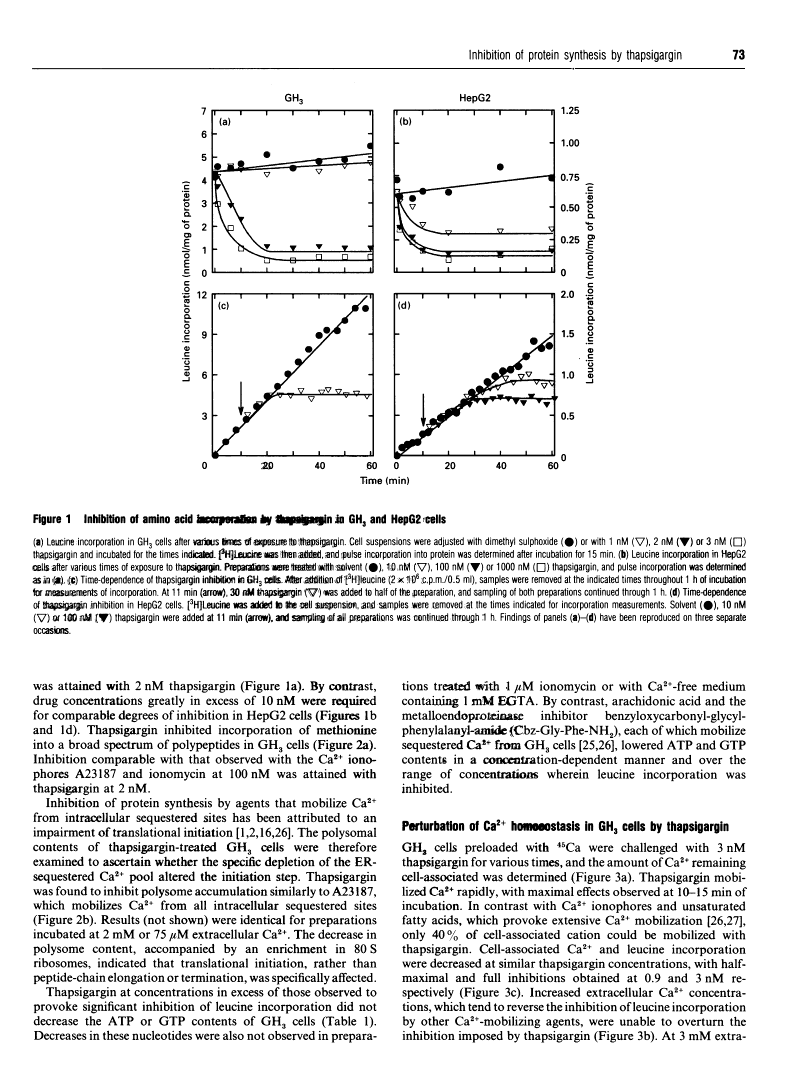
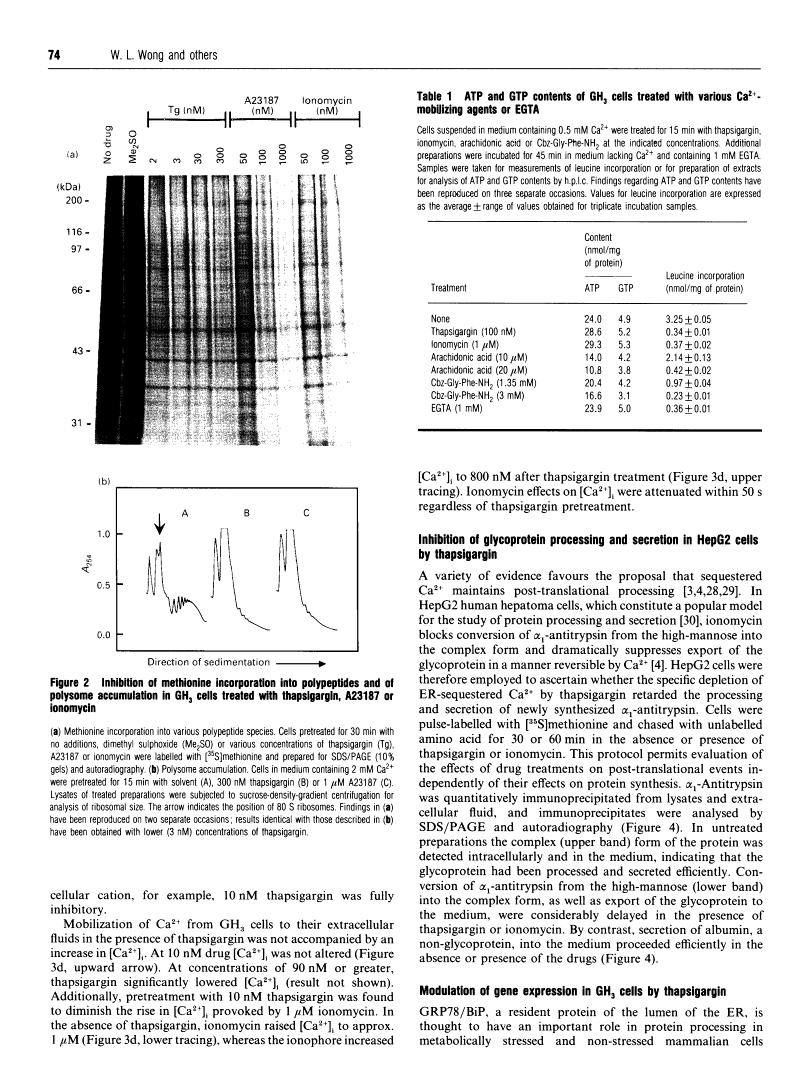
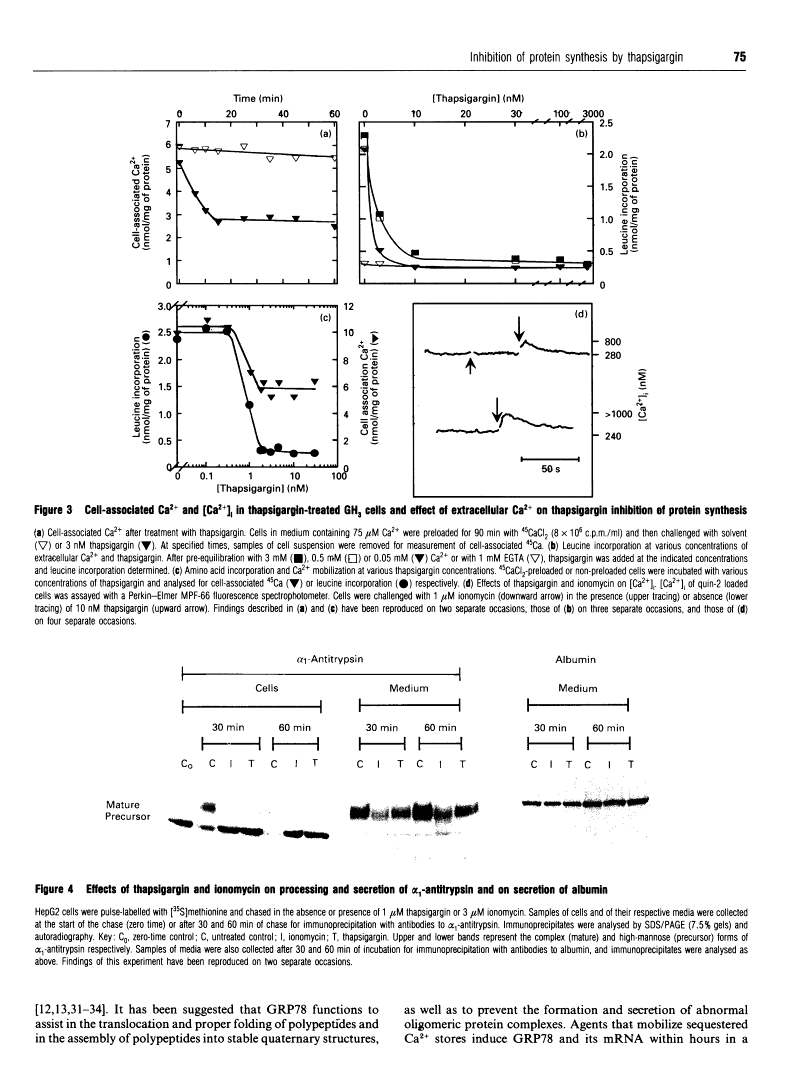
