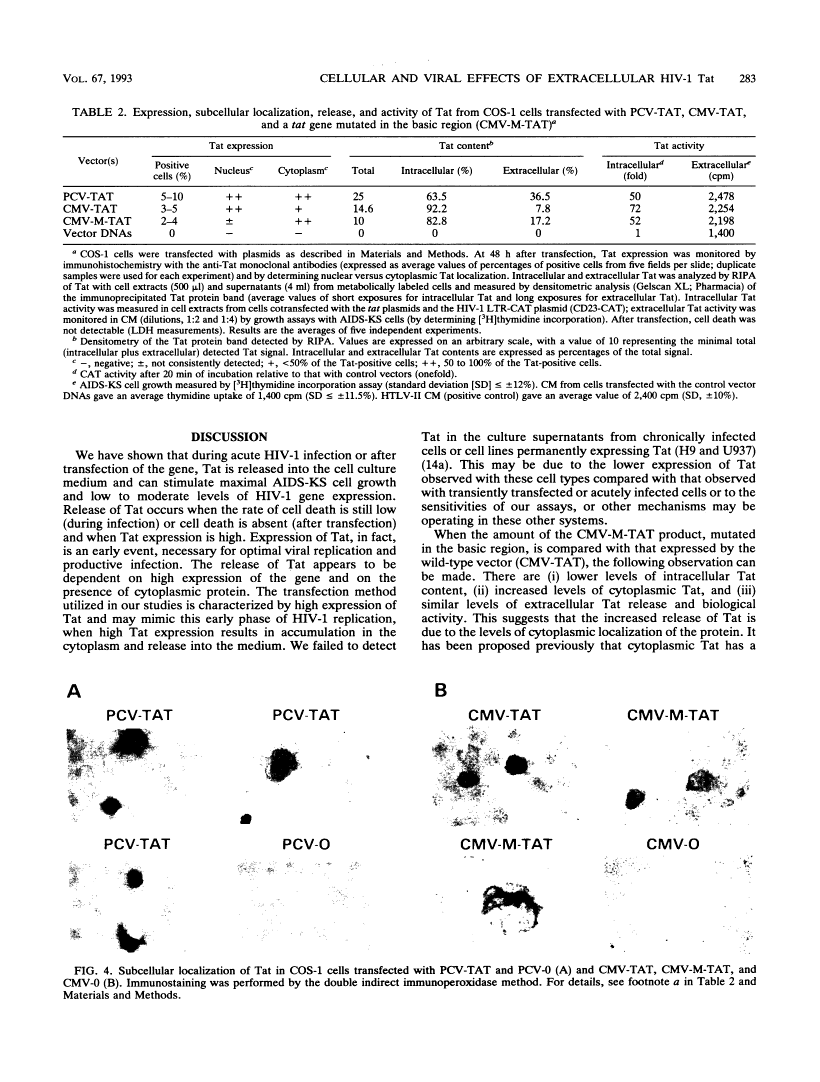
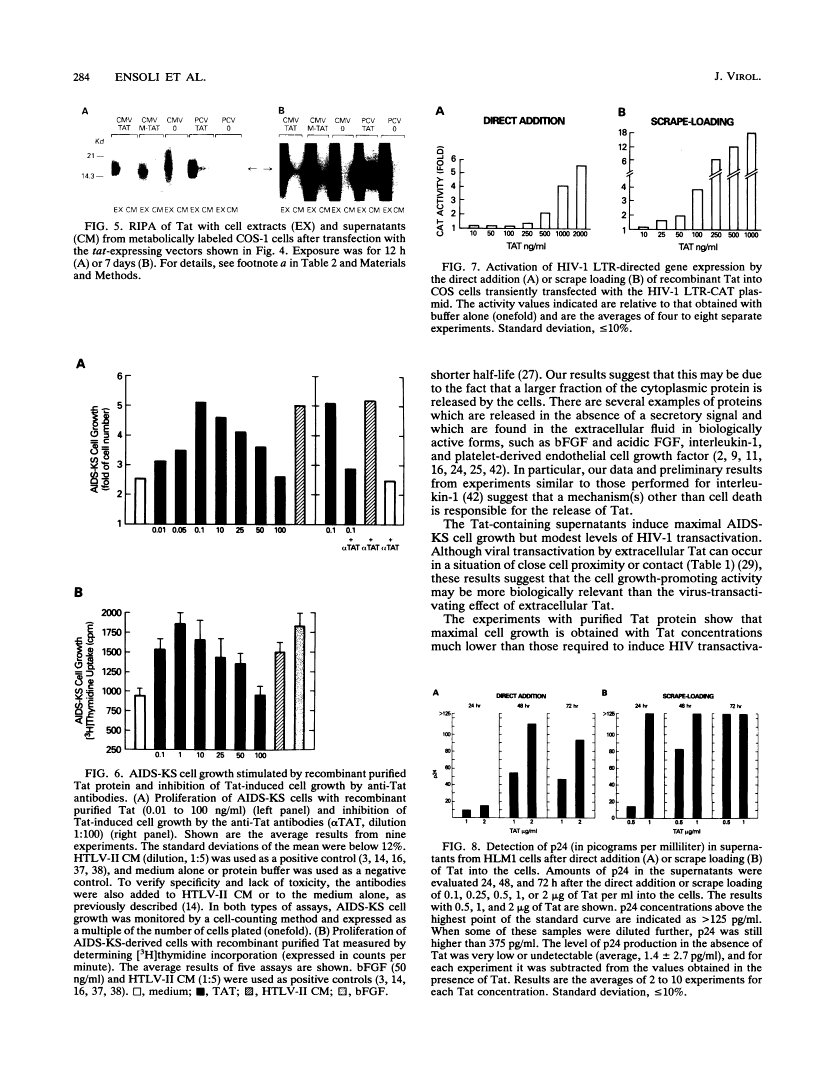
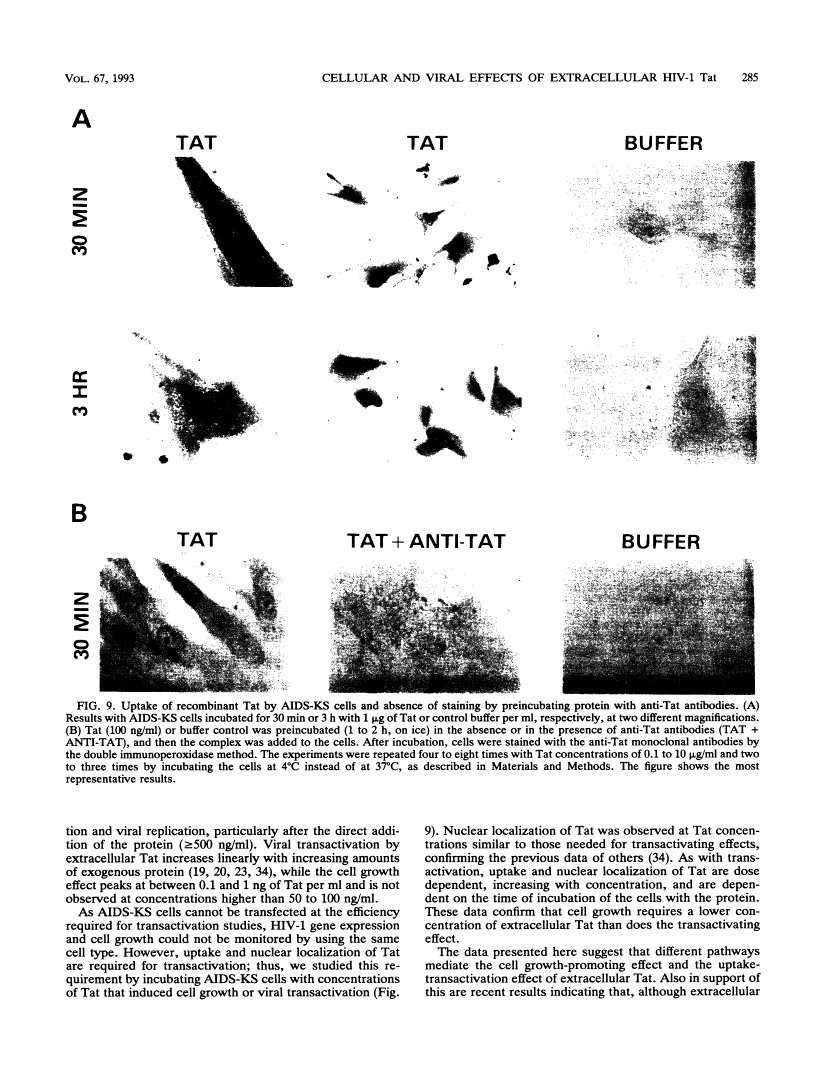
Abstract
Free full text

Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation.
