Abstract
Free full text

Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells.
Abstract
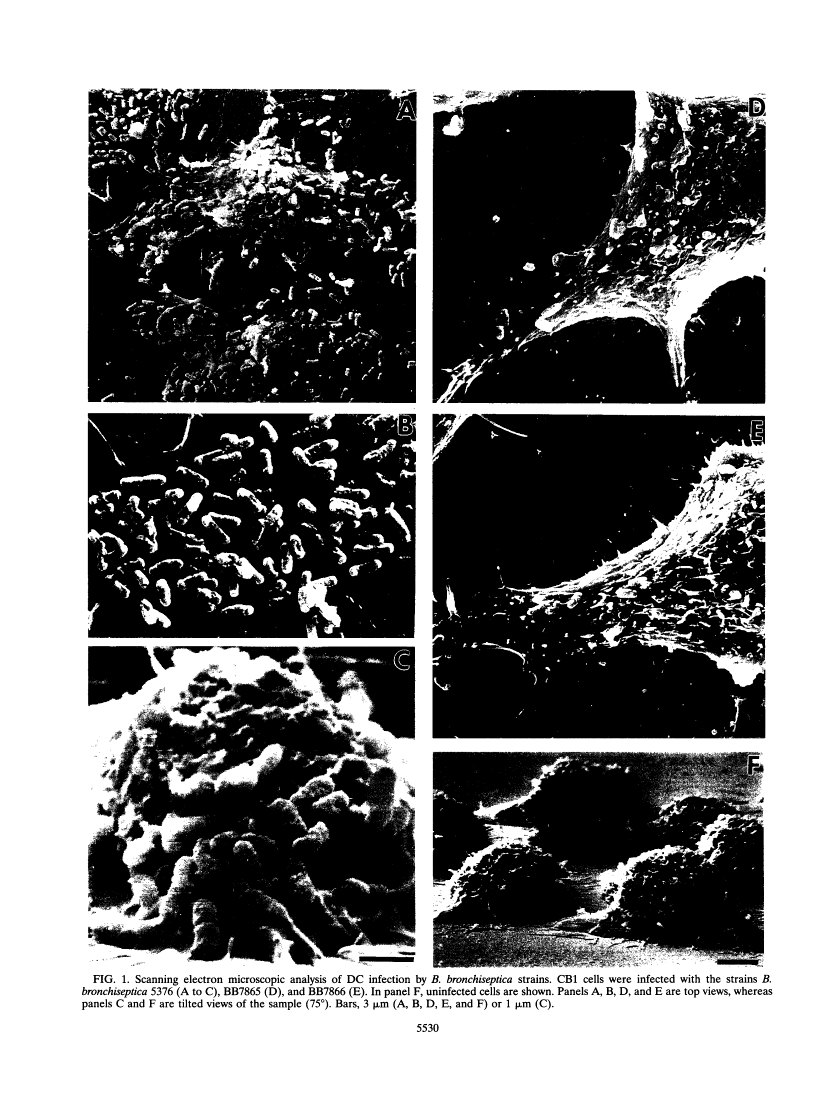
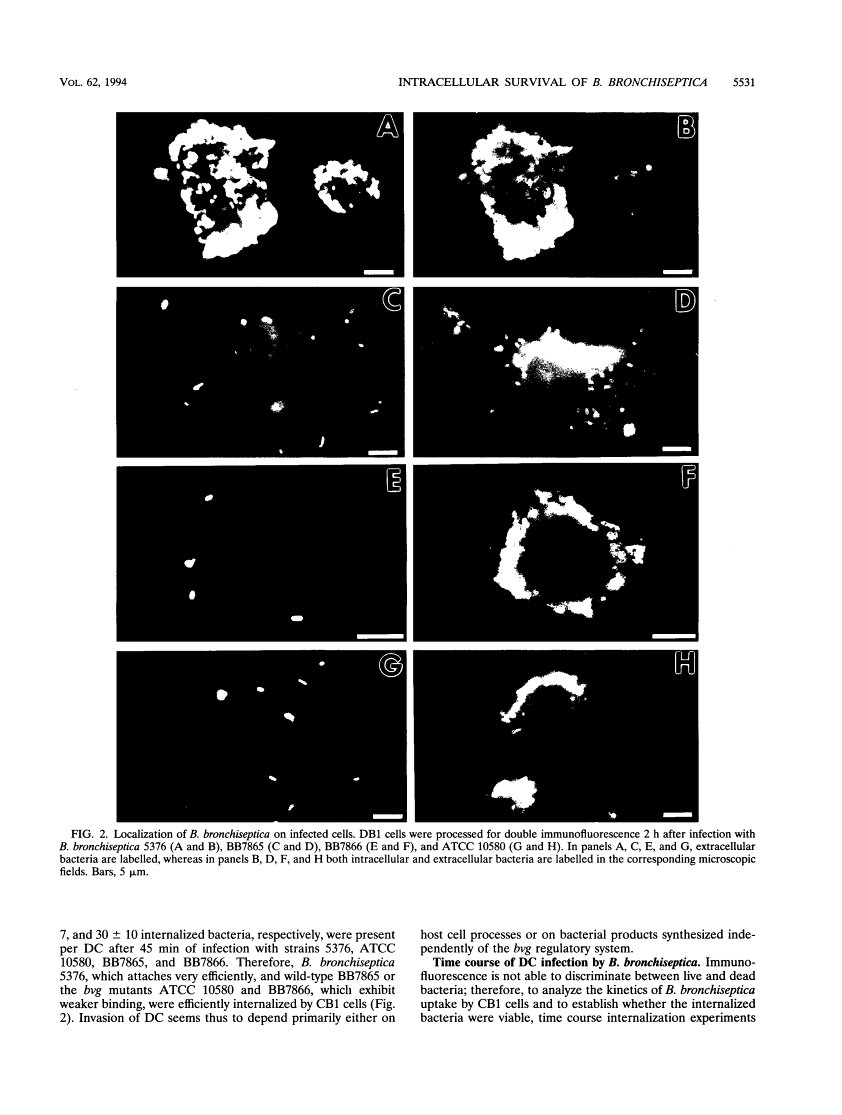
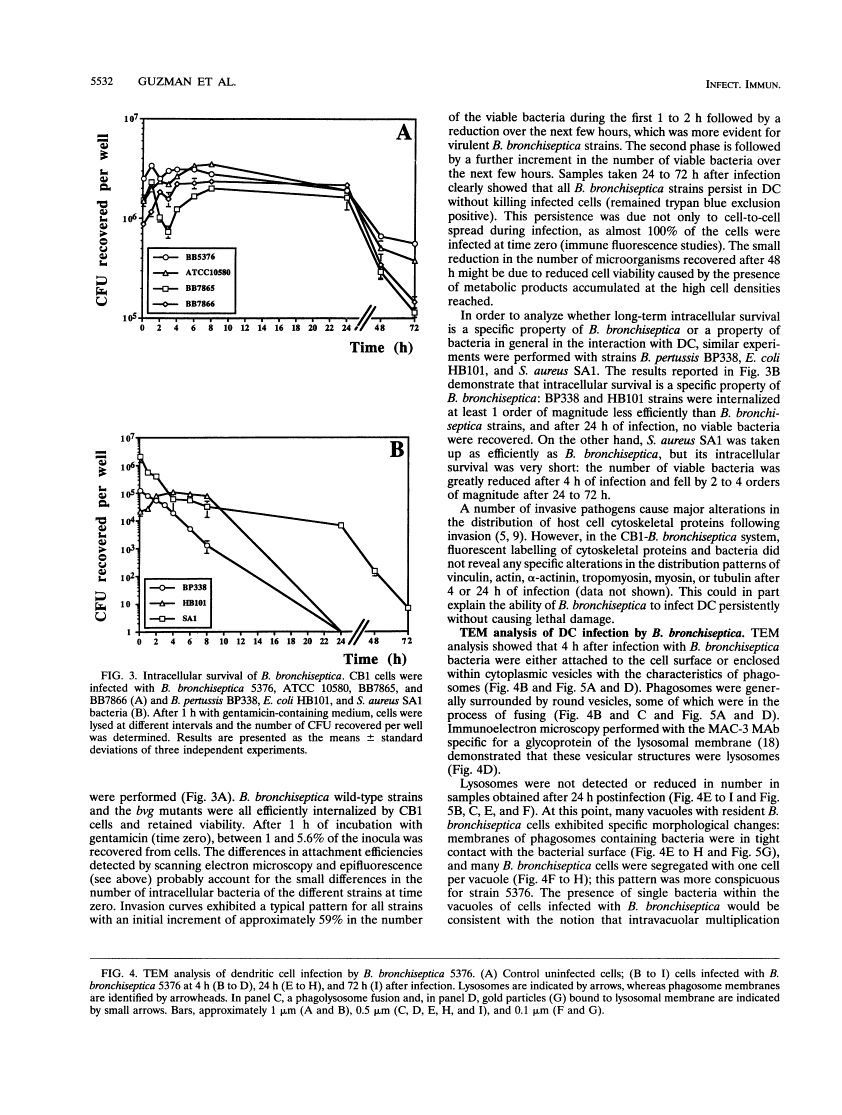
We have studied the interaction between the respiratory pathogen Bordetella bronchiseptica and murine spleen dendritic cells, important antigen-presenting cells that are found in the airway epithelium. Wild-type B. bronchiseptica 5376 attached very efficiently to dendritic cells, whereas the bvg mutant ATCC 10580, wild-type strain BB7865, and its spontaneous delta bvgS mutant BB7866 bound less efficiently. However, all tested B. bronchiseptica strains were able to invade dendritic cells and survive intracellularly for at least 72 h. These results suggest that bvg-independent or bvg-downregulated products are involved in the uptake and intracellular survival. Transmission electron microscopic analysis revealed that bacteria grew and replicated intracellularly and were present in typical phagosomes, which fused with lysosomes during the initial infection period. However, in later infection stages some bacteria seemed to escape into an unfused endocytic compartment, where individual bacteria were tightly surrounded by a membrane. The in vitro interaction of B. bronchiseptica with dendritic cells reported here may be relevant to natural infections caused by this organism that lead to chronicity or an altered immune response.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (4.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bemis DA, Wilson SA. Influence of potential virulence determinants on Bordetella bronchiseptica-induced ciliostasis. Infect Immun. 1985 Oct;50(1):35–42. [Europe PMC free article] [Abstract] [Google Scholar]
- Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. [Abstract] [Google Scholar]
- Bromberg K, Tannis G, Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991 Dec;59(12):4715–4719. [Europe PMC free article] [Abstract] [Google Scholar]
- Cheers C, Gray DF. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology. 1969 Dec;17(6):875–887. [Abstract] [Google Scholar]
- Clerc P, Sansonetti PJ. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. [Europe PMC free article] [Abstract] [Google Scholar]
