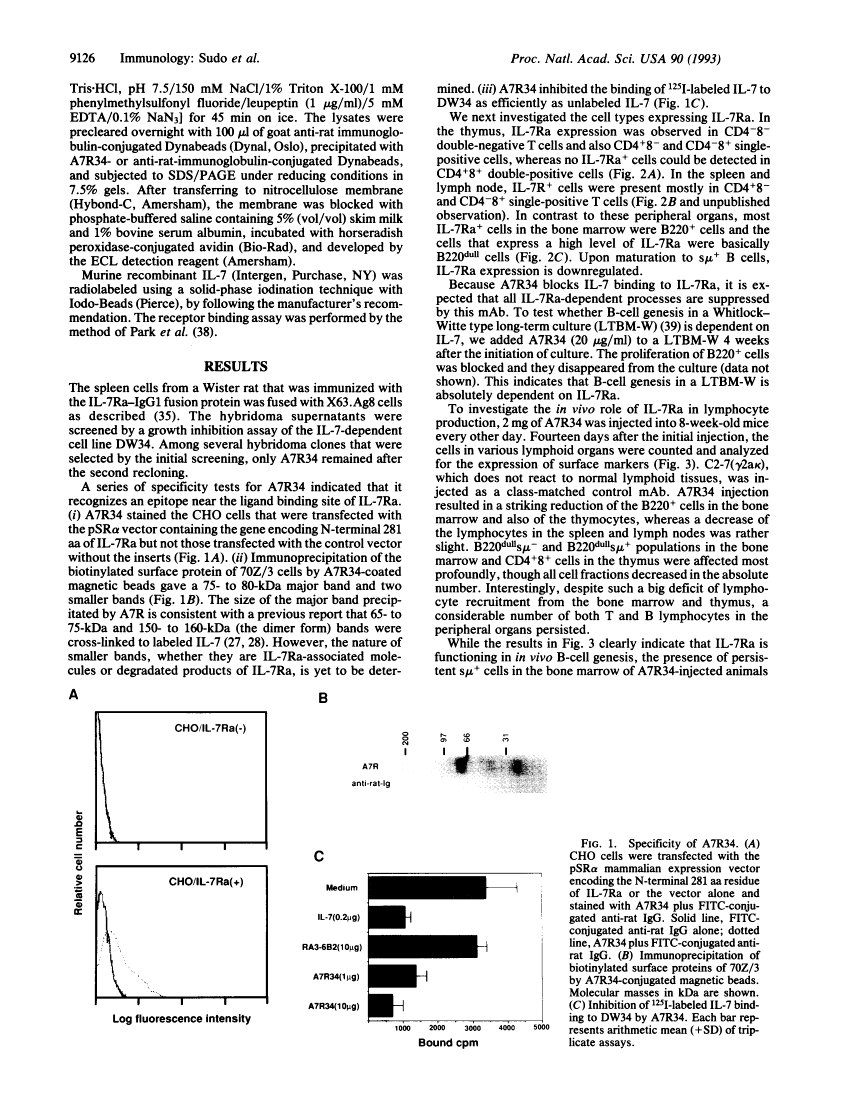
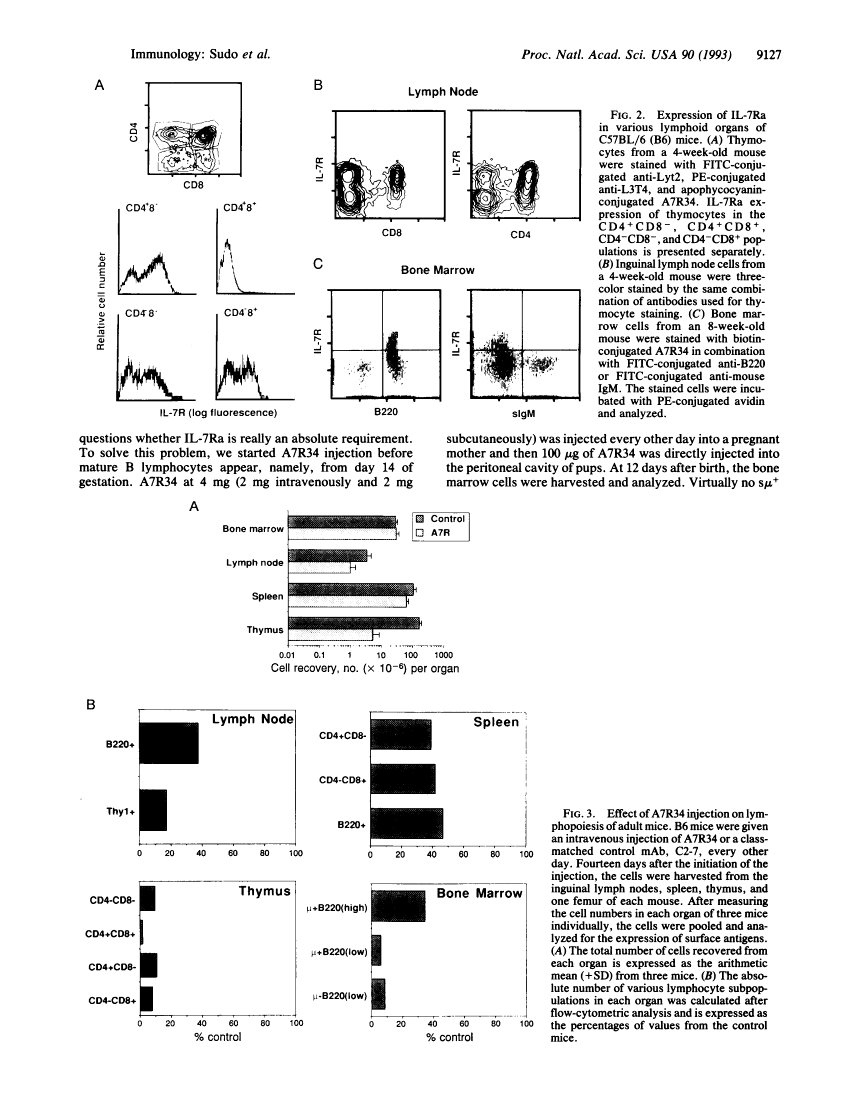
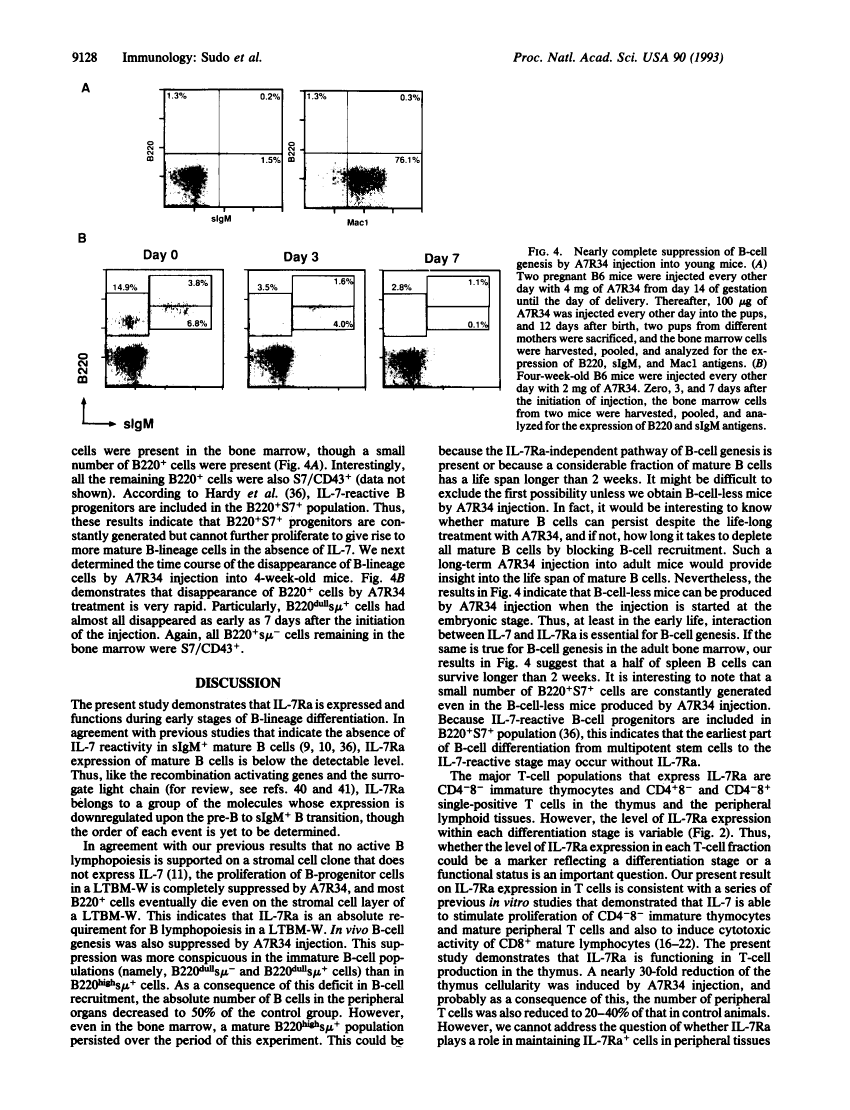
Abstract
Free full text

Expression and function of the interleukin 7 receptor in murine lymphocytes.
