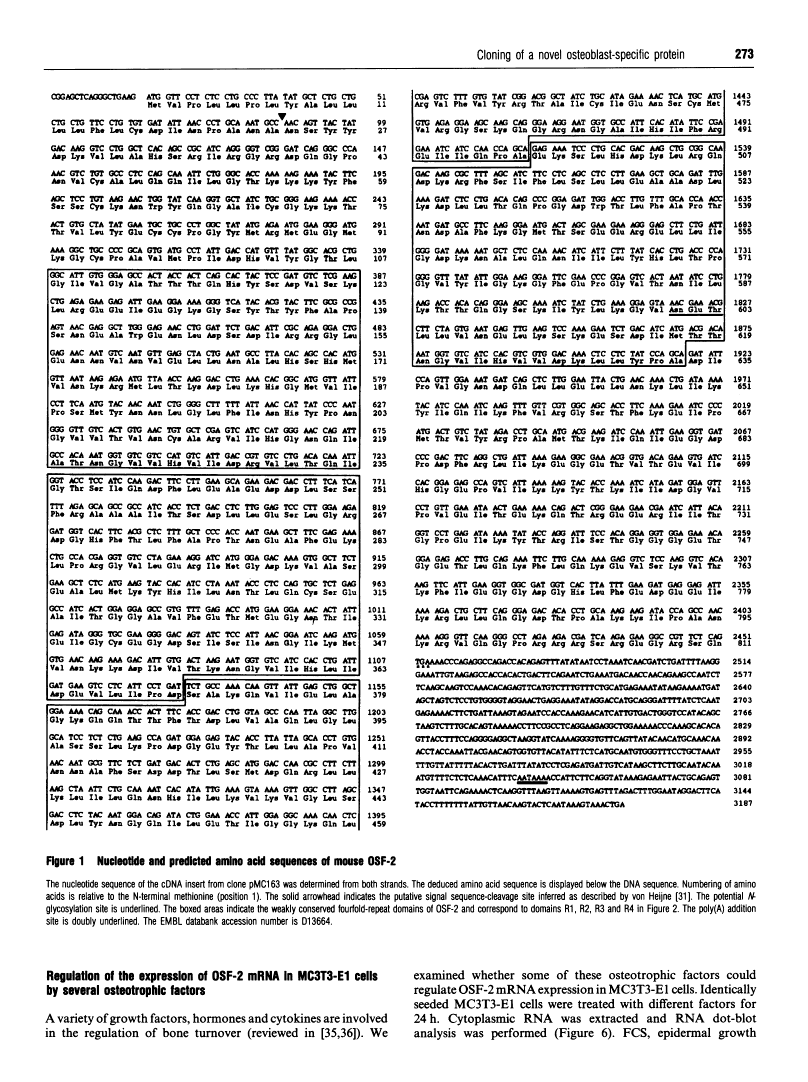
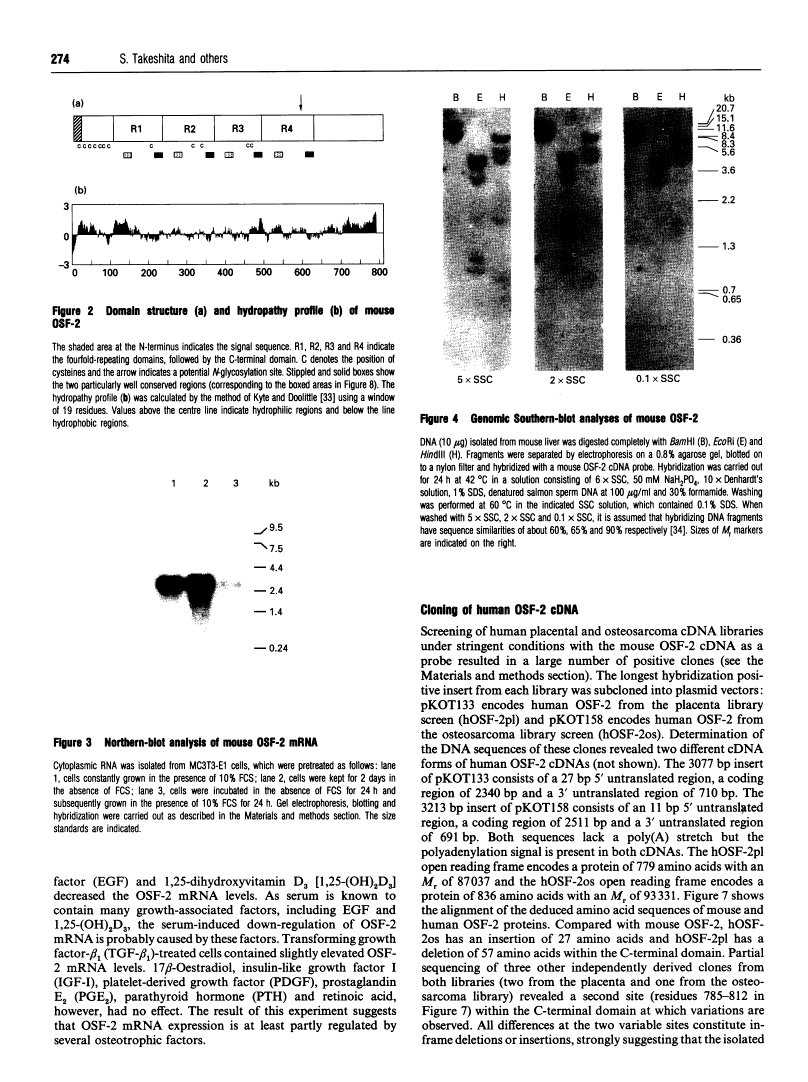
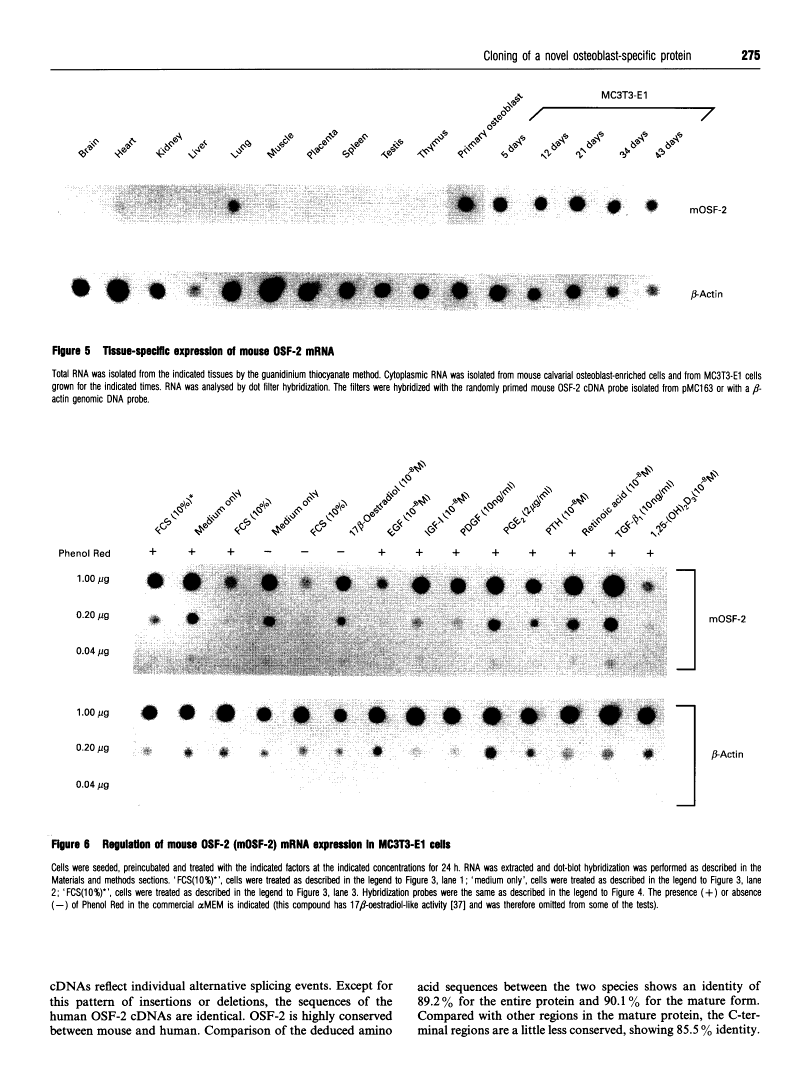
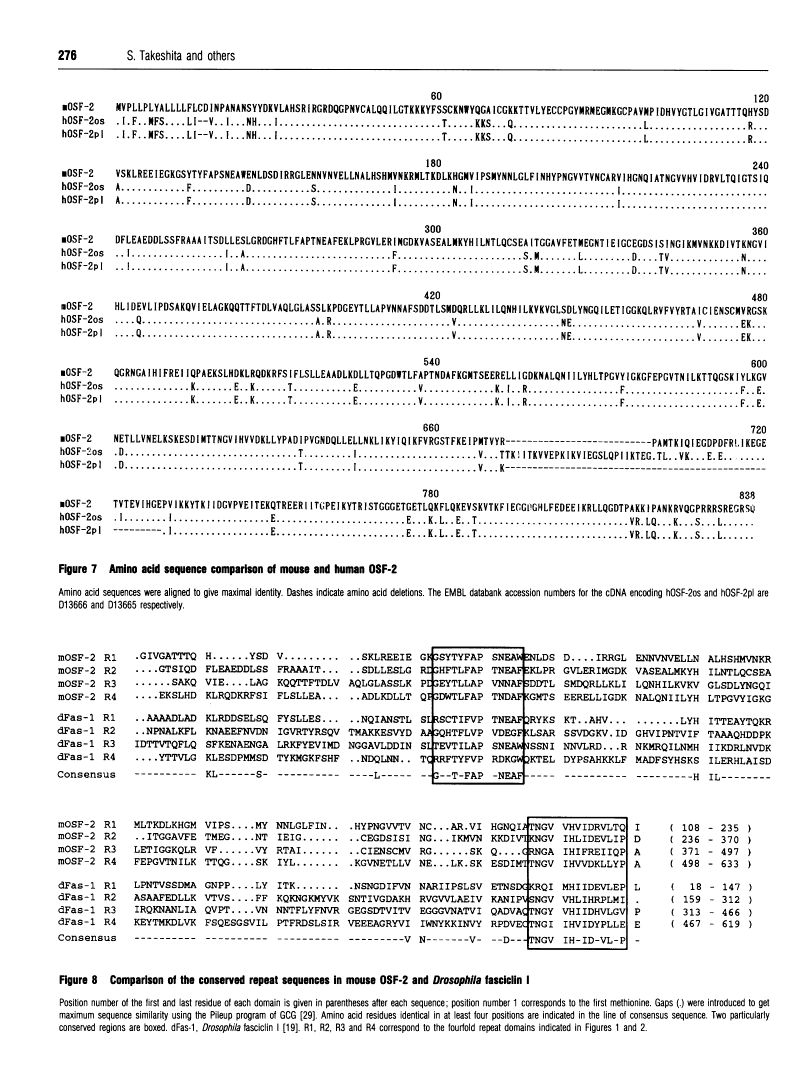
Abstract
Free full text

Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I.
Abstract
A cDNA library prepared from the mouse osteoblastic cell line MC3T3-E1 was screened for the presence of specifically expressed genes by employing a combined subtraction hybridization/differential screening approach. A cDNA was identified and sequenced which encodes a protein designated osteoblast-specific factor 2 (OSF-2) comprising 811 amino acids. OSF-2 has a typical signal sequence, followed by a cysteine-rich domain, a fourfold repeated domain and a C-terminal domain. The protein lacks a typical transmembrane region. The fourfold repeated domain of OSF-2 shows homology with the insect protein fasciclin I. RNA analyses revealed that OSF-2 is expressed in bone and to a lesser extent in lung, but not in other tissues. Mouse OSF-2 cDNA was subsequently used as a probe to clone the human counterpart. Mouse and human OSF-2 show a high amino acid sequence conservation except for the signal sequence and two regions in the C-terminal domain in which 'in-frame' insertions or deletions are observed, implying alternative splicing events. On the basis of the amino acid sequence homology with fasciclin I, we suggest that OSF-2 functions as a homophilic adhesion molecule in bone formation.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992 Jan 24;68(2):303–322. [Abstract] [Google Scholar]
- Horton MA, Lewis D, McNulty K, Pringle JA, Chambers TJ. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [Abstract] [Google Scholar]
- Horton MA, Chambers TJ. Human osteoclast-specific antigens are expressed by osteoclasts in a wide range of non-human species. Br J Exp Pathol. 1986 Feb;67(1):95–104. [Europe PMC free article] [Abstract] [Google Scholar]
- Argraves WS, Suzuki S, Arai H, Thompson K, Pierschbacher MD, Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. [Europe PMC free article] [Abstract] [Google Scholar]