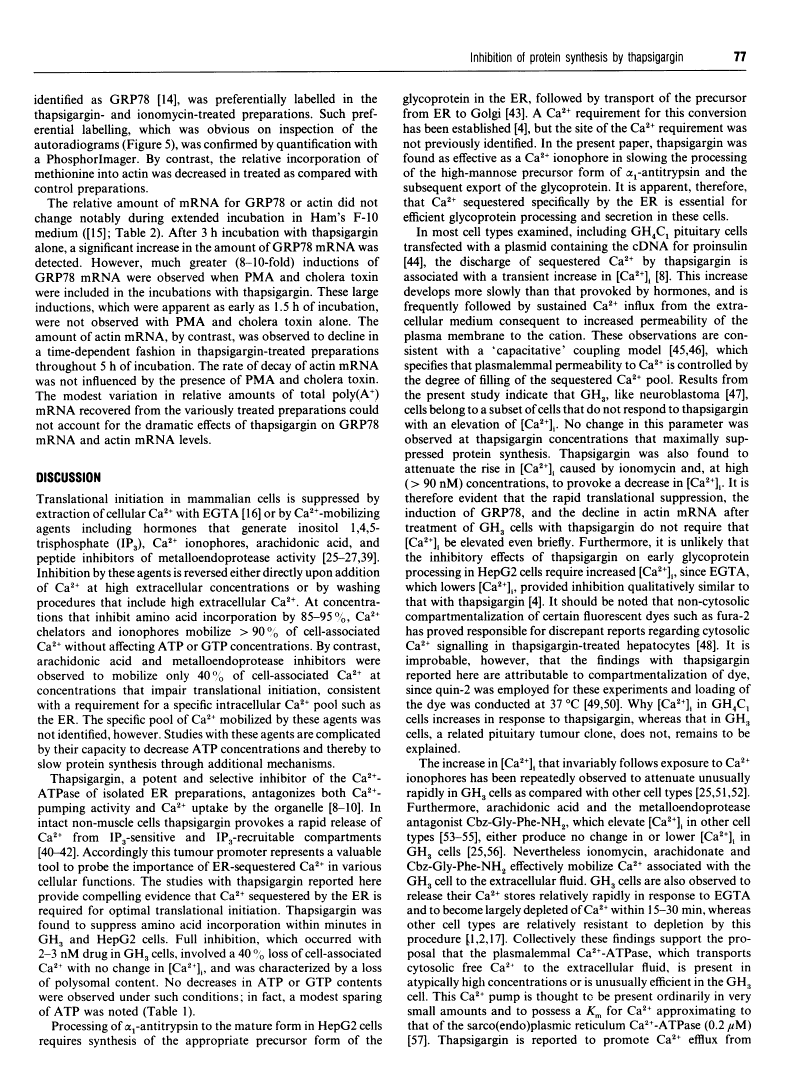
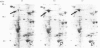Abstract
Free full text

Inhibition of protein synthesis and early protein processing by thapsigargin in cultured cells.