- Ewanowich CA, Melton AR, Weiss AA, Sherburne RK, Peppler MS. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. [Europe PMC free article] [Abstract] [Google Scholar]
- Ewanowich CA, Sherburne RK, Man SF, Peppler MS. Bordetella parapertussis invasion of HeLa 229 cells and human respiratory epithelial cells in primary culture. Infect Immun. 1989 Apr;57(4):1240–1247. [Europe PMC free article] [Abstract] [Google Scholar]
- Friedman RL, Fiederlein RL, Glasser L, Galgiani JN. Bordetella pertussis adenylate cyclase: effects of affinity-purified adenylate cyclase on human polymorphonuclear leukocyte functions. Infect Immun. 1987 Jan;55(1):135–140. [Europe PMC free article] [Abstract] [Google Scholar]
- Friedman RL, Nordensson K, Wilson L, Akporiaye ET, Yocum DE. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect Immun. 1992 Nov;60(11):4578–4585. [Europe PMC free article] [Abstract] [Google Scholar]
- Goodnow RA. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980 Dec;44(4):722–738. [Europe PMC free article] [Abstract] [Google Scholar]
- Gross R, Rappuoli R. Pertussis toxin promoter sequences involved in modulation. J Bacteriol. 1989 Jul;171(7):4026–4030. [Europe PMC free article] [Abstract] [Google Scholar]
- Gueirard P, Guiso N. Virulence of Bordetella bronchiseptica: role of adenylate cyclase-hemolysin. Infect Immun. 1993 Oct;61(10):4072–4078. [Europe PMC free article] [Abstract] [Google Scholar]
- Heesemann J, Laufs R. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J Clin Microbiol. 1985 Aug;22(2):168–175. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoepelman AI, Tuomanen EI. Consequences of microbial attachment: directing host cell functions with adhesins. Infect Immun. 1992 May;60(5):1729–1733. [Europe PMC free article] [Abstract] [Google Scholar]
- Horiguchi Y, Matsuda H, Koyama H, Nakai T, Kume K. Bordetella bronchiseptica dermonecrotizing toxin suppresses in vivo antibody responses in mice. FEMS Microbiol Lett. 1992 Jan 15;69(3):229–234. [Abstract] [Google Scholar]
- Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3038–3042. [Europe PMC free article] [Abstract] [Google Scholar]
- Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993 Aug 1;178(2):479–488. [Europe PMC free article] [Abstract] [Google Scholar]
- King PD, Katz DR. Mechanisms of dendritic cell function. Immunol Today. 1990 Jun;11(6):206–211. [Abstract] [Google Scholar]