- Horton MA, Davies J. Perspectives: adhesion receptors in bone. J Bone Miner Res. 1989 Dec;4(6):803–808. [Abstract] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. [Abstract] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. [Abstract] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. [Europe PMC free article] [Abstract] [Google Scholar]
- Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [Abstract] [Google Scholar]
- Oldberg A, Franzén A, Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. [Europe PMC free article] [Abstract] [Google Scholar]
- Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. [Abstract] [Google Scholar]
- Clezardin P, Jouishomme H, Chavassieux P, Marie PJ. Thrombospondin is synthesized and secreted by human osteoblasts and osteosarcoma cells. A model to study the different effects of thrombospondin in cell adhesion. Eur J Biochem. 1989 May 15;181(3):721–726. [Abstract] [Google Scholar]
- Robey PG, Young MF, Fisher LW, McClain TD. Thrombospondin is an osteoblast-derived component of mineralized extracellular matrix. J Cell Biol. 1989 Feb;108(2):719–727. [Europe PMC free article] [Abstract] [Google Scholar]
- Helfrich MH, Nesbitt SA, Dorey EL, Horton MA. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J Bone Miner Res. 1992 Mar;7(3):335–343. [Abstract] [Google Scholar]
- Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990 Oct 19;63(2):437–445. [Abstract] [Google Scholar]
- Tezuka K, Takeshita S, Hakeda Y, Kumegawa M, Kikuno R, Hashimoto-Gotoh T. Isolation of mouse and human cDNA clones encoding a protein expressed specifically in osteoblasts and brain tissues. Biochem Biophys Res Commun. 1990 Nov 30;173(1):246–251. [Abstract] [Google Scholar]
- Zinn K, McAllister L, Goodman CS. Sequence analysis and neuronal expression of fasciclin I in grasshopper and Drosophila. Cell. 1988 May 20;53(4):577–587. [Abstract] [Google Scholar]