Abstract
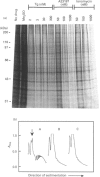
Thapsigargin, a tumour-promoting sesquiterpene lactone, selectively inhibits the Ca(2+)-ATPase responsible for Ca2+ accumulation by the endoplasmic reticulum (ER). Mobilization of ER-sequestered Ca2+ to the cytosol and to the extracellular fluid subsequently ensues, with concomitant alteration of cellular functions. Thapsigargin was found to serve as a rapid, potent and efficacious inhibitor of amino acid incorporation in cultured mammalian cells. At concentrations mobilizing cell-associated Ca2+ to the extracellular fluid, thapsigargin provoked extensive inhibition of protein synthesis within 10 min. The inhibition in GH3 pituitary cells involved the synthesis of almost all polypeptides, was not associated with increased cytosolic free Ca2+ concentration ([Ca2+]i), and was not reversed at high extracellular Ca2+. The transient rise in [Ca2+]i triggered by ionomycin was diminished by thapsigargin. Polysomes failed to accumulate in the presence of the drug, indicative of impaired translational initiation. With longer (1-3 h) exposures to thapsigargin, recovery of translational activity was observed accompanied by increased synthesis of the ER protein glucose-regulated stress protein 78 or immunoglobulin heavy-chain binding protein ('GRP78/BiP') and its mRNA. Such inductions were comparable with those observed previously with Ca2+ ionophores which mobilize the cation from all intracellular sequestered sites. Actin mRNA concentrations declined significantly during such treatments. In HepG2 cells processing and secretion of the glycoprotein alpha 1-antitrypsin were rapidly suppressed by thapsigargin. Ca2+ sequestered specifically by the ER is concluded to be essential for optimal protein synthesis and processing. These rapid effects of thapsigargin on mRNA translation, protein processing and gene expression should be considered when evaluating potential mechanisms by which this tumour promoter influences cellular events.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brostrom CO, Brostrom MA. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. [Abstract] [Google Scholar]
- Lodish HF, Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990 Jul 5;265(19):10893–10899. [Abstract] [Google Scholar]
- Kuznetsov G, Brostrom MA, Brostrom CO. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J Biol Chem. 1992 Feb 25;267(6):3932–3939. [Abstract] [Google Scholar]
- Walz B, Baumann O. Calcium-sequestering cell organelles: in situ localization, morphological and functional characterization. Prog Histochem Cytochem. 1989;20(2):1–47. [Abstract] [Google Scholar]
- Meldolesi J, Madeddu L, Pozzan T. Intracellular Ca2+ storage organelles in non-muscle cells: heterogeneity and functional assignment. Biochim Biophys Acta. 1990 Nov 12;1055(2):130–140. [Abstract] [Google Scholar]
- Rossier MF, Putney JW., Jr The identity of the calcium-storing, inositol 1,4,5-trisphosphate-sensitive organelle in non-muscle cells: calciosome, endoplasmic reticulum ... or both? Trends Neurosci. 1991 Jul;14(7):310–314. [Abstract] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. [Abstract] [Google Scholar]
- Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. [Europe PMC free article] [Abstract] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [Abstract] [Google Scholar]
- Marks F, Hanke B, Thastrup O, Fürstenberger G. Stimulatory effect of thapsigargin, a non-TPA-type tumor promoter, on arachidonic acid metabolism in the murine keratinocyte line HEL30 and on epidermal cell proliferation in vivo as compared to the effects of phorbol ester TPA. Carcinogenesis. 1991 Aug;12(8):1491–1497. [Abstract] [Google Scholar]
- Gething MJ, Sambrook J. Transport and assembly processes in the endoplasmic reticulum. Semin Cell Biol. 1990 Feb;1(1):65–72. [Abstract] [Google Scholar]
- Haas IG. BiP--a heat shock protein involved in immunoglobulin chain assembly. Curr Top Microbiol Immunol. 1991;167:71–82. [Abstract] [Google Scholar]
