles | PMID: 35975910 | PMCID: PMC9486931
Targeted deletion of TGFβ1 in basal keratinocytes causes profound defects in stratified squamous epithelia and aberrant melanocyte migration.
Dev Biol, 485:9-23, 25 Feb 2022
Cited by: 1 article | PMID: 35227671 | PMCID: PMC8969113
Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers.
Cancers (Basel), 14(7):1681, 25 Mar 2022
Cited by: 75 articles | PMID: 35406451 | PMCID: PMC8996887
Review Free full text in Europe PMC
Go to all (116) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Influence of beta1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: alpha3beta1, but not alpha2beta1, suppresses malignant conversion.
Cancer Res, 61(13):5248-5254, 01 Jul 2001
Cited by: 40 articles | PMID: 11431366
Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis.
Cancer Res, 48(11):3245-3252, 01 Jun 1988
Cited by: 107 articles | PMID: 2452688
Expression of the TGF-beta coreceptor endoglin in epidermal keratinocytes and its dual role in multistage mouse skin carcinogenesis.
Oncogene, 22(38):5976-5985, 01 Sep 2003
Cited by: 37 articles | PMID: 12955076
Differential regulation of integrins and extracellular matrix binding in epidermal differentiation and squamous tumor progression.
J Investig Dermatol Symp Proc, 1(2):157-161, 01 Apr 1996
Cited by: 9 articles | PMID: 9627711
Review