Abstract
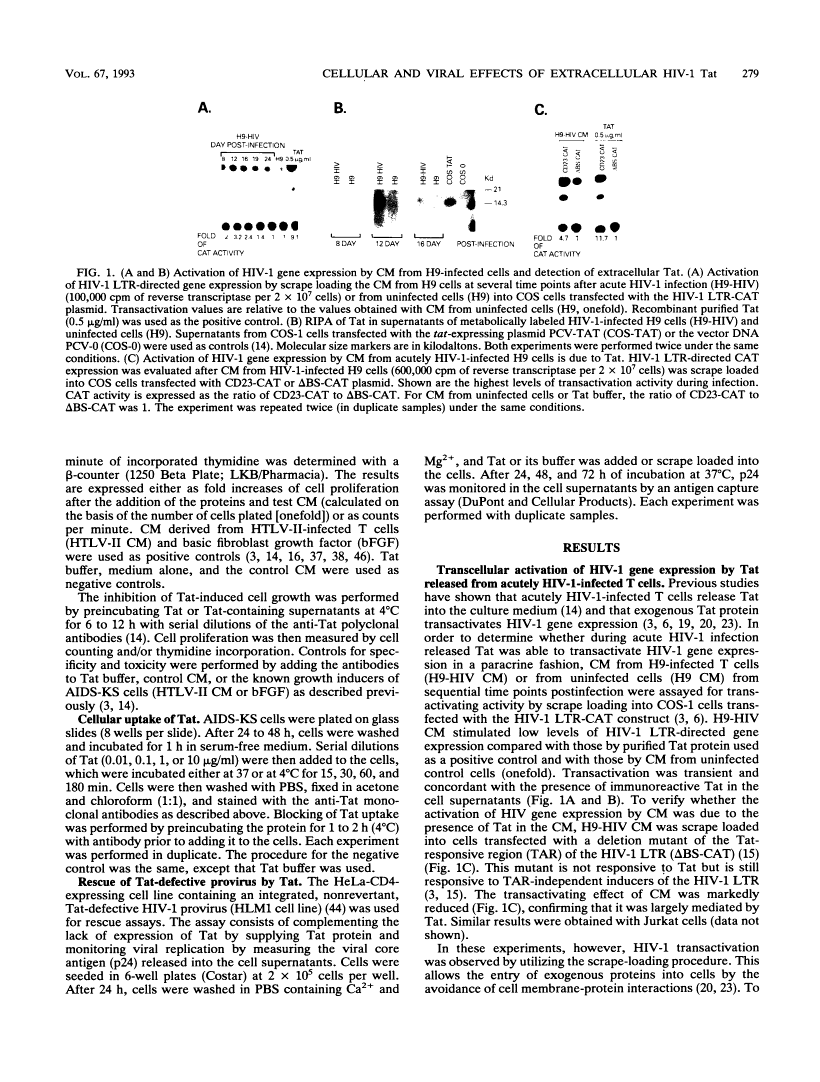
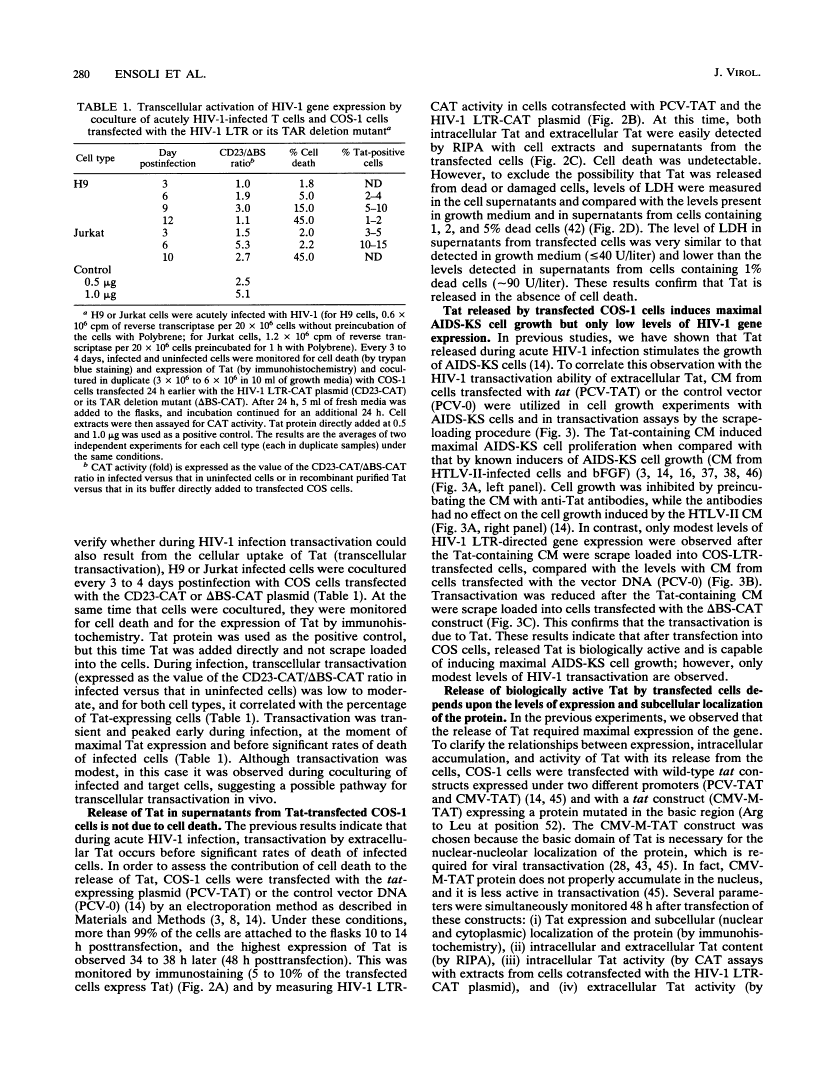
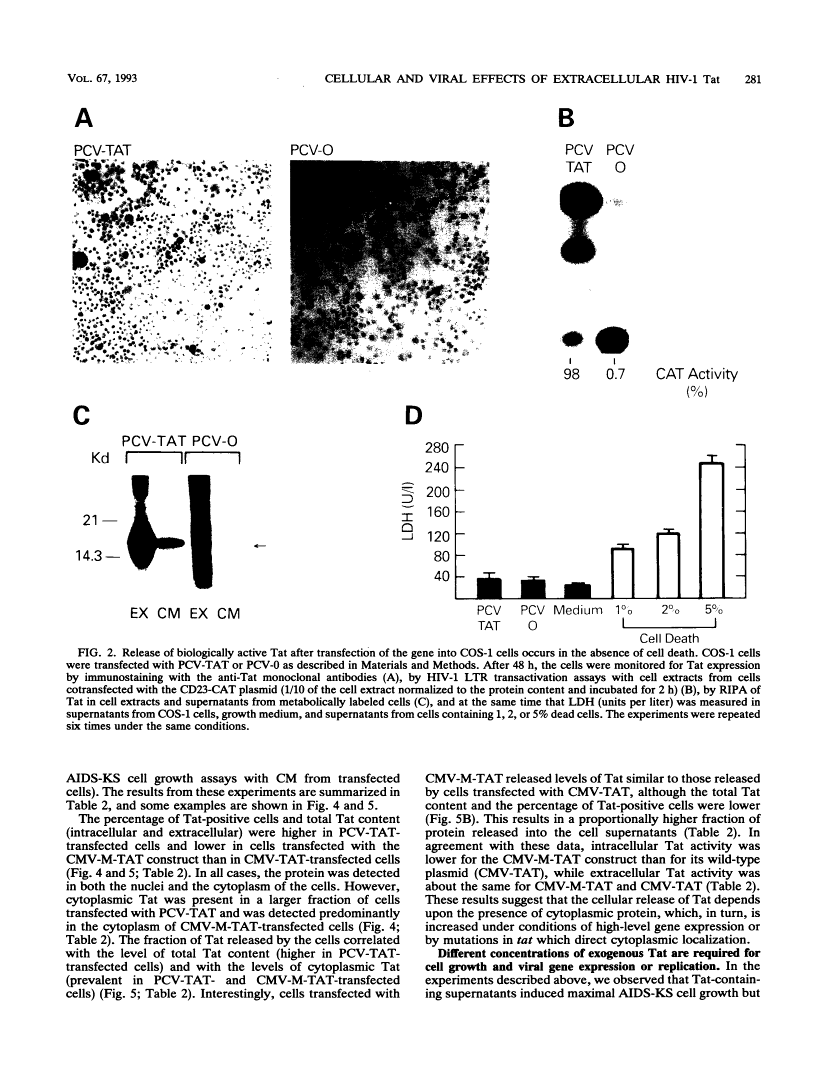
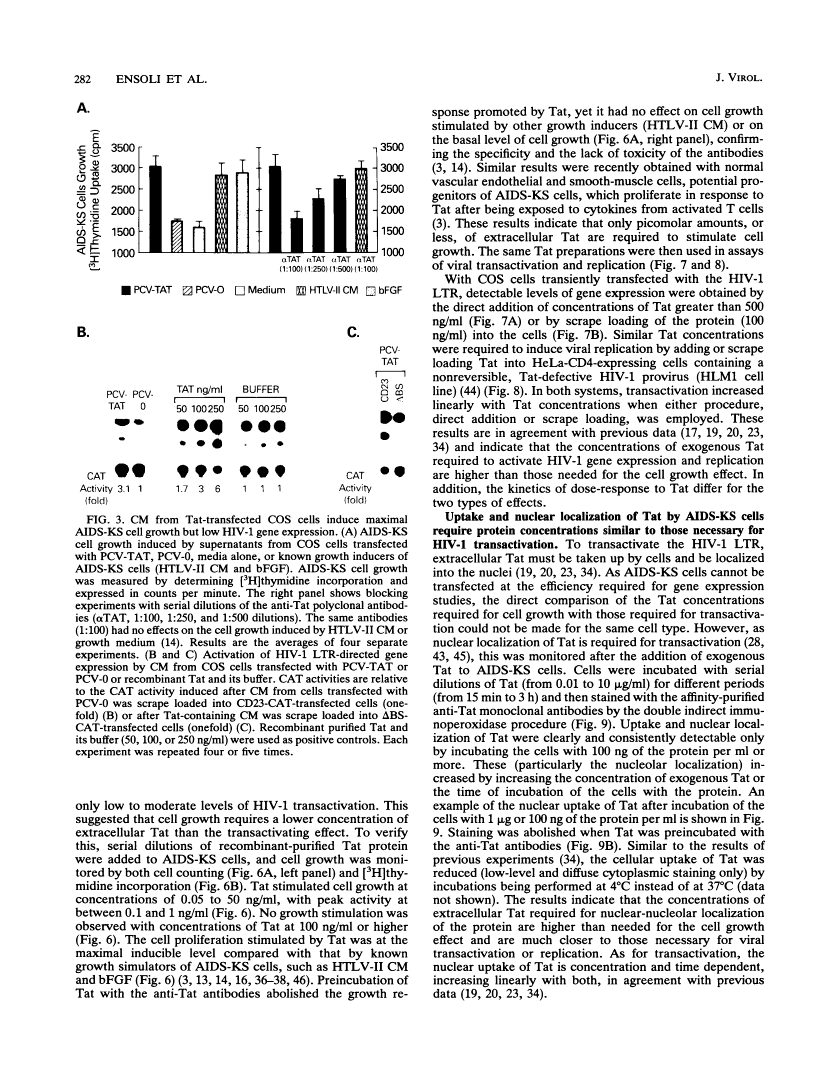
During acute human immunodeficiency virus type 1 (HIV-1) infection or after transfection of the tat gene, Tat protein is released into the cell culture supernatant. In this extracellular form, Tat stimulates both HIV-1 gene expression and the growth of cells derived from Kaposi's sarcoma (KS) lesions of HIV-1-infected individuals (AIDS-KS cells). Tat protein and its biological activities appear in the cell supernatants at the peak of Tat expression, when the rate of cell death is low (infection) or cell death is undetectable (transfection) and increased levels of cytoplasmic Tat are present. Tat-containing supernatants stimulate maximal AIDS-KS cell growth but only low to moderate levels of HIV-1 gene expression. This is due to the different concentrations of exogenous Tat required for the two effects. The cell growth-promoting effects of Tat peak at between 0.1 and 1 ng of purified recombinant protein per ml in the cell growth medium and do not increase with concentration. In contrast, both the detection of nuclear-localized Tat taken up by cells and the induction of HIV-1 gene expression or replication require higher Tat concentrations (> or = 100 ng/ml), and all increase linearly with increasing amounts of the exogenous protein. These data suggest that Tat can be released by a mechanism(s) other than cell death and that the cell growth-promoting activity and the virus-transactivating effect of extracellular Tat are mediated by different pathways.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya SK, Guo C, Josephs SF, Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. [Abstract] [Google Scholar]
- Bacle F, Haeffner-Cavaillon N, Laude M, Couturier C, Kazatchkine MD. Induction of IL-1 release through stimulation of the C3b/C4b complement receptor type one (CR1, CD35) on human monocytes. J Immunol. 1990 Jan 1;144(1):147–152. [Abstract] [Google Scholar]
- Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. [Abstract] [Google Scholar]
- Brake DA, Debouck C, Biesecker G. Identification of an Arg-Gly-Asp (RGD) cell adhesion site in human immunodeficiency virus type 1 transactivation protein, tat. J Cell Biol. 1990 Sep;111(3):1275–1281. [Europe PMC free article] [Abstract] [Google Scholar]
- Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992 Dec;66(12):7159–7167. [Europe PMC free article] [Abstract] [Google Scholar]
- D'Amore PA. Modes of FGF release in vivo and in vitro. Cancer Metastasis Rev. 1990 Nov;9(3):227–238. [Abstract] [Google Scholar]
- Dayton AI, Sodroski JG, Rosen CA, Goh WC, Haseltine WA. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. [Abstract] [Google Scholar]
- Dinarello CA, Savage N. Interleukin-1 and its receptor. Crit Rev Immunol. 1989;9(1):1–20. [Abstract] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [Abstract] [Google Scholar]
- Ensoli B, Barillari G, Gallo RC. Pathogenesis of AIDS-associated Kaposi's sarcoma. Hematol Oncol Clin North Am. 1991 Apr;5(2):281–295. [Abstract] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. [Abstract] [Google Scholar]
- Ensoli B, Lusso P, Schachter F, Josephs SF, Rappaport J, Negro F, Gallo RC, Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J. 1989 Oct;8(10):3019–3027. [Europe PMC free article] [Abstract] [Google Scholar]
- Ensoli B, Nakamura S, Salahuddin SZ, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo RC. AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989 Jan 13;243(4888):223–226. [Abstract] [Google Scholar]
- Feinberg MB, Baltimore D, Frankel AD. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4045–4049. [Europe PMC free article] [Abstract] [Google Scholar]
- Fisher AG, Feinberg MB, Josephs SF, Harper ME, Marselle LM, Reyes G, Gonda MA, Aldovini A, Debouk C, Gallo RC, et al. The trans-activator gene of HTLV-III is essential for virus replication. Nature. 320(6060):367–371. [Abstract] [Google Scholar]
