Abstract
Free full text

La proteins from Drosophila melanogaster and Saccharomyces cerevisiae: a yeast homolog of the La autoantigen is dispensable for growth.
Abstract
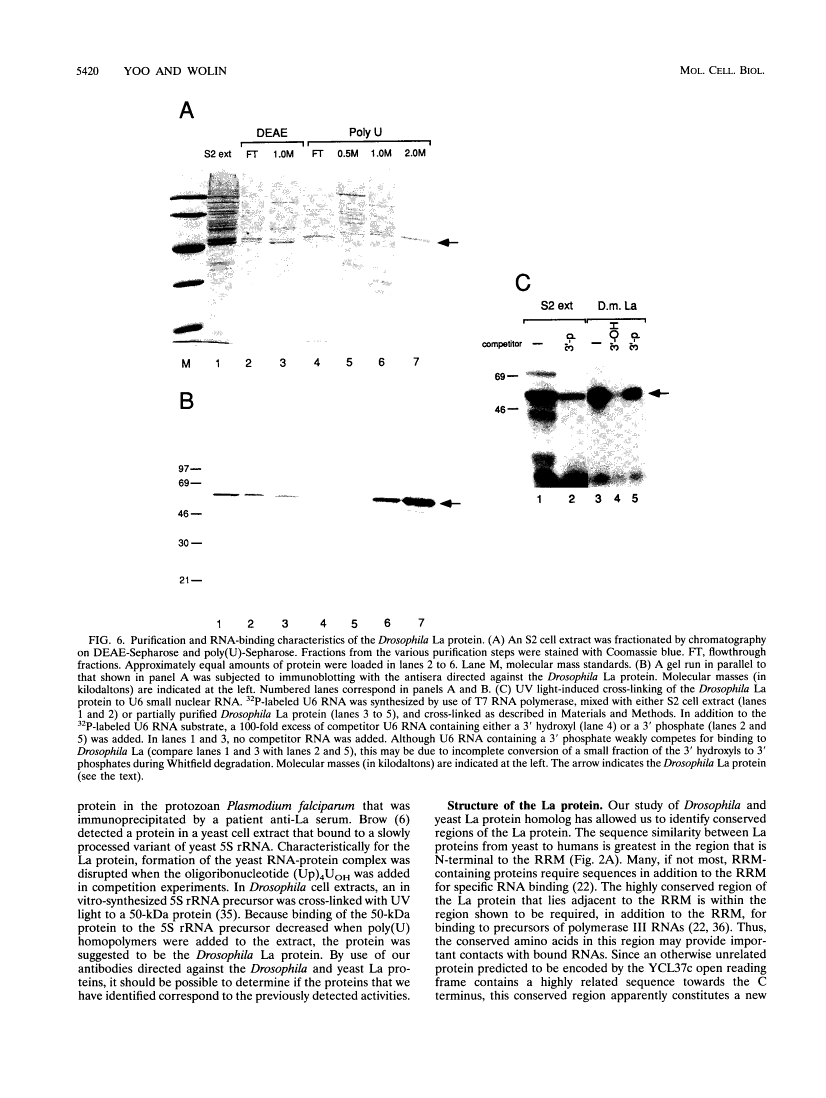
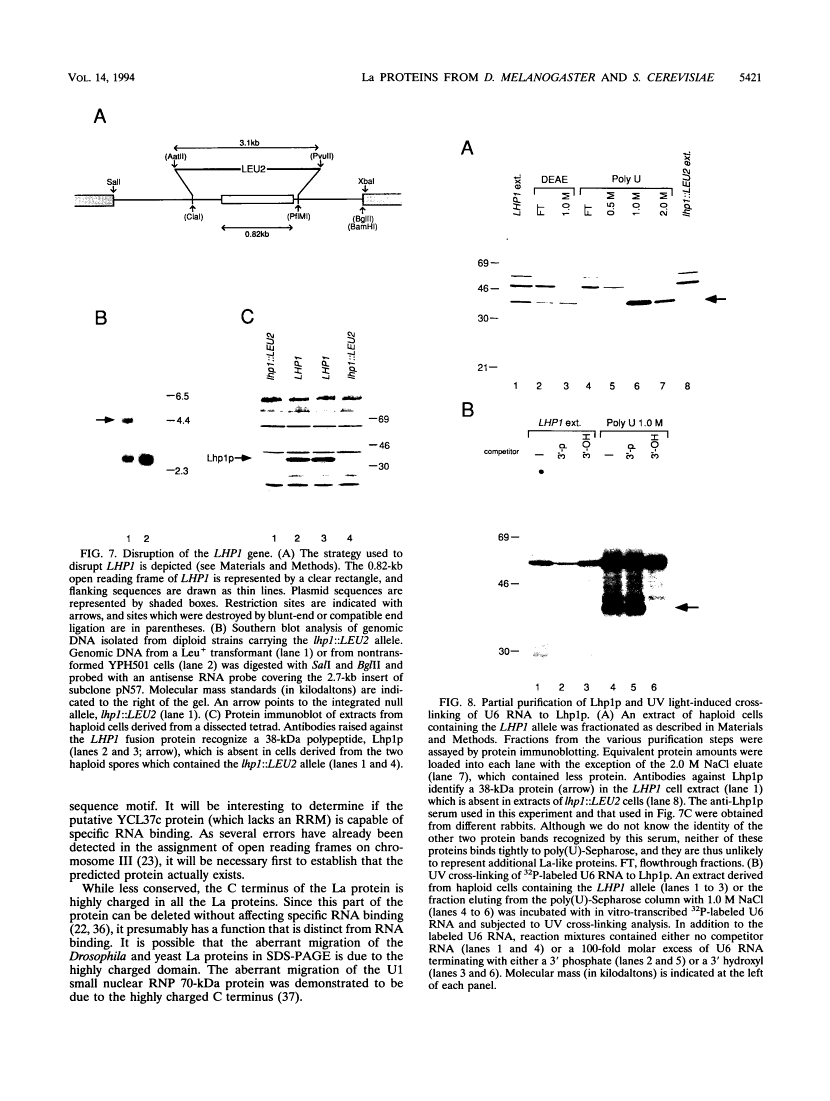
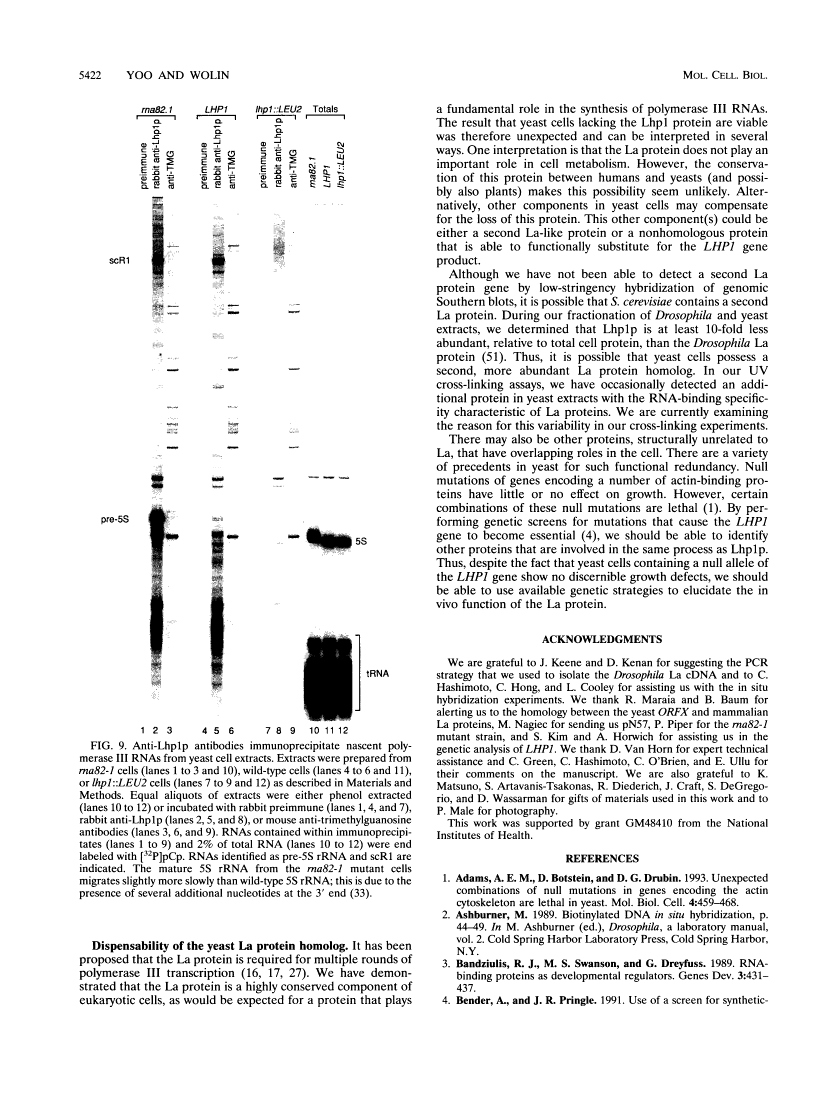
The human autoantigen La is a 50-kDa protein which binds to the 3' termini of virtually all nascent polymerase III transcripts. Experiments with mammalian transcription extracts have led to the proposal that the La protein is required for multiple rounds of transcription by RNA polymerase III (E. Gottlieb and J. A. Steitz, EMBO J. 8:851-861, 1989; R. J. Maraia, D. J. Kenan, and J. D. Keene, Mol. Cell. Biol. 14:2147-2158, 1994). Although La protein homologs have been identified in a variety of vertebrate species, the protein has not been identified in invertebrates. In order to begin a genetic analysis of La protein function, we have characterized homologs of the La protein in the fruit fly Drosophila melanogaster and the yeast Saccharomyces cerevisiae. We show that both the Drosophila and yeast La proteins are bound to precursors of polymerase III RNAs in vivo. The Drosophila and yeast proteins resemble the human La protein in their biochemical properties, as both proteins can be partially purified from cells by a procedure previously devised to purify the human protein. Similarly to vertebrate La proteins, the Drosophila and yeast homologs preferentially bind RNAs that terminate with a 3' hydroxyl. Despite the fact that the La protein is conserved between humans and Saccharomyces cerevisiae, yeast cells containing a null allele of the gene encoding the La protein are viable, suggesting that another protein(s) plays a functionally redundant role.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
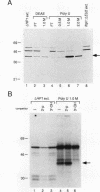
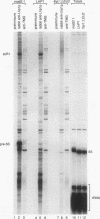
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams AE, Cooper JA, Drubin DG. Unexpected combinations of null mutations in genes encoding the actin cytoskeleton are lethal in yeast. Mol Biol Cell. 1993 May;4(5):459–468. [Europe PMC free article] [Abstract] [Google Scholar]
- Bandziulis RJ, Swanson MS, Dreyfuss G. RNA-binding proteins as developmental regulators. Genes Dev. 1989 Apr;3(4):431–437. [Abstract] [Google Scholar]
- Bender A, Pringle JR. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Mar;11(3):1295–1305. [Europe PMC free article] [Abstract] [Google Scholar]
