Abstract
Free full text

Purification and characterization of human 3-methyladenine-DNA glycosylase.
Abstract
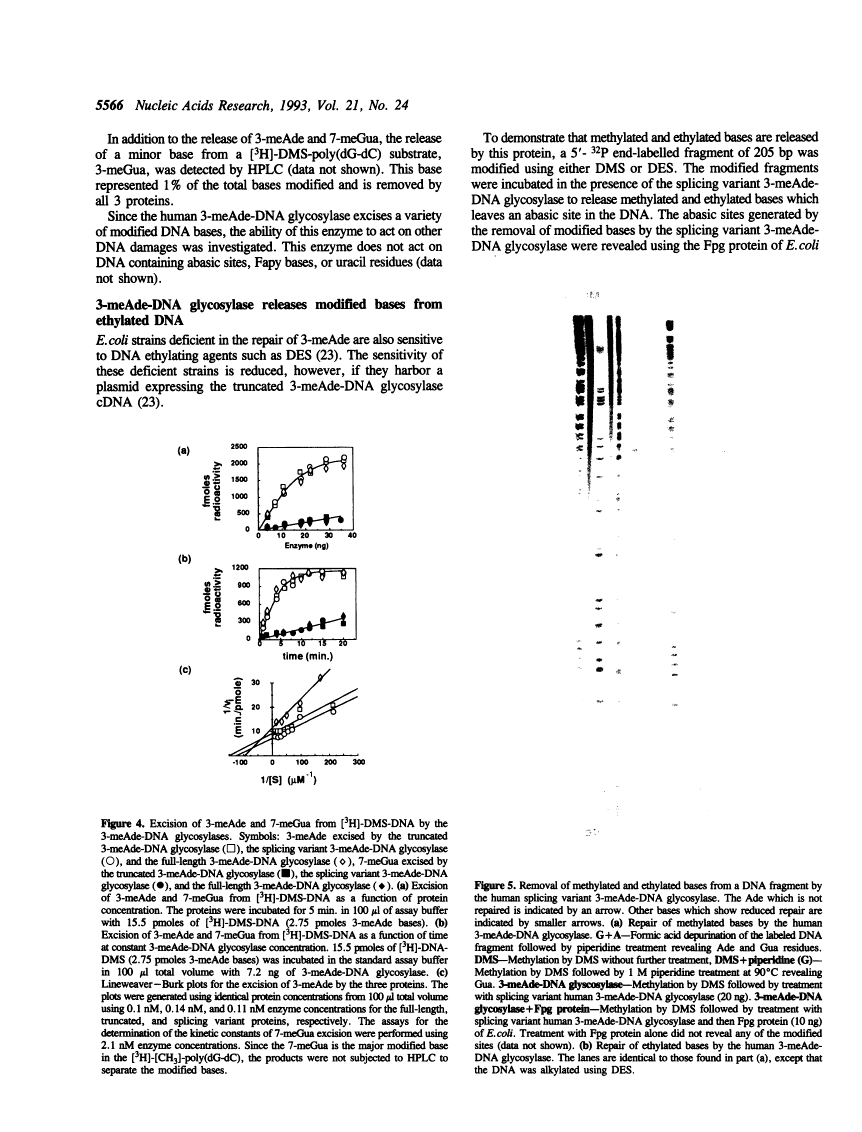
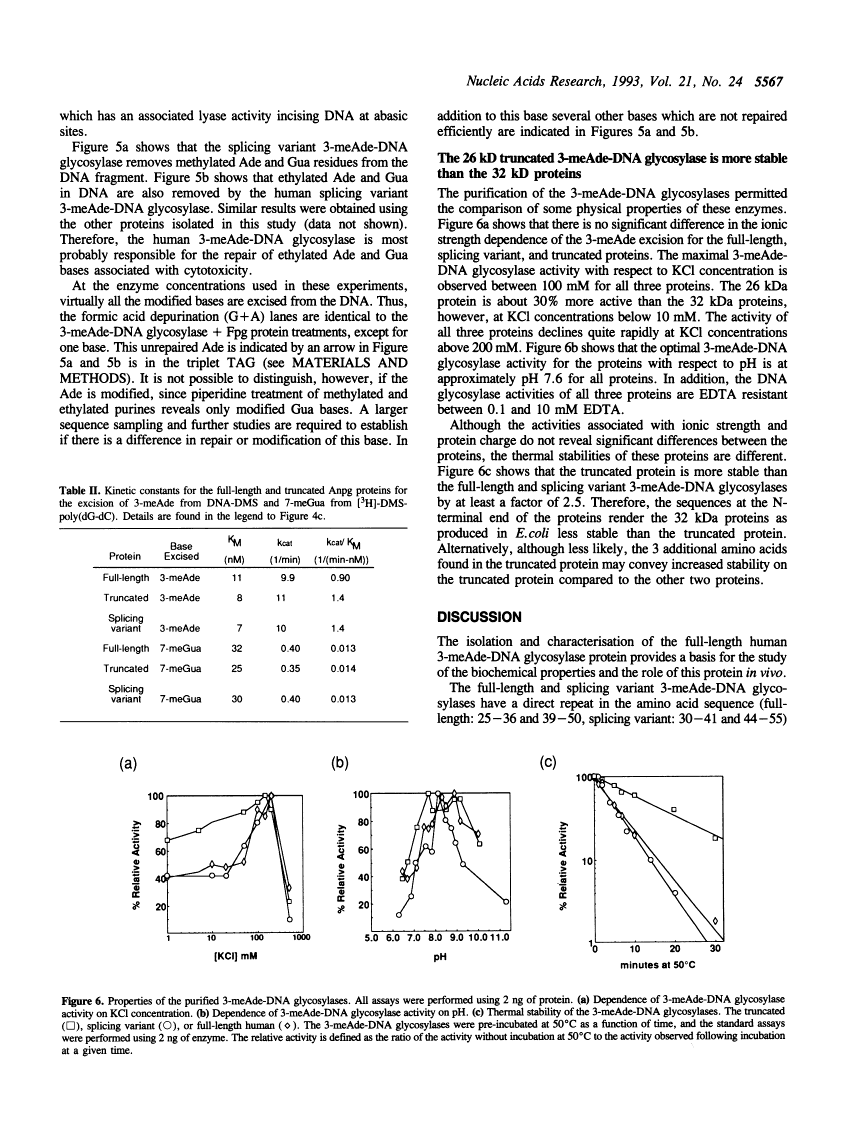
A human cDNA coding sequence for a 3-methyladenine-DNA glycosylase was expressed in Escherichia coli. In addition to the full-length 3-methyladenine-DNA glycosylase coding sequence, two other sequences (resulting from differential RNA splicing and the truncated anpg cDNA) derived from that sequence were also expressed. All three proteins were purified to physical homogeneity and their N-terminal amino acid sequences are identical to those predicted by the nucleic acid sequences. The full-length protein has 293 amino acids coding for a protein with a molecular mass of 32 kDa. Polyclonal antibodies against one of the proteins react with the other two proteins, and a murine 3-methyladenine-DNA glycosylase, but not with several other E. coli DNA repair proteins. All three proteins excise 3-methyl-adenine, 7-methylguanine, and 3-methylguanine as well as ethylated bases from DNA. The activities of the proteins with respect to ionic strength (optimum 100 mM KCl), pH (optimum 7.6), and kinetics for 3-methyladenine and 7-methylguanine excision (average values: 3-methyladenine: Km 9 nM and kcat 10 min-1, 7-methylguanine: Km 29 nM and kcat 0.38 min-1) are comparable. In contrast to these results, however, the thermal stability of the full-length and splicing variant proteins at 50 degrees C is less than that of the truncated protein.