- Frankel AD, Biancalana S, Hudson D. Activity of synthetic peptides from the Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7397–7401. [Europe PMC free article] [Abstract] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. [Abstract] [Google Scholar]
- Friedman-Kien AE. Disseminated Kaposi's sarcoma syndrome in young homosexual men. J Am Acad Dermatol. 1981 Oct;5(4):468–471. [Abstract] [Google Scholar]
- Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Haynes BF, Palker TJ, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. [Abstract] [Google Scholar]
- Gentz R, Chen CH, Rosen CA. Bioassay for trans-activation using purified human immunodeficiency virus tat-encoded protein: trans-activation requires mRNA synthesis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):821–824. [Europe PMC free article] [Abstract] [Google Scholar]
- Globus RK, Plouet J, Gospodarowicz D. Cultured bovine bone cells synthesize basic fibroblast growth factor and store it in their extracellular matrix. Endocrinology. 1989 Mar;124(3):1539–1547. [Abstract] [Google Scholar]
- Goh K, Furusawa S, Kawa Y, Negishi-Okitsu S, Mizoguchi M. Production of interleukin-1-alpha and -beta by human peripheral polymorphonuclear neutrophils. Int Arch Allergy Appl Immunol. 1989;88(3):297–303. [Abstract] [Google Scholar]
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. [Europe PMC free article] [Abstract] [Google Scholar]
- Hauber J, Malim MH, Cullen BR. Mutational analysis of the conserved basic domain of human immunodeficiency virus tat protein. J Virol. 1989 Mar;63(3):1181–1187. [Europe PMC free article] [Abstract] [Google Scholar]
- Hauber J, Perkins A, Heimer EP, Cullen BR. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6364–6368. [Europe PMC free article] [Abstract] [Google Scholar]
- Helland DE, Welles JL, Caputo A, Haseltine WA. Transcellular transactivation by the human immunodeficiency virus type 1 tat protein. J Virol. 1991 Aug;65(8):4547–4549. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaplan MH, Susin M, Pahwa SG, Fetten J, Allen SL, Lichtman S, Sarngadharan MG, Gallo RC. Neoplastic complications of HTLV-III infection. Lymphomas and solid tumors. Am J Med. 1987 Mar;82(3):389–396. [Abstract] [Google Scholar]