- Black DL, Pinto AL. U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/U6. Mol Cell Biol. 1989 Aug;9(8):3350–3359. [Europe PMC free article] [Abstract] [Google Scholar]
- Brow DA. In vitro transcripts of a yeast variant 5 S rRNA gene exhibit alterations in 3'-end processing and protein binding. J Biol Chem. 1987 Oct 15;262(29):13959–13965. [Abstract] [Google Scholar]
- Campbell FE, Jr, Setzer DR. Transcription termination by RNA polymerase III: uncoupling of polymerase release from termination signal recognition. Mol Cell Biol. 1992 May;12(5):2260–2272. [Europe PMC free article] [Abstract] [Google Scholar]
- Chambers JC, Kenan D, Martin BJ, Keene JD. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [Abstract] [Google Scholar]
- Chambers JC, Kurilla MG, Keene JD. Association between the 7 S RNA and the lupus La protein varies among cell types. J Biol Chem. 1983 Oct 10;258(19):11438–11441. [Abstract] [Google Scholar]
- Chan EK, Sullivan KF, Tan EM. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. [Europe PMC free article] [Abstract] [Google Scholar]
- Cozzarelli NR, Gerrard SP, Schlissel M, Brown DD, Bogenhagen DF. Purified RNA polymerase III accurately and efficiently terminates transcription of 5S RNA genes. Cell. 1983 Oct;34(3):829–835. [Abstract] [Google Scholar]
- Driever W, Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988 Jul 1;54(1):83–93. [Abstract] [Google Scholar]
- England TE, Bruce AG, Uhlenbeck OC. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. [Abstract] [Google Scholar]
- Felici F, Cesareni G, Hughes JM. The most abundant small cytoplasmic RNA of Saccharomyces cerevisiae has an important function required for normal cell growth. Mol Cell Biol. 1989 Aug;9(8):3260–3268. [Europe PMC free article] [Abstract] [Google Scholar]
- Francoeur AM, Gritzmacher CA, Peebles CL, Reese RT, Tan EM. Synthesis of small nuclear ribonucleoprotein particles by the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3635–3639. [Europe PMC free article] [Abstract] [Google Scholar]
- Gottlieb E, Steitz JA. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 1989 Mar;8(3):841–850. [Europe PMC free article] [Abstract] [Google Scholar]
- Gottlieb E, Steitz JA. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. [Europe PMC free article] [Abstract] [Google Scholar]
- Hann BC, Walter P. The signal recognition particle in S. cerevisiae. Cell. 1991 Oct 4;67(1):131–144. [Abstract] [Google Scholar]
- Hendrick JP, Wolin SL, Rinke J, Lerner MR, Steitz JA. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. [Europe PMC free article] [Abstract] [Google Scholar]