- Lee CK, Roberts AL, Finn TM, Knapp S, Mekalanos JJ. A new assay for invasion of HeLa 229 cells by Bordetella pertussis: effects of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990 Aug;58(8):2516–2522. [Europe PMC free article] [Abstract] [Google Scholar]
- Leininger E, Ewanowich CA, Bhargava A, Peppler MS, Kenimer JG, Brennan MJ. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992 Jun;60(6):2380–2385. [Europe PMC free article] [Abstract] [Google Scholar]
- Little TW. Respiratory disease in pigs: a study. Vet Rec. 1975 Jun 21;96(25):540–544. [Abstract] [Google Scholar]
- McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993 Jul;61(7):2763–2773. [Europe PMC free article] [Abstract] [Google Scholar]
- Mekalanos JJ. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. [Europe PMC free article] [Abstract] [Google Scholar]
- Monack DM, Arico B, Rappuoli R, Falkow S. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol. 1989 Dec;3(12):1719–1728. [Abstract] [Google Scholar]
- Nicod LP, Lipscomb MF, Weissler JC, Toews GB. Mononuclear cells from human lung parenchyma support antigen-induced T lymphocyte proliferation. J Leukoc Biol. 1989 Apr;45(4):336–344. [Abstract] [Google Scholar]
- Paglia P, Girolomoni G, Robbiati F, Granucci F, Ricciardi-Castagnoli P. Immortalized dendritic cell line fully competent in antigen presentation initiates primary T cell responses in vivo. J Exp Med. 1993 Dec 1;178(6):1893–1901. [Europe PMC free article] [Abstract] [Google Scholar]
- Redhead K, Watkins J, Barnard A, Mills KH. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993 Aug;61(8):3190–3198. [Europe PMC free article] [Abstract] [Google Scholar]
- Relman D, Tuomanen E, Falkow S, Golenbock DT, Saukkonen K, Wright SD. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. [Abstract] [Google Scholar]
- Roop RM, 2nd, Veit HP, Sinsky RJ, Veit SP, Hewlett EL, Kornegay ET. Virulence factors of Bordetella bronchiseptica associated with the production of infectious atrophic rhinitis and pneumonia in experimentally infected neonatal swine. Infect Immun. 1987 Jan;55(1):217–222. [Europe PMC free article] [Abstract] [Google Scholar]
- Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. [Europe PMC free article] [Abstract] [Google Scholar]