- Brostrom MA, Cade C, Prostko CR, Gmitter-Yellen D, Brostrom CO. Accommodation of protein synthesis to chronic deprivation of intracellular sequestered calcium. A putative role for GRP78. J Biol Chem. 1990 Nov 25;265(33):20539–20546. [Abstract] [Google Scholar]
- Prostko CR, Brostrom MA, Galuska-Malara EM, Brostrom CO. Stimulation of GRP78 gene transcription by phorbol ester and cAMP in GH3 pituitary cells. The accommodation of protein synthesis to chronic deprivation of intracellular sequestered calcium. J Biol Chem. 1991 Oct 15;266(29):19790–19795. [Abstract] [Google Scholar]
- Chin KV, Cade C, Brostrom CO, Galuska EM, Brostrom MA. Calcium-dependent regulation of protein synthesis at translational initiation in eukaryotic cells. J Biol Chem. 1987 Dec 5;262(34):16509–16514. [Abstract] [Google Scholar]
- Brostrom CO, Bocckino SB, Brostrom MA. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983 Dec 10;258(23):14390–14399. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Brostrom MA, Chin KV, Cade C, Gmitter D, Brostrom CO. Stimulation of protein synthesis in pituitary cells by phorbol esters and cyclic AMP. Evidence for rapid induction of a component of translational initiation. J Biol Chem. 1987 Dec 5;262(34):16515–16523. [Abstract] [Google Scholar]
- Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. [Abstract] [Google Scholar]
- Gross V, Geiger T, Tran-Thi TA, Gauthier F, Heinrich PC. Biosynthesis and secretion of alpha 1-antitrypsin in primary cultures of rat hepatocytes. Characterization of differently glycosylated intracellular and extracellular forms. Eur J Biochem. 1982 Dec 15;129(2):317–323. [Abstract] [Google Scholar]
- Paulik M, Nowack DD, Morré DJ. Isolation of a vesicular intermediate in the cell-free transfer of membrane from transitional elements of the endoplasmic reticulum to Golgi apparatus cisternae of rat liver. J Biol Chem. 1988 Nov 25;263(33):17738–17748. [Abstract] [Google Scholar]
- Hollander MC, Fornace AJ., Jr Estimation of relative mRNA content by filter hybridization to a polythymidylate probe. Biotechniques. 1990 Aug;9(2):174–179. [Abstract] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. [Abstract] [Google Scholar]
- Brostrom MA, Prostko CR, Gmitter-Yellen D, Grandison LJ, Kuznetsov G, Wong WL, Brostrom CO. Inhibition of translational initiation by metalloendoprotease antagonists. Evidence for involvement of sequestered Ca2+ stores. J Biol Chem. 1991 Apr 15;266(11):7037–7043. [Abstract] [Google Scholar]
- Rotman EI, Brostrom MA, Brostrom CO. Inhibition of protein synthesis in intact mammalian cells by arachidonic acid. Biochem J. 1992 Mar 1;282(Pt 2):487–494. [Europe PMC free article] [Abstract] [Google Scholar]
- Brostrom CO, Chin KV, Wong WL, Cade C, Brostrom MA. Inhibition of translational initiation in eukaryotic cells by calcium ionophore. J Biol Chem. 1989 Jan 25;264(3):1644–1649. [Abstract] [Google Scholar]
- Schutzbach JS, Forsee WT. Calcium ion activation of rabbit liver alpha 1,2-mannosidase. J Biol Chem. 1990 Feb 15;265(5):2546–2549. [Abstract] [Google Scholar]
- Wileman T, Kane LP, Carson GR, Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J Biol Chem. 1991 Mar 5;266(7):4500–4507. [Abstract] [Google Scholar]
- Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. [Abstract] [Google Scholar]
- Bole DG, Hendershot LM, Kearney JF. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. [Europe PMC free article] [Abstract] [Google Scholar]
- Ng DT, Randall RE, Lamb RA. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989 Dec;109(6 Pt 2):3273–3289. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanders SL, Whitfield KM, Vogel JP, Rose MD, Schekman RW. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. [Abstract] [Google Scholar]
- Drummond IA, Lee AS, Resendez E, Jr, Steinhardt RA. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987 Sep 15;262(26):12801–12805. [Abstract] [Google Scholar]
- Lin AY, Chang SC, Lee AS. A calcium ionophore-inducible cellular promoter is highly active and has enhancerlike properties. Mol Cell Biol. 1986 Apr;6(4):1235–1243. [Europe PMC free article] [Abstract] [Google Scholar]
