Abstract
Free full text

Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells.
Abstract
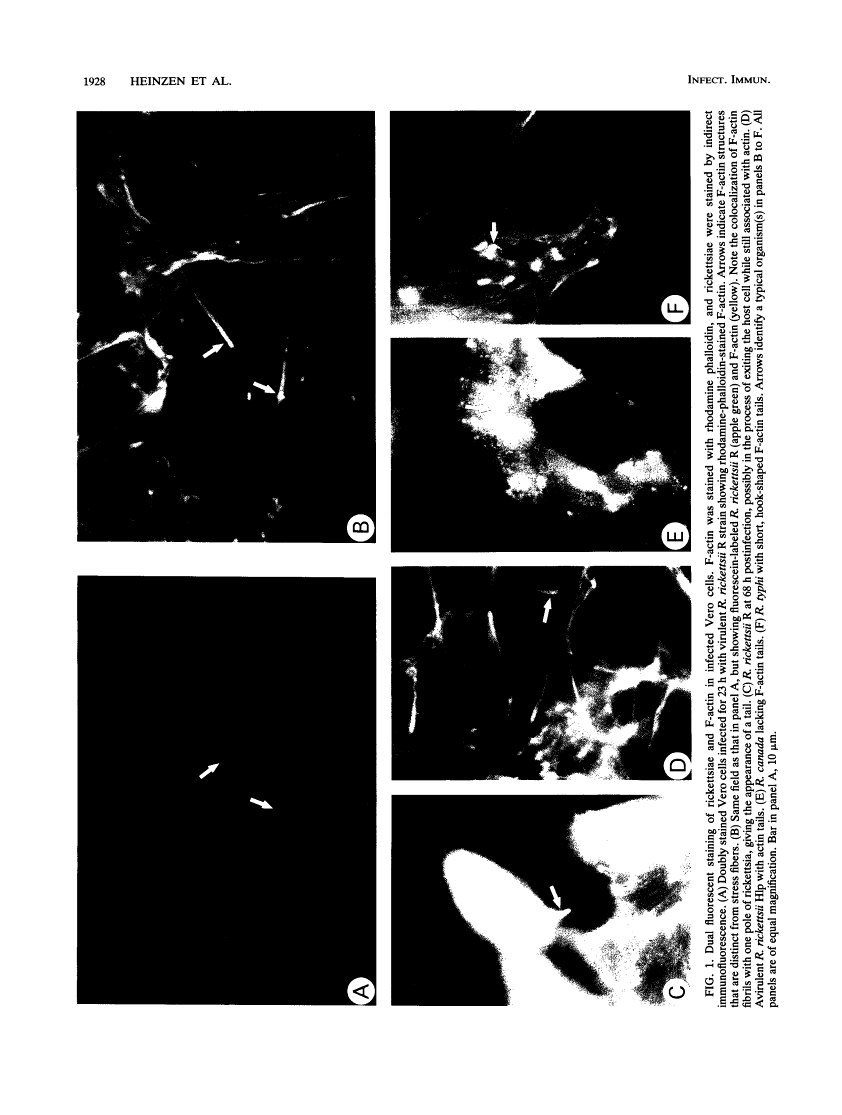
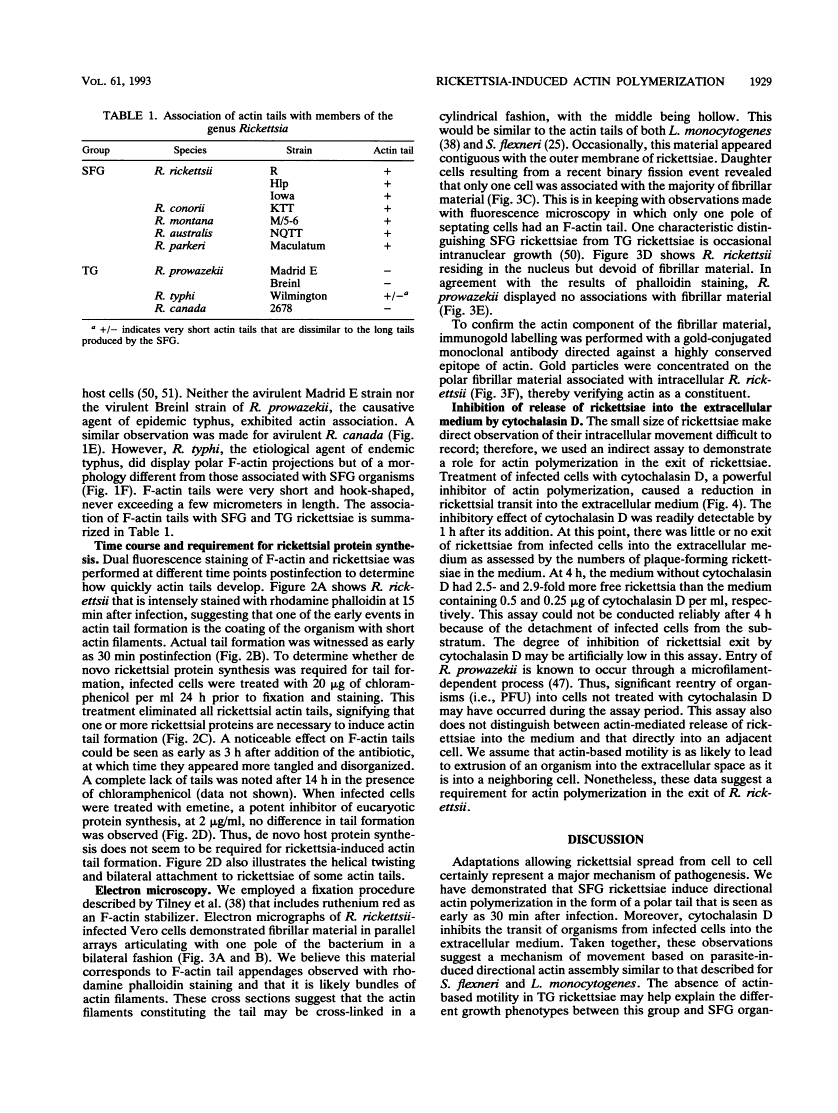
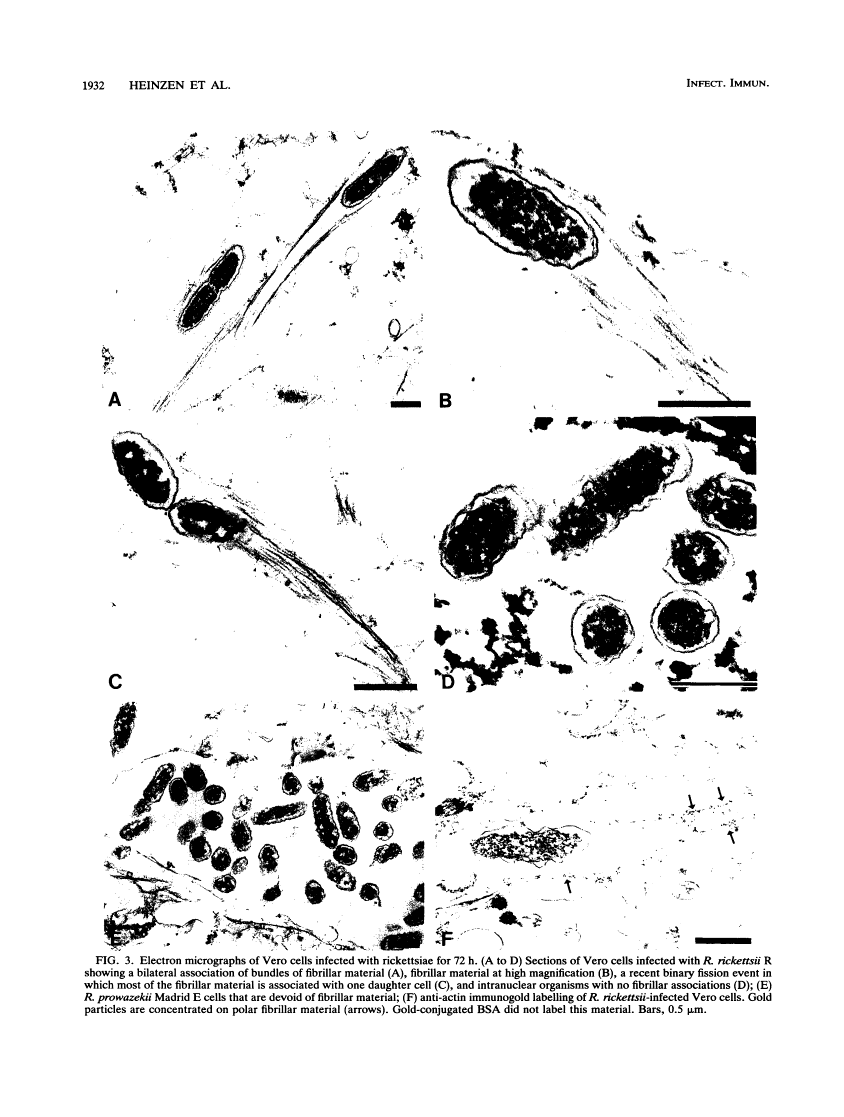