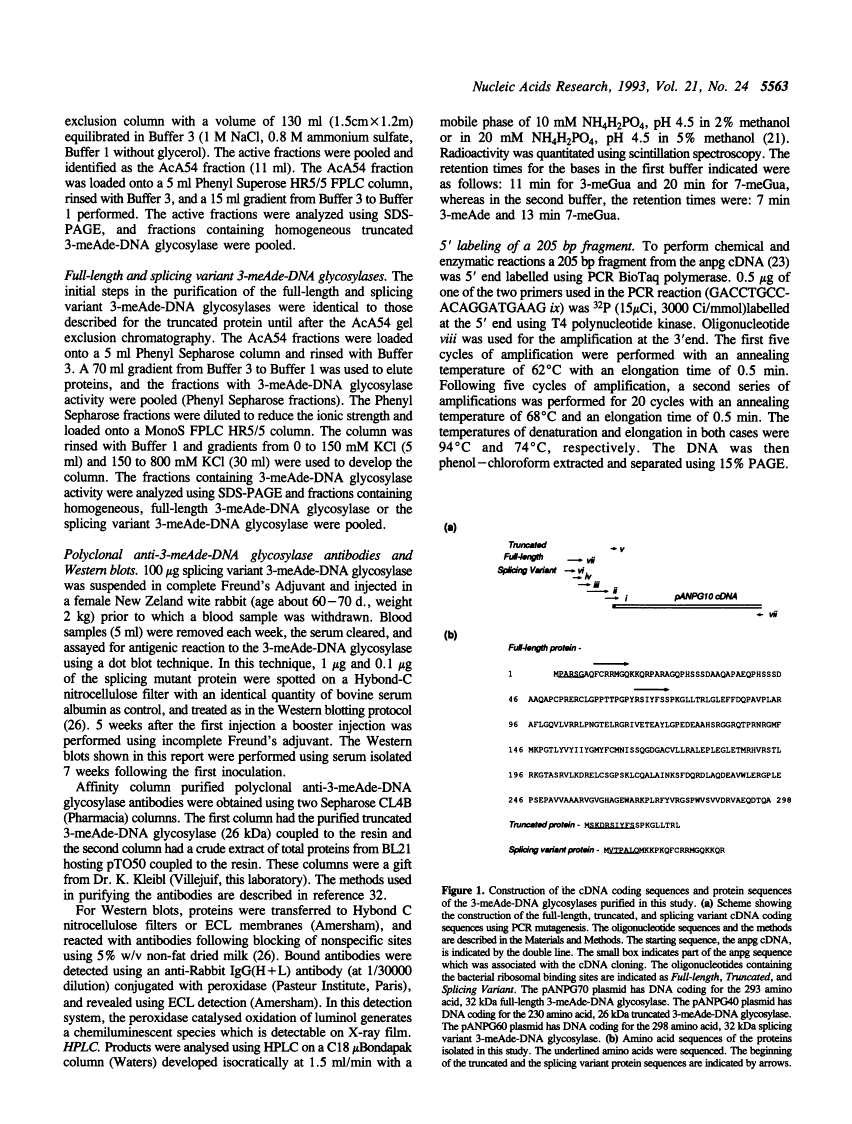
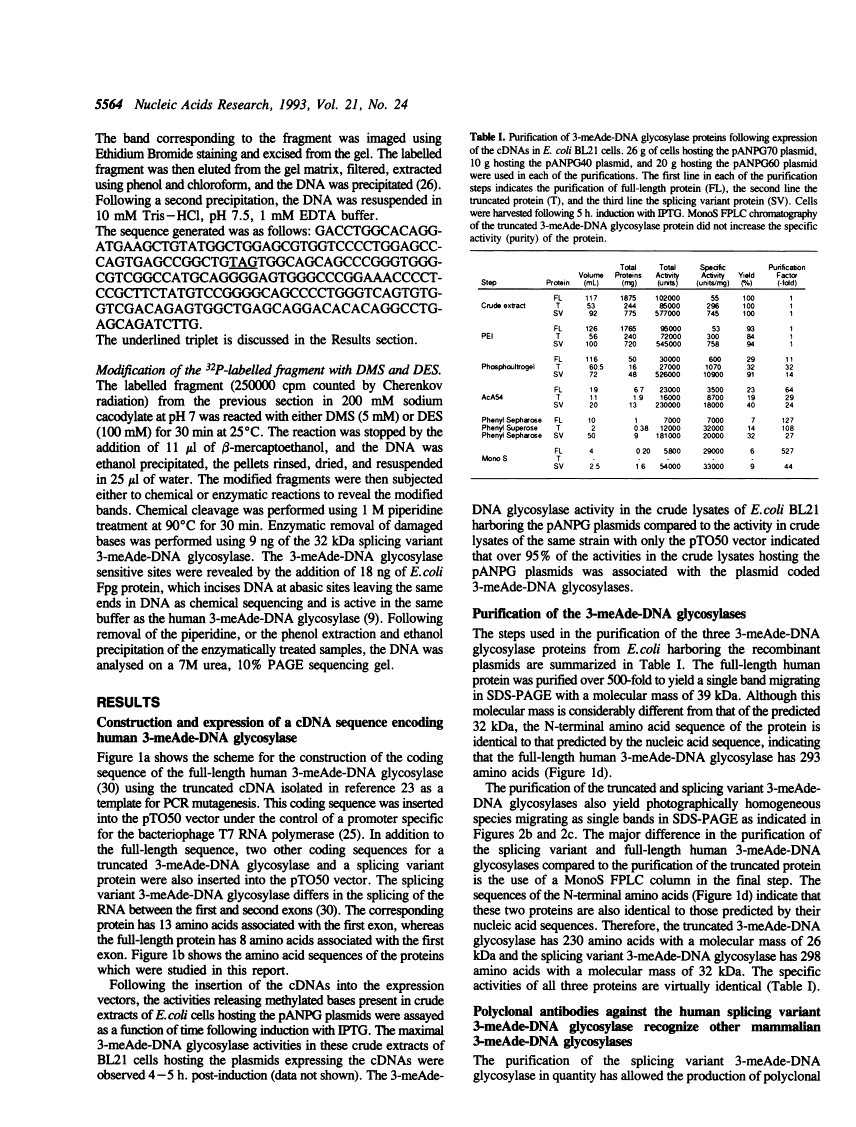
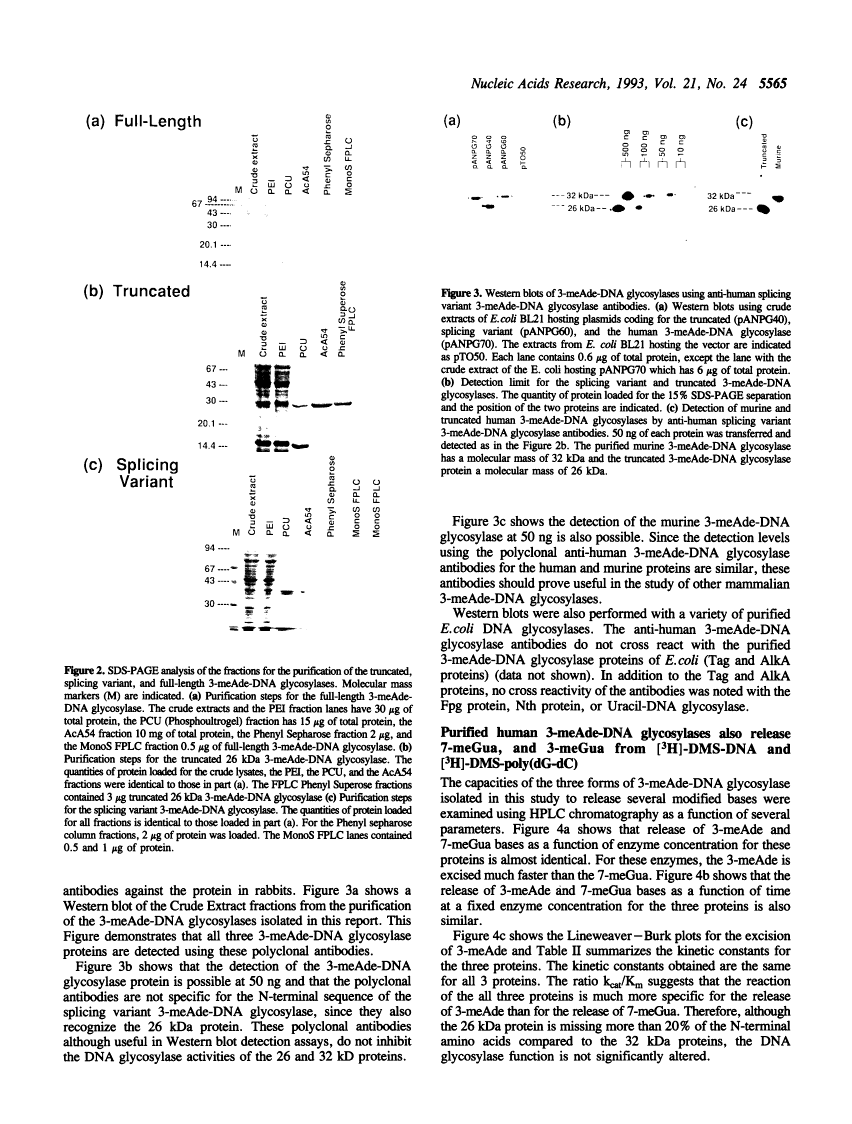
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709–715. [Abstract] [Google Scholar]
- Xiao W, Samson L. In vivo evidence for endogenous DNA alkylation damage as a source of spontaneous mutation in eukaryotic cells. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2117–2121. [Europe PMC free article] [Abstract] [Google Scholar]
- Laval J. Two enzymes are required from strand incision in repair of alkylated DNA. Nature. 1977 Oct 27;269(5631):829–832. [Abstract] [Google Scholar]
- Sakumi K, Sekiguchi M. Structures and functions of DNA glycosylases. Mutat Res. 1990 Sep-Nov;236(2-3):161–172. [Abstract] [Google Scholar]
- Doetsch PW, Cunningham RP. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. [Abstract] [Google Scholar]
- Franklin WA, Lindahl T. DNA deoxyribophosphodiesterase. EMBO J. 1988 Nov;7(11):3617–3622. [Europe PMC free article] [Abstract] [Google Scholar]
- Price A, Lindahl T. Enzymatic release of 5'-terminal deoxyribose phosphate residues from damaged DNA in human cells. Biochemistry. 1991 Sep 3;30(35):8631–8637. [Abstract] [Google Scholar]
- Graves RJ, Felzenszwalb I, Laval J, O'Connor TR. Excision of 5'-terminal deoxyribose phosphate from damaged DNA is catalyzed by the Fpg protein of Escherichia coli. J Biol Chem. 1992 Jul 15;267(20):14429–14435. [Abstract] [Google Scholar]
- Levin JD, Johnson AW, Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J Biol Chem. 1988 Jun 15;263(17):8066–8071. [Abstract] [Google Scholar]
- RICHARDSON CC, KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND CHARACTERIZATION OF THE PHOSPHATASE ACTIVITY. J Biol Chem. 1964 Jan;239:242–250. [Abstract] [Google Scholar]
- Bailly V, Verly WG. The multiple activities of Escherichia coli endonuclease IV and the extreme lability of 5'-terminal base-free deoxyribose 5-phosphates. Biochem J. 1989 May 1;259(3):761–768. [Europe PMC free article] [Abstract] [Google Scholar]