- Leeuwenberg JF, von Asmuth EJ, Jeunhomme TM, Buurman WA. IFN-gamma regulates the expression of the adhesion molecule ELAM-1 and IL-6 production by human endothelial cells in vitro. J Immunol. 1990 Oct 1;145(7):2110–2114. [Abstract] [Google Scholar]
- Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Oshiro LS. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. [Abstract] [Google Scholar]
- Lindholm PF, Marriott SJ, Gitlin SD, Bohan CA, Brady JN. Induction of nuclear NF-kappa B DNA binding activity after exposure of lymphoid cells to soluble tax1 protein. New Biol. 1990 Nov;2(11):1034–1043. [Abstract] [Google Scholar]
- Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991 Jul;10(7):1733–1739. [Europe PMC free article] [Abstract] [Google Scholar]
- Marriott SJ, Lindholm PF, Reid RL, Brady JN. Soluble HTLV-I Tax1 protein stimulates proliferation of human peripheral blood lymphocytes. New Biol. 1991 Jul;3(7):678–686. [Abstract] [Google Scholar]
- Miles SA, Martínez-Maza O, Rezai A, Magpantay L, Kishimoto T, Nakamura S, Radka SF, Linsley PS. Oncostatin M as a potent mitogen for AIDS-Kaposi's sarcoma-derived cells. Science. 1992 Mar 13;255(5050):1432–1434. [Abstract] [Google Scholar]
- Nair BC, DeVico AL, Nakamura S, Copeland TD, Chen Y, Patel A, O'Neil T, Oroszlan S, Gallo RC, Sarngadharan MG. Identification of a major growth factor for AIDS-Kaposi's sarcoma cells as oncostatin M. Science. 1992 Mar 13;255(5050):1430–1432. [Abstract] [Google Scholar]
- Nakamura S, Salahuddin SZ, Biberfeld P, Ensoli B, Markham PD, Wong-Staal F, Gallo RC. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science. 1988 Oct 21;242(4877):426–430. [Abstract] [Google Scholar]
- Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. [Abstract] [Google Scholar]
- Rosen CA, Sodroski JG, Haseltine WA. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. [Abstract] [Google Scholar]