- Jacq B, Jourdan R, Jordan BR. Structure and processing of precursor 5 S RNA in Drosophila melanogaster. J Mol Biol. 1977 Dec 15;117(3):785–795. [Abstract] [Google Scholar]
- Karpen GH, Spradling AC. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics. 1992 Nov;132(3):737–753. [Europe PMC free article] [Abstract] [Google Scholar]
- Kenan DJ, Query CC, Keene JD. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. [Abstract] [Google Scholar]
- Koonin EV, Bork P, Sander C. Yeast chromosome III: new gene functions. EMBO J. 1994 Feb 1;13(3):493–503. [Europe PMC free article] [Abstract] [Google Scholar]
- Lerner MR, Andrews NC, Miller G, Steitz JA. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. [Europe PMC free article] [Abstract] [Google Scholar]
- Lerner MR, Boyle JA, Hardin JA, Steitz JA. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. [Abstract] [Google Scholar]
- Maraia RJ, Kenan DJ, Keene JD. Eukaryotic transcription termination factor La mediates transcript release and facilitates reinitiation by RNA polymerase III. Mol Cell Biol. 1994 Mar;14(3):2147–2158. [Europe PMC free article] [Abstract] [Google Scholar]
- Mathews MB, Francoeur AM. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. [Europe PMC free article] [Abstract] [Google Scholar]
- Meerovitch K, Svitkin YV, Lee HS, Lejbkowicz F, Kenan DJ, Chan EK, Agol VI, Keene JD, Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. [Europe PMC free article] [Abstract] [Google Scholar]
- Myer VE, Lee SI, Steitz JA. Viral small nuclear ribonucleoproteins bind a protein implicated in messenger RNA destabilization. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1296–1300. [Europe PMC free article] [Abstract] [Google Scholar]
- Nagiec MM, Wells GB, Lester RL, Dickson RC. A suppressor gene that enables Saccharomyces cerevisiae to grow without making sphingolipids encodes a protein that resembles an Escherichia coli fatty acyltransferase. J Biol Chem. 1993 Oct 15;268(29):22156–22163. [Abstract] [Google Scholar]
- Oliver SG, van der Aart QJ, Agostoni-Carbone ML, Aigle M, Alberghina L, Alexandraki D, Antoine G, Anwar R, Ballesta JP, Benit P, et al. The complete DNA sequence of yeast chromosome III. Nature. 1992 May 7;357(6373):38–46. [Abstract] [Google Scholar]
- Piper PW, Bellatin JA, Lockheart A. Altered maturation of sequences at the 3' terminus of 5S gene transcripts in a Saccharomyces cerevisiae mutant that lacks a RNA processing endonuclease. EMBO J. 1983;2(3):353–359. [Europe PMC free article] [Abstract] [Google Scholar]