- Savelkoul PH, Kremer B, Kusters JG, van der Zeijst BA, Gaastra W. Invasion of HeLa cells by Bordetella bronchiseptica. Microb Pathog. 1993 Feb;14(2):161–168. [Abstract] [Google Scholar]
- Schipper H, Krohne GF, Gross R. Epithelial cell invasion and survival of Bordetella bronchiseptica. Infect Immun. 1994 Jul;62(7):3008–3011. [Europe PMC free article] [Abstract] [Google Scholar]
- Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986 Feb 1;163(2):436–451. [Europe PMC free article] [Abstract] [Google Scholar]
- Spangrude GJ, Sacchi F, Hill HR, Van Epps DE, Daynes RA. Inhibition of lymphocyte and neutrophil chemotaxis by pertussis toxin. J Immunol. 1985 Dec;135(6):4135–4143. [Abstract] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. [Abstract] [Google Scholar]
- Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. [Abstract] [Google Scholar]
- Steed LL, Akporiaye ET, Friedman RL. Bordetella pertussis induces respiratory burst activity in human polymorphonuclear leukocytes. Infect Immun. 1992 May;60(5):2101–2105. [Europe PMC free article] [Abstract] [Google Scholar]
- Steed LL, Setareh M, Friedman RL. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J Leukoc Biol. 1991 Oct;50(4):321–330. [Abstract] [Google Scholar]
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. [Abstract] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. [Europe PMC free article] [Abstract] [Google Scholar]
- Straley SC, Harmon PA. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984 Sep;45(3):655–659. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker MJ, Guzmán CA, Rohde M, Timmis KN. Production of recombinant Bordetella pertussis serotype 2 fimbriae in Bordetella parapertussis and Bordetella bronchiseptica: utility of Escherichia coli gene expression signals. Infect Immun. 1991 May;59(5):1739–1746. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker MJ, Rohde M, Timmis KN, Guzmán CA. Specific lung mucosal and systemic immune responses after oral immunization of mice with Salmonella typhimurium aroA, Salmonella typhi Ty21a, and invasive Escherichia coli expressing recombinant pertussis toxin S1 subunit. Infect Immun. 1992 Oct;60(10):4260–4268. [Europe PMC free article] [Abstract] [Google Scholar]
- Weiss AA, Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.62.12.5528-5537.1994