- Roy S, Delling U, Chen CH, Rosen CA, Sonenberg N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated trans-activation. Genes Dev. 1990 Aug;4(8):1365–1373. [Abstract] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruben S, Perkins A, Purcell R, Joung K, Sia R, Burghoff R, Haseltine WA, Rosen CA. Structural and functional characterization of human immunodeficiency virus tat protein. J Virol. 1989 Jan;63(1):1–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Sadaie MR, Tschachler E, Valerie K, Rosenberg M, Felber BK, Pavlakis GN, Klotman ME, Wong-Staal F. Activation of tat-defective human immunodeficiency virus by ultraviolet light. New Biol. 1990 May;2(5):479–486. [Abstract] [Google Scholar]
- Sadaie MR, Mukhopadhyaya R, Benaissa ZN, Pavlakis GN, Wong-Staal F. Conservative mutations in the putative metal-binding region of human immunodeficiency virus tat disrupt virus replication. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1257–1263. [Abstract] [Google Scholar]
- Salahuddin SZ, Nakamura S, Biberfeld P, Kaplan MH, Markham PD, Larsson L, Gallo RC. Angiogenic properties of Kaposi's sarcoma-derived cells after long-term culture in vitro. Science. 1988 Oct 21;242(4877):430–433. [Abstract] [Google Scholar]
- Siekevitz M, Feinberg MB, Holbrook N, Wong-Staal F, Greene WC. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. [Europe PMC free article] [Abstract] [Google Scholar]
- Viscidi RP, Mayur K, Lederman HM, Frankel AD. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science. 1989 Dec 22;246(4937):1606–1608. [Abstract] [Google Scholar]
- Vogel J, Hinrichs SH, Reynolds RK, Luciw PA, Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. [Abstract] [Google Scholar]
- Wright CM, Felber BK, Paskalis H, Pavlakis GN. Expression and characterization of the trans-activator of HTLV-III/LAV virus. Science. 1986 Nov 21;234(4779):988–992. [Abstract] [Google Scholar]
- Yohn JJ, Critelli M, Lyons MB, Norris DA. Modulation of melanocyte intercellular adhesion molecule-1 by immune cytokines. J Invest Dermatol. 1990 Aug;95(2):233–237. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.67.1.277-287.1993