- Elkins T, Hortsch M, Bieber AJ, Snow PM, Goodman CS. Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. J Cell Biol. 1990 May;110(5):1825–1832. [Europe PMC free article] [Abstract] [Google Scholar]
- Elkins T, Zinn K, McAllister L, Hoffmann FM, Goodman CS. Genetic analysis of a Drosophila neural cell adhesion molecule: interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell. 1990 Feb 23;60(4):565–575. [Abstract] [Google Scholar]
- Hortsch M, Goodman CS. Drosophila fasciclin I, a neural cell adhesion molecule, has a phosphatidylinositol lipid membrane anchor that is developmentally regulated. J Biol Chem. 1990 Sep 5;265(25):15104–15109. [Abstract] [Google Scholar]
- McAllister L, Rehm EJ, Goodman GS, Zinn K. Alternative splicing of micro-exons creates multiple forms of the insect cell adhesion molecule fasciclin I. J Neurosci. 1992 Mar;12(3):895–905. [Abstract] [Google Scholar]
- Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. [Abstract] [Google Scholar]
- Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. [Abstract] [Google Scholar]
- Kiefer MC, Ioh RS, Bauer DM, Zapf J. Molecular cloning of a new human insulin-like growth factor binding protein. Biochem Biophys Res Commun. 1991 Apr 15;176(1):219–225. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. [Europe PMC free article] [Abstract] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. [Europe PMC free article] [Abstract] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. [Europe PMC free article] [Abstract] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. [Abstract] [Google Scholar]
- Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. [Abstract] [Google Scholar]
- Centrella M, Canalis E. Local regulators of skeletal growth: a perspective. Endocr Rev. 1985 Fall;6(4):544–551. [Abstract] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. [Europe PMC free article] [Abstract] [Google Scholar]
- Ferguson MA, Williams AF. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. [Abstract] [Google Scholar]
- Low MG, Saltiel AR. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. [Abstract] [Google Scholar]