Abstract
A monoclonal antibody, A7R34, that recognizes the high-affinity interleukin 7 receptor (IL-7Ra) and blocks the binding between IL-7 and IL-7Ra has been produced. Cell surface staining with A7R34 demonstrated that IL-7Ra is expressed in both B- and T-cell lineages. In the bone marrow, immature B-lineage cells that do not express surface IgM were IL-7Ra+. In the thymus, IL-7Ra was detected in CD4-8- T cells and also in CD4 or CD8 single-positive cells but not in CD4+8+ double-positive cells. In the peripheral lymphoid tissues, both CD4 and CD8 single-positive cells were the major cell types that express IL-7Ra. Addition of A7R34 to a long-term B-precursor-cell culture inhibited proliferation of the B-lineage cells, indicating that IL-7 is an absolute requirement for in vitro B-cell genesis. Consistent with this in vitro result, continuous injection of A7R34 into an adult mouse resulted in a decrease of B-precursor cells and also of thymocytes, whereas a considerable fraction of mature B and T cells in the peripheral tissues persisted over 2 weeks of the experiment. When A7R34 injection is started from day 14 of gestation, it is possible to produce mice that lack B cells. These results indicate that IL-7 is an essential molecule for generation of both B and T cells in murine bone marrow and thymus, respectively. Moreover, IL-7Ra would be the sole receptor system regulating these processes.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kincade PW, Lee G, Pietrangeli CE, Hayashi S, Gimble JM. Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol. 1989;7:111–143. [Abstract] [Google Scholar]
- Osmond DG. B cell development in the bone marrow. Semin Immunol. 1990 May;2(3):173–180. [Abstract] [Google Scholar]
- Hunt P, Robertson D, Weiss D, Rennick D, Lee F, Witte ON. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. [Abstract] [Google Scholar]
- Whitlock CA, Tidmarsh GF, Muller-Sieburg C, Weissman IL. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. [Abstract] [Google Scholar]
- Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987 Feb 15;138(4):1082–1087. [Abstract] [Google Scholar]
- Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K, Nishikawa S. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988 May;7(5):1337–1343. [Europe PMC free article] [Abstract] [Google Scholar]
- Namen AE, Schmierer AE, March CJ, Overell RW, Park LS, Urdal DL, Mochizuki DY. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988 Mar 1;167(3):988–1002. [Europe PMC free article] [Abstract] [Google Scholar]
- Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. [Abstract] [Google Scholar]
- Lee G, Namen AE, Gillis S, Ellingsworth LR, Kincade PW. Normal B cell precursors responsive to recombinant murine IL-7 and inhibition of IL-7 activity by transforming growth factor-beta. J Immunol. 1989 Jun 1;142(11):3875–3883. [Abstract] [Google Scholar]
- Suda T, Okada S, Suda J, Miura Y, Ito M, Sudo T, Hayashi S, Nishikawa S, Nakauchi H. A stimulatory effect of recombinant murine interleukin-7 (IL-7) on B-cell colony formation and an inhibitory effect of IL-1 alpha. Blood. 1989 Nov 1;74(6):1936–1941. [Abstract] [Google Scholar]
- Hayashi S, Kunisada T, Ogawa M, Sudo T, Kodama H, Suda T, Nishikawa S, Nishikawa S. Stepwise progression of B lineage differentiation supported by interleukin 7 and other stromal cell molecules. J Exp Med. 1990 May 1;171(5):1683–1695. [Europe PMC free article] [Abstract] [Google Scholar]
- Cumano A, Dorshkind K, Gillis S, Paige CJ. The influence of S17 stromal cells and interleukin 7 on B cell development. Eur J Immunol. 1990 Oct;20(10):2183–2189. [Abstract] [Google Scholar]