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/67/1/277.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/67/1/277
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/67/1/277
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.67.1.277-287.1993
Article citations
Mechanisms and Cardiorenal Complications of Chronic Anemia in People with HIV.
Viruses, 16(4):542, 30 Mar 2024
Cited by: 1 article | PMID: 38675885 | PMCID: PMC11053456
Review Free full text in Europe PMC
Friends and Foes: The Ambivalent Role of Autophagy in HIV-1 Infection.
Viruses, 16(4):500, 25 Mar 2024
Cited by: 1 article | PMID: 38675843 | PMCID: PMC11054699
Review Free full text in Europe PMC
Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis.
Int J Mol Sci, 25(3):1704, 30 Jan 2024
Cited by: 2 articles | PMID: 38338977 | PMCID: PMC10855115
Review Free full text in Europe PMC
Heterologous DNA Prime/Protein Boost Immunization Targeting Nef-Tat Fusion Antigen Induces Potent T-cell Activity and in vitro Anti-SCR HIV-1 Effects.
Curr HIV Res, 22(2):109-119, 01 Jan 2024
Cited by: 0 articles | PMID: 38712371
HIV-1 Tat endocytosis and retention in endolysosomes affects HIV-1 Tat-induced LTR transactivation in astrocytes.
FASEB J, 36(3):e22184, 01 Mar 2022
Cited by: 5 articles | PMID: 35113458 | PMCID: PMC9627655
Go to all (507) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients.
Nature, 345(6270):84-86, 01 May 1990
Cited by: 546 articles | PMID: 2184372
Latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells.
J Virol, 75(18):8761-8771, 01 Sep 2001
Cited by: 45 articles | PMID: 11507221 | PMCID: PMC115121
Effects of the tat and nef gene products of human immunodeficiency virus type 1 (HIV-1) on transcription controlled by the HIV-1 long terminal repeat and on cell growth in macrophages.
J Virol, 67(12):6956-6964, 01 Dec 1993
Cited by: 26 articles | PMID: 8230418 | PMCID: PMC238154
Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi's sarcoma.
Clin Microbiol Rev, 15(2):310-326, 01 Apr 2002
Cited by: 74 articles | PMID: 11932235 | PMCID: PMC118071
Review Free full text in Europe PMC