- Resendez E, Jr, Wooden SK, Lee AS. Identification of highly conserved regulatory domains and protein-binding sites in the promoters of the rat and human genes encoding the stress-inducible 78-kilodalton glucose-regulated protein. Mol Cell Biol. 1988 Oct;8(10):4579–4584. [Europe PMC free article] [Abstract] [Google Scholar]
- Chin KV, Cade C, Brostrom MA, Brostrom CO. Regulation of protein synthesis in intact rat liver by calcium mobilizing agents. Int J Biochem. 1988;20(11):1313–1319. [Abstract] [Google Scholar]
- Bian JH, Ghosh TK, Wang JC, Gill DL. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [Abstract] [Google Scholar]
- Menniti FS, Bird GS, Takemura H, Thastrup O, Potter BV, Putney JW., Jr Mobilization of calcium by inositol trisphosphates from permeabilized rat parotid acinar cells. Evidence for translocation of calcium from uptake to release sites within the inositol 1,4,5-trisphosphate- and thapsigargin-sensitive calcium pool. J Biol Chem. 1991 Jul 25;266(21):13646–13653. [Abstract] [Google Scholar]
- Ely JA, Ambroz C, Baukal AJ, Christensen SB, Balla T, Catt KJ. Relationship between agonist- and thapsigargin-sensitive calcium pools in adrenal glomerulosa cells. Thapsigargin-induced Ca2+ mobilization and entry. J Biol Chem. 1991 Oct 5;266(28):18635–18641. [Abstract] [Google Scholar]
- Lodish HF, Kong N, Hirani S, Rasmussen J. A vesicular intermediate in the transport of hepatoma secretory proteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1987 Feb;104(2):221–230. [Europe PMC free article] [Abstract] [Google Scholar]
- Law GJ, Pachter JA, Thastrup O, Hanley MR, Dannies PS. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochem J. 1990 Apr 15;267(2):359–364. [Europe PMC free article] [Abstract] [Google Scholar]
- Takemura H, Hughes AR, Thastrup O, Putney JW., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [Abstract] [Google Scholar]
- Mason MJ, Garcia-Rodriguez C, Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [Abstract] [Google Scholar]
- Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. [Europe PMC free article] [Abstract] [Google Scholar]
- Glennon MC, Bird GS, Kwan CY, Putney JW., Jr Actions of vasopressin and the Ca(2+)-ATPase inhibitor, thapsigargin, on Ca2+ signaling in hepatocytes. J Biol Chem. 1992 Apr 25;267(12):8230–8233. [Abstract] [Google Scholar]
- Tsien R, Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. [Abstract] [Google Scholar]
- Tsien RY. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. [Abstract] [Google Scholar]
- Albert PR, Tashjian AH., Jr Relationship of thyrotropin-releasing hormone-induced spike and plateau phases in cytosolic free Ca2+ concentrations to hormone secretion. Selective blockade using ionomycin and nifedipine. J Biol Chem. 1984 Dec 25;259(24):15350–15363. [Abstract] [Google Scholar]
- Albert PR, Tashjian AH., Jr Ionomycin acts as an ionophore to release TRH-regulated Ca2+ stores from GH4C1 cells. Am J Physiol. 1986 Dec;251(6 Pt 1):C887–C891. [Abstract] [Google Scholar]
- Kruger MC, Booyens J, Malan NT. A biochemical analysis of the effects of arachidonic acid on sarcoplasmic reticulum function. Prostaglandins Leukot Essent Fatty Acids. 1988 Jul;33(1):41–47. [Abstract] [Google Scholar]
- Chow SC, Jondal M. Polyunsaturated free fatty acids stimulate an increase in cytosolic Ca2+ by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in T cells through a mechanism independent of phosphoinositide turnover. J Biol Chem. 1990 Jan 15;265(2):902–907. [Abstract] [Google Scholar]
- Lelkes PI, Pollard HB. Oligopeptide inhibitors of metalloendoprotease activity inhibit catecholamine secretion from bovine adrenal chromaffin cells by modulating intracellular calcium homeostasis. J Biol Chem. 1987 Nov 15;262(32):15496–15505. [Abstract] [Google Scholar]
- Kolesnick RN, Gershengorn MC. Arachidonic acid inhibits thyrotropin-releasing hormone-induced elevation of cytoplasmic free calcium in GH3 pituitary cells. J Biol Chem. 1985 Jan 25;260(2):707–713. [Abstract] [Google Scholar]