- Boiteux S, Huisman O, Laval J. 3-Methyladenine residues in DNA induce the SOS function sfiA in Escherichia coli. EMBO J. 1984 Nov;3(11):2569–2573. [Europe PMC free article] [Abstract] [Google Scholar]
- Larson K, Sahm J, Shenkar R, Strauss B. Methylation-induced blocks to in vitro DNA replication. Mutat Res. 1985 Jun-Jul;150(1-2):77–84. [Abstract] [Google Scholar]
- O'Connor TR, Boiteux S, Laval J. Ring-opened 7-methylguanine residues in DNA are a block to in vitro DNA synthesis. Nucleic Acids Res. 1988 Jul 11;16(13):5879–5894. [Europe PMC free article] [Abstract] [Google Scholar]
- Clarke ND, Kvaal M, Seeberg E. Cloning of Escherichia coli genes encoding 3-methyladenine DNA glycosylases I and II. Mol Gen Genet. 1984;197(3):368–372. [Abstract] [Google Scholar]
- Nakabeppu Y, Miyata T, Kondo H, Iwanaga S, Sekiguchi M. Structure and expression of the alkA gene of Escherichia coli involved in adaptive response to alkylating agents. J Biol Chem. 1984 Nov 25;259(22):13730–13736. [Abstract] [Google Scholar]
- Karran P, Lindahl T, Ofsteng I, Evensen GB, Seeberg E. Escherichia coli mutants deficient in 3-methyladenine-DNA glycosylase. J Mol Biol. 1980 Jun 15;140(1):101–127. [Abstract] [Google Scholar]
- Habraken Y, Laval F. Increased resistance of the Chinese hamster mutant irs1 cells to monofunctional alkylating agents by transfection of the E. coli or mammalian N3-methyladenine-DNA-glycosylase genes. Mutat Res. 1993 Mar;293(3):187–195. [Abstract] [Google Scholar]
- Klungland A, Fairbairn L, Watson AJ, Margison GP, Seeberg E. Expression of the E.coli 3-methyladenine DNA glycosylase I gene in mammalian cells reduces the toxic and mutagenic effects of methylating agents. EMBO J. 1992 Dec;11(12):4439–4444. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Connor TR, Laval F. Isolation and structure of a cDNA expressing a mammalian 3-methyladenine-DNA glycosylase. EMBO J. 1990 Oct;9(10):3337–3342. [Europe PMC free article] [Abstract] [Google Scholar]