- Piper PW, Patel N, Lockheart A. Processing of the 3' sequence extensions upon the 5S rRNA of a mutant yeast in Xenopus laevis germinal vesicle extract. Eur J Biochem. 1984 May 15;141(1):115–118. [Abstract] [Google Scholar]
- Preiser P, Vasisht V, Birk A, Levinger L. Poly(U)-binding protein inhibits Drosophila pre-5 S RNA 3'-exonuclease digestion. J Biol Chem. 1993 Jun 5;268(16):11553–11557. [Abstract] [Google Scholar]
- Pruijn GJ, Slobbe RL, van Venrooij WJ. Analysis of protein--RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991 Oct 11;19(19):5173–5180. [Europe PMC free article] [Abstract] [Google Scholar]
- Query CC, Bentley RC, Keene JD. A common RNA recognition motif identified within a defined U1 RNA binding domain of the 70K U1 snRNP protein. Cell. 1989 Apr 7;57(1):89–101. [Abstract] [Google Scholar]
- Reddy R, Henning D, Tan E, Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J Biol Chem. 1983 Jul 10;258(13):8352–8356. [Abstract] [Google Scholar]
- Rinke J, Steitz JA. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. [Abstract] [Google Scholar]
- Rinke J, Steitz JA. Association of the lupus antigen La with a subset of U6 snRNA molecules. Nucleic Acids Res. 1985 Apr 11;13(7):2617–2629. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosa MD, Gottlieb E, Lerner MR, Steitz JA. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. [Europe PMC free article] [Abstract] [Google Scholar]
- Scherly D, Stutz F, Lin-Marq N, Clarkson SG. La proteins from Xenopus laevis. cDNA cloning and developmental expression. J Mol Biol. 1993 May 20;231(2):196–204. [Abstract] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Stefano JE. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. [Abstract] [Google Scholar]
- Terns MP, Lund E, Dahlberg JE. 3'-end-dependent formation of U6 small nuclear ribonucleoprotein particles in Xenopus laevis oocyte nuclei. Mol Cell Biol. 1992 Jul;12(7):3032–3040. [Europe PMC free article] [Abstract] [Google Scholar]
- Topfer F, Gordon T, McCluskey J. Characterization of the mouse autoantigen La (SS-B). Identification of conserved RNA-binding motifs, a putative ATP binding site and reactivity of recombinant protein with poly(U) and human autoantibodies. J Immunol. 1993 Apr 1;150(7):3091–3100. [Abstract] [Google Scholar]