- Margalit H, Spouge JL, Cornette JL, Cease KB, Delisi C, Berzofsky JA. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [Abstract] [Google Scholar]
- Vogel H, Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2940271
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1134594?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Periostin<sup>+</sup> myeloid cells improved long bone regeneration in a mechanosensitive manner.
Bone Res, 12(1):59, 15 Oct 2024
Cited by: 0 articles | PMID: 39406726 | PMCID: PMC11480347
Correlation between Periostin Expression and Pro-Angiogenic Factors in Non-Small-Cell Lung Carcinoma.
Cells, 13(17):1406, 23 Aug 2024
Cited by: 0 articles | PMID: 39272978 | PMCID: PMC11394527
Development of a top-down MS assay for specific identification of human periostin isoforms.
Front Mol Biosci, 11:1399225, 19 Jun 2024
Cited by: 0 articles | PMID: 38962283 | PMCID: PMC11220192
Thick Skin on the Dorsal Spine in Osteoproliferative Disease: Ossification of the Posterior Longitudinal Ligament and Diffuse Idiopathic Skeletal Hyperostosis.
Cureus, 16(6):e62235, 12 Jun 2024
Cited by: 0 articles | PMID: 38868545
Targeted Antisense Oligonucleotide-Mediated Skipping of Murine Postn Exon 17 Partially Addresses Fibrosis in D2.mdx Mice.
Int J Mol Sci, 25(11):6113, 01 Jun 2024
Cited by: 2 articles | PMID: 38892298 | PMCID: PMC11172600
Go to all (413) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cloning and characterization of OSF-3, a new member of the MER5 family, expressed in mouse osteoblastic cells.
J Biochem, 115(4):641-643, 01 Apr 1994
Cited by: 26 articles | PMID: 8089076
Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta.
J Bone Miner Res, 14(7):1239-1249, 01 Jul 1999
Cited by: 628 articles | PMID: 10404027
Isolation of mouse and human cDNA clones encoding a protein expressed specifically in osteoblasts and brain tissues.
Biochem Biophys Res Commun, 173(1):246-251, 01 Nov 1990
Cited by: 73 articles | PMID: 1701634