- O'Connor TR, Laval J. Human cDNA expressing a functional DNA glycosylase excising 3-methyladenine and 7-methylguanine. Biochem Biophys Res Commun. 1991 May 15;176(3):1170–1177. [Abstract] [Google Scholar]
- Chakravarti D, Ibeanu GC, Tano K, Mitra S. Cloning and expression in Escherichia coli of a human cDNA encoding the DNA repair protein N-methylpurine-DNA glycosylase. J Biol Chem. 1991 Aug 25;266(24):15710–15715. [Abstract] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. [Abstract] [Google Scholar]
- Le Caer JP, Rossier J. On the use of polyethylenimine as a carrier for protein sequencing: comparison with polybrene. Anal Biochem. 1988 Mar;169(2):246–252. [Abstract] [Google Scholar]
- Zagorski W, Castaing B, Herbert CJ, Labouesse M, Martin R, Slonimski PP. Purification and characterization of the Saccharomyces cerevisiae mitochondrial leucyl-tRNA synthetase. J Biol Chem. 1991 Feb 5;266(4):2537–2541. [Abstract] [Google Scholar]
- Samson L, Derfler B, Boosalis M, Call K. Cloning and characterization of a 3-methyladenine DNA glycosylase cDNA from human cells whose gene maps to chromosome 16. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9127–9131. [Europe PMC free article] [Abstract] [Google Scholar]
- Vickers MA, Vyas P, Harris PC, Simmons DL, Higgs DR. Structure of the human 3-methyladenine DNA glycosylase gene and localization close to the 16p telomere. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3437–3441. [Europe PMC free article] [Abstract] [Google Scholar]
- Asahara H, Wistort PM, Bank JF, Bakerian RH, Cunningham RP. Purification and characterization of Escherichia coli endonuclease III from the cloned nth gene. Biochemistry. 1989 May 16;28(10):4444–4449. [Abstract] [Google Scholar]
- Gallagher PE, Brent TP. Partial purification and characterization of 3-methyladenine-DNA glycosylase from human placenta. Biochemistry. 1982 Dec 7;21(25):6404–6409. [Abstract] [Google Scholar]