- Carafoli E, Chiesi M. Calcium pumps in the plasma and intracellular membranes. Curr Top Cell Regul. 1992;32:209–241. [Abstract] [Google Scholar]
- Sagara Y, Wade JB, Inesi G. A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J Biol Chem. 1992 Jan 15;267(2):1286–1292. [Abstract] [Google Scholar]
- Ghosh TK, Bian JH, Short AD, Rybak SL, Gill DL. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991 Dec 25;266(36):24690–24697. [Abstract] [Google Scholar]
- Schönthal A, Sugarman J, Brown JH, Hanley MR, Feramisco JR. Regulation of c-fos and c-jun protooncogene expression by the Ca(2+)-ATPase inhibitor thapsigargin. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7096–7100. [Europe PMC free article] [Abstract] [Google Scholar]
- Makino R, Hayashi K, Sugimura T. C-myc transcript is induced in rat liver at a very early stage of regeneration or by cycloheximide treatment. Nature. 1984 Aug 23;310(5979):697–698. [Abstract] [Google Scholar]
- Müller R, Bravo R, Burckhardt J, Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. [Abstract] [Google Scholar]
- Reed JC, Alpers JD, Nowell PC, Hoover RG. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. [Europe PMC free article] [Abstract] [Google Scholar]
- Rozenval'd IB, Makarova GF, Epifanova OI. Priobretenie pokoiashchimisia kletkami kompetentnosti k vstupleniiu v period sinteza DNK posle inkubatsii s faktorami rosta i ingibitorami sinteza belka. Dokl Akad Nauk SSSR. 1989;305(4):977–980. [Abstract] [Google Scholar]
- Li XA, Lee AS. Competitive inhibition of a set of endoplasmic reticulum protein genes (GRP78, GRP94, and ERp72) retards cell growth and lowers viability after ionophore treatment. Mol Cell Biol. 1991 Jul;11(7):3446–3453. [Europe PMC free article] [Abstract] [Google Scholar]
- Macejak DG, Sarnow P. Internal initiation of translation mediated by the 5' leader of a cellular mRNA. Nature. 1991 Sep 5;353(6339):90–94. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2890071
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1132132?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1042/bj2890071
Article citations
Cardiovascular protection of YiyiFuzi powder and the potential mechanisms through modulating mitochondria-endoplasmic reticulum interactions.
Front Pharmacol, 15:1405545, 24 Jun 2024
Cited by: 0 articles | PMID: 38978978 | PMCID: PMC11228702
Review Free full text in Europe PMC
Cyclin-dependent kinase inhibitor 1 plays a more prominent role than activating transcription factor 4 or the p53 tumour suppressor in thapsigargin-induced G1 arrest.
PeerJ, 11:e16683, 18 Dec 2023
Cited by: 1 article | PMID: 38130926 | PMCID: PMC10734451
ER Ca2+ overload activates the IRE1α signaling and promotes cell survival.
Cell Biosci, 13(1):123, 03 Jul 2023
Cited by: 5 articles | PMID: 37400935 | PMCID: PMC10318635
Dyskerin Downregulation Can Induce ER Stress and Promote Autophagy via AKT-mTOR Signaling Deregulation.
Biomedicines, 10(5):1092, 08 May 2022
Cited by: 4 articles | PMID: 35625829 | PMCID: PMC9138296
IRE1/XBP1 and endoplasmic reticulum signaling - from basic to translational research for cardiovascular disease.
Curr Opin Physiol, 28:100552, 27 May 2022
Cited by: 4 articles | PMID: 37207249 | PMCID: PMC10195104
Go to all (96) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Inhibition of translational initiation by metalloendoprotease antagonists. Evidence for involvement of sequestered Ca2+ stores.
J Biol Chem, 266(11):7037-7043, 01 Apr 1991
Cited by: 12 articles | PMID: 1901861
Role of endoplasmic reticular calcium in oligosaccharide processing of alpha 1-antitrypsin.
J Biol Chem, 268(3):2001-2008, 01 Jan 1993
Cited by: 24 articles | PMID: 8380585
Reversible phosphorylation of eukaryotic initiation factor 2 alpha in response to endoplasmic reticular signaling.
Mol Cell Biochem, 127-128:255-265, 01 Nov 1993
Cited by: 53 articles | PMID: 7935356
Transactivation of the grp78 promoter by Ca2+ depletion. A comparative analysis with A23187 and the endoplasmic reticulum Ca(2+)-ATPase inhibitor thapsigargin.
J Biol Chem, 268(16):12003-12009, 01 Jun 1993
Cited by: 144 articles | PMID: 8505325