- McNiece IK, Langley KE, Zsebo KM. The role of recombinant stem cell factor in early B cell development. Synergistic interaction with IL-7. J Immunol. 1991 Jun 1;146(11):3785–3790. [Abstract] [Google Scholar]
- Rolink A, Streb M, Nishikawa S, Melchers F. The c-kit-encoded tyrosine kinase regulates the proliferation of early pre-B cells. Eur J Immunol. 1991 Oct;21(10):2609–2612. [Abstract] [Google Scholar]
- Hirayama F, Shih JP, Awgulewitsch A, Warr GW, Clark SC, Ogawa M. Clonal proliferation of murine lymphohemopoietic progenitors in culture. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5907–5911. [Europe PMC free article] [Abstract] [Google Scholar]
- Chazen GD, Pereira GM, LeGros G, Gillis S, Shevach EM. Interleukin 7 is a T-cell growth factor. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5923–5927. [Europe PMC free article] [Abstract] [Google Scholar]
- Morrissey PJ, Goodwin RG, Nordan RP, Anderson D, Grabstein KH, Cosman D, Sims J, Lupton S, Acres B, Reed SG. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989 Mar 1;169(3):707–716. [Europe PMC free article] [Abstract] [Google Scholar]
- Grabstein KH, Namen AE, Shanebeck K, Voice RF, Reed SG, Widmer MB. Regulation of T cell proliferation by IL-7. J Immunol. 1990 Apr 15;144(8):3015–3020. [Abstract] [Google Scholar]
- Chantry D, Turner M, Feldmann M. Interleukin 7 (murine pre-B cell growth factor/lymphopoietin 1) stimulates thymocyte growth: regulation by transforming growth factor beta. Eur J Immunol. 1989 Apr;19(4):783–786. [Abstract] [Google Scholar]
- Conlon PJ, Morrissey PJ, Nordan RP, Grabstein KH, Prickett KS, Reed SG, Goodwin R, Cosman D, Namen AE. Murine thymocytes proliferate in direct response to interleukin-7. Blood. 1989 Sep;74(4):1368–1373. [Abstract] [Google Scholar]
- Murray R, Suda T, Wrighton N, Lee F, Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;1(5):526–531. [Abstract] [Google Scholar]
- Suda T, Zlotnik A. IL-7 maintains the T cell precursor potential of CD3-CD4-CD8- thymocytes. J Immunol. 1991 May 1;146(9):3068–3073. [Abstract] [Google Scholar]
- Morrissey PJ, Conlon P, Braddy S, Williams DE, Namen AE, Mochizuki DY. Administration of IL-7 to mice with cyclophosphamide-induced lymphopenia accelerates lymphocyte repopulation. J Immunol. 1991 Mar 1;146(5):1547–1552. [Abstract] [Google Scholar]
- Samaridis J, Casorati G, Traunecker A, Iglesias A, Gutierrez JC, Müller U, Palacios R. Development of lymphocytes in interleukin 7-transgenic mice. Eur J Immunol. 1991 Feb;21(2):453–460. [Abstract] [Google Scholar]
- Damia G, Komschlies KL, Faltynek CR, Ruscetti FW, Wiltrout RH. Administration of recombinant human interleukin-7 alters the frequency and number of myeloid progenitor cells in the bone marrow and spleen of mice. Blood. 1992 Mar 1;79(5):1121–1129. [Abstract] [Google Scholar]
- Faltynek CR, Wang S, Miller D, Young E, Tiberio L, Kross K, Kelley M, Kloszewski E. Administration of human recombinant IL-7 to normal and irradiated mice increases the numbers of lymphocytes and some immature cells of the myeloid lineage. J Immunol. 1992 Aug 15;149(4):1276–1282. [Abstract] [Google Scholar]
- Armitage RJ, Ziegler SF, Friend DJ, Park LS, Fanslow WC. Identification of a novel low-affinity receptor for human interleukin-7. Blood. 1992 Apr 1;79(7):1738–1745. [Abstract] [Google Scholar]
- Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, et al. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. [Abstract] [Google Scholar]
- Nishikawa S, Ogawa M, Nishikawa S, Kunisada T, Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988 Nov;18(11):1767–1771. [Abstract] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. [Abstract] [Google Scholar]