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/62/12/5528.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/62/12/5528
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/62/12/5528
Citations & impact
Impact metrics
Citations of article over time
Article citations
Bordetella bronchiseptica and Bordetella pertussis: Similarities and Differences in Infection, Immuno-Modulation, and Vaccine Considerations.
Clin Microbiol Rev, 36(3):e0016422, 12 Jun 2023
Cited by: 4 articles | PMID: 37306571
Review
Natural History and Ecology of Interactions Between Bordetella Species and Amoeba.
Front Cell Infect Microbiol, 12:798317, 09 Feb 2022
Cited by: 3 articles | PMID: 35223538 | PMCID: PMC8863592
Evolution and Conservation of Bordetella Intracellular Survival in Eukaryotic Host Cells.
Front Microbiol, 11:557819, 15 Oct 2020
Cited by: 2 articles | PMID: 33178148 | PMCID: PMC7593398
Review Free full text in Europe PMC
Respiratory Bordetella bronchiseptica Carriage is Associated with Broad Phenotypic Alterations of Peripheral CD4⁺CD25⁺ T Cells and Differentially Affects Immune Responses to Secondary Non-Infectious and Infectious Stimuli in Mice.
Int J Mol Sci, 19(9):E2602, 01 Sep 2018
Cited by: 3 articles | PMID: 30200513 | PMCID: PMC6165163
Bordetella bronchiseptica exploits the complex life cycle of Dictyostelium discoideum as an amplifying transmission vector.
PLoS Biol, 15(4):e2000420, 12 Apr 2017
Cited by: 34 articles | PMID: 28403138 | PMCID: PMC5389573
Go to all (43) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mechanisms involved in uptake of Bordetella bronchiseptica by mouse dendritic cells.
Infect Immun, 62(12):5538-5544, 01 Dec 1994
Cited by: 24 articles | PMID: 7960136 | PMCID: PMC303299
Effect of temperature modulation and bvg mutation of Bordetella bronchiseptica on adhesion, intracellular survival and cytotoxicity for swine alveolar macrophages.
Vet Microbiol, 73(1):1-12, 01 Apr 2000
Cited by: 7 articles | PMID: 10731613
A highly adherent phenotype associated with virulent Bvg+-phase swine isolates of Bordetella bronchiseptica grown under modulating conditions.
Infect Immun, 65(12):5295-5300, 01 Dec 1997
Cited by: 10 articles | PMID: 9393829 | PMCID: PMC175762
Environmental sensing mechanisms in Bordetella.
Adv Microb Physiol, 44:141-181, 01 Jan 2001
Cited by: 23 articles | PMID: 11407112
Review