- Gallagher PE, Brent TP. Further purification and characterization of human 3-methyladenine-DNA glycosylase. Evidence for broad specificity. Biochim Biophys Acta. 1984 Sep 10;782(4):394–401. [Abstract] [Google Scholar]
- Male R, Helland DE, Kleppe K. Purification and characterization of 3-methyladenine-DNA glycosylase from calf thymus. J Biol Chem. 1985 Feb 10;260(3):1623–1629. [Abstract] [Google Scholar]
- Lefebvre P, Zak P, Laval F. Induction of O6-methylguanine-DNA-methyltransferase and N3-methyladenine-DNA-glycosylase in human cells exposed to DNA-damaging agents. DNA Cell Biol. 1993 Apr;12(3):233–241. [Abstract] [Google Scholar]
- Male R, Haukanes BI, Helland DE, Kleppe K. Substrate specificity of 3-methyladenine-DNA glycosylase from calf thymus. Eur J Biochem. 1987 May 15;165(1):13–19. [Abstract] [Google Scholar]
- Thomas L, Yang CH, Goldthwait DA. Two DNA glycosylases in Escherichia coli which release primarily 3-methyladenine. Biochemistry. 1982 Mar 16;21(6):1162–1169. [Abstract] [Google Scholar]
- Habraken Y, Carter CA, Sekiguchi M, Ludlum DB. Release of N2,3-ethanoguanine from haloethylnitrosourea-treated DNA by Escherichia coli 3-methyladenine DNA glycosylase II. Carcinogenesis. 1991 Oct;12(10):1971–1973. [Abstract] [Google Scholar]
- Matijasevic Z, Sekiguchi M, Ludlum DB. Release of N2,3-ethenoguanine from chloroacetaldehyde-treated DNA by Escherichia coli 3-methyladenine DNA glycosylase II. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9331–9334. [Europe PMC free article] [Abstract] [Google Scholar]
- Singer B, Antoccia A, Basu AK, Dosanjh MK, Fraenkel-Conrat H, Gallagher PE, Kuśmierek JT, Qiu ZH, Rydberg B. Both purified human 1,N6-ethenoadenine-binding protein and purified human 3-methyladenine-DNA glycosylase act on 1,N6-ethenoadenine and 3-methyladenine. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9386–9390. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/21.24.5561