- Urlaub G, Chasin LA. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. [Europe PMC free article] [Abstract] [Google Scholar]
- Scahill SJ, Devos R, Van der Heyden J, Fiers W. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4654–4658. [Europe PMC free article] [Abstract] [Google Scholar]
- Coffman RL. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. [Abstract] [Google Scholar]
- Springer T, Galfré G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. [Abstract] [Google Scholar]
- Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991 Jul 1;174(1):63–71. [Europe PMC free article] [Abstract] [Google Scholar]
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. [Europe PMC free article] [Abstract] [Google Scholar]
- Ingalls HM, Goodloe-Holland CM, Luna EJ. Junctional plasma membrane domains isolated from aggregating Dictyostelium discoideum amebae. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4779–4783. [Europe PMC free article] [Abstract] [Google Scholar]
- Park LS, Friend D, Gillis S, Urdal DL. Characterization of the cell surface receptor for granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1986 Mar 25;261(9):4177–4183. [Abstract] [Google Scholar]
- Whitlock CA, Witte ON. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. [Europe PMC free article] [Abstract] [Google Scholar]
- Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. [Abstract] [Google Scholar]
- Rolink A, Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell. 1991 Sep 20;66(6):1081–1094. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.90.19.9125
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc47514?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.90.19.9125
Article citations
Food antigens suppress small intestinal tumorigenesis.
Front Immunol, 15:1373766, 18 Sep 2024
Cited by: 0 articles | PMID: 39359724 | PMCID: PMC11445177
Induction of Thymus Atrophy and Disruption of Thymocyte Development by Fipronil through Dysregulation of IL-7-Associated Genes.
Chem Res Toxicol, 37(9):1488-1500, 14 Aug 2024
Cited by: 0 articles | PMID: 39141674 | PMCID: PMC11409377
Notch Partners in the Long Journey of T-ALL Pathogenesis.
Int J Mol Sci, 24(2):1383, 10 Jan 2023
Cited by: 6 articles | PMID: 36674902 | PMCID: PMC9866461
Review Free full text in Europe PMC
Multi-modular structure of the gene regulatory network for specification and commitment of murine T cells.
Front Immunol, 14:1108368, 31 Jan 2023
Cited by: 7 articles | PMID: 36817475 | PMCID: PMC9928580
Review Free full text in Europe PMC
Understanding the Roles of the Hedgehog Signaling Pathway during T-Cell Lymphopoiesis and in T-Cell Acute Lymphoblastic Leukemia (T-ALL).
Int J Mol Sci, 24(3):2962, 03 Feb 2023
Cited by: 4 articles | PMID: 36769284 | PMCID: PMC9917970
Review Free full text in Europe PMC
Go to all (288) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interleukin-7 signaling in human B cell precursor acute lymphoblastic leukemia cells and murine BAF3 cells involves activation of STAT1 and STAT5 mediated via the interleukin-7 receptor alpha chain.
Leukemia, 10(8):1317-1325, 01 Aug 1996
Cited by: 36 articles | PMID: 8709637
Influence of polysaccharide fractions isolated from Caltha palustris L. on the cellular immune response in collagen-induced arthritis (CIA) in mice. A comparison with methotrexate.
J Ethnopharmacol, 145(1):109-117, 01 Nov 2012
Cited by: 18 articles | PMID: 23123796
Expression and function of mouse Fas antigen on immature and mature T cells.
J Immunol, 154(9):4395-4403, 01 May 1995
Cited by: 78 articles | PMID: 7536770
Fidelity and infidelity in commitment to B-lymphocyte lineage development.
Immunol Rev, 175:104-111, 01 Jun 2000
Cited by: 38 articles | PMID: 10933595
Review