- Watson JB, Chandler DW, Gralla JD. Specific termination of in vitro transcription by calf thymus RNA polymerase III. Nucleic Acids Res. 1984 Jul 11;12(13):5369–5384. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolin SL, Steitz JA. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. [Europe PMC free article] [Abstract] [Google Scholar]
- Yisraeli JK, Melton DA. Synthesis of long, capped transcripts in vitro by SP6 and T7 RNA polymerases. Methods Enzymol. 1989;180:42–50. [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.14.8.5412-5424.1994
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/14/8/5412
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/14/8/5412
Citations & impact
Impact metrics
Citations of article over time
Article citations
miR-125-chinmo pathway regulates dietary restriction-dependent enhancement of lifespan in Drosophila.
Elife, 10:e62621, 08 Jun 2021
Cited by: 10 articles | PMID: 34100717 | PMCID: PMC8233039
Revisiting tRNA chaperones: New players in an ancient game.
RNA, rna.078428.120, 16 Feb 2021
Cited by: 15 articles | PMID: 33593999 | PMCID: PMC8051267
Review Free full text in Europe PMC
La proteins couple use of sequence-specific and non-specific binding modes to engage RNA substrates.
RNA Biol, 18(2):168-177, 18 Mar 2019
Cited by: 5 articles | PMID: 30777481 | PMCID: PMC7928037
Review Free full text in Europe PMC
Structural basis for recognition of human 7SK long noncoding RNA by the La-related protein Larp7.
Proc Natl Acad Sci U S A, 115(28):E6457-E6466, 26 Jun 2018
Cited by: 33 articles | PMID: 29946027 | PMCID: PMC6048529
La Deletion from Mouse Brain Alters Pre-tRNA Metabolism and Accumulation of Pre-5.8S rRNA, with Neuron Death and Reactive Astrocytosis.
Mol Cell Biol, 37(10):e00588-16, 02 May 2017
Cited by: 3 articles | PMID: 28223366 | PMCID: PMC5477551
Go to all (85) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The La protein in Schizosaccharomyces pombe: a conserved yet dispensable phosphoprotein that functions in tRNA maturation.
RNA, 3(12):1434-1443, 01 Dec 1997
Cited by: 71 articles | PMID: 9404894 | PMCID: PMC1369584
A yeast RNA binding protein that resembles the human autoantigen La.
J Mol Biol, 245(2):81-85, 01 Jan 1995
Cited by: 24 articles | PMID: 7799435
A misfolded form of 5S rRNA is complexed with the Ro and La autoantigens.
RNA, 2(8):769-784, 01 Aug 1996
Cited by: 50 articles | PMID: 8752087 | PMCID: PMC1369414
La protein and its associated small nuclear and nucleolar precursor RNAs.
Gene Expr, 10(1-2):41-57, 01 Jan 2002
Cited by: 33 articles | PMID: 11868987 | PMCID: PMC5977531
Review Free full text in Europe PMC