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc310516?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Structural and mechanistic insights into the DNA glycosylase AAG-mediated base excision in nucleosome.
Cell Discov, 9(1):62, 20 Jun 2023
Cited by: 3 articles | PMID: 37339965 | PMCID: PMC10281986
Measuring DNA modifications with the comet assay: a compendium of protocols.
Nat Protoc, 18(3):929-989, 27 Jan 2023
Cited by: 46 articles | PMID: 36707722 | PMCID: PMC10281087
Review Free full text in Europe PMC
Toxicological Properties of 7-Methylguanine, and Preliminary Data on its Anticancer Activity.
Front Pharmacol, 13:842316, 06 Jul 2022
Cited by: 4 articles | PMID: 35873588 | PMCID: PMC9299380
Validation of the in vitro comet assay for DNA cross-links and altered bases detection.
Arch Toxicol, 95(8):2825-2838, 01 Jul 2021
Cited by: 8 articles | PMID: 34196753 | PMCID: PMC8298235
Molecular characterization of Plasmodium falciparum DNA-3-methyladenine glycosylase.
Malar J, 19(1):284, 06 Aug 2020
Cited by: 0 articles | PMID: 32762689 | PMCID: PMC7409487
Go to all (71) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cloning and characterization of a mouse 3-methyladenine/7-methyl-guanine/3-methylguanine DNA glycosylase cDNA whose gene maps to chromosome 11.
Carcinogenesis, 14(2):175-181, 01 Feb 1993
Cited by: 38 articles | PMID: 8435858
Purification and biochemical characterization of recombinant N-methylpurine-DNA glycosylase of the mouse.
Biochemistry, 33(50):15131-15140, 01 Dec 1994
Cited by: 29 articles | PMID: 7999773
A novel 3-methyladenine DNA glycosylase from Helicobacter pylori defines a new class within the endonuclease III family of base excision repair glycosylases.
J Biol Chem, 275(26):20077-20083, 01 Jun 2000
Cited by: 28 articles | PMID: 10777493
Tobacco BY-2 cells excise both 3-methyladenine and 7-methylguanine from methylated DNA.
Mutat Res, 409(2):91-95, 01 Nov 1998
Cited by: 2 articles | PMID: 9838925