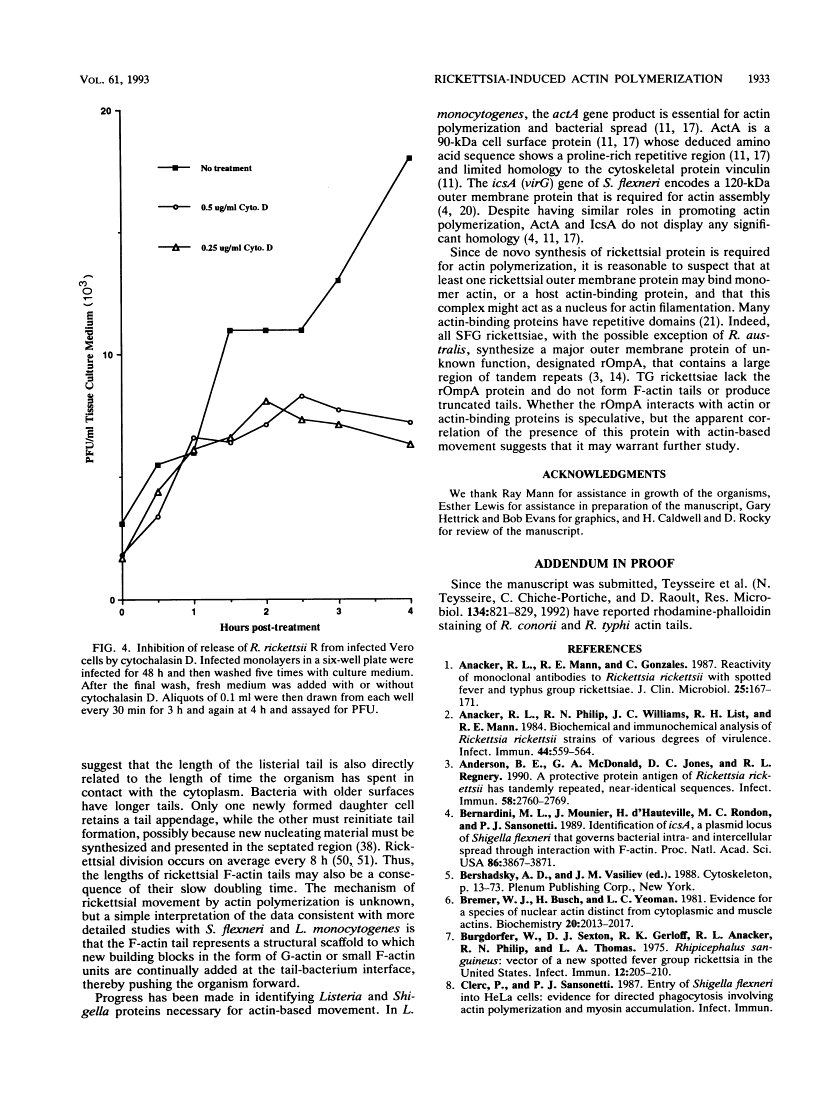
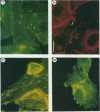
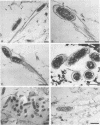
Members of the spotted fever group (SFG) of rickettsiae spread rapidly from cell to cell by an unknown mechanism(s). Staining of Rickettsia rickettsii-infected Vero cells with rhodamine phalloidin demonstrated unique actin filaments associated with one pole of intracellular rickettsiae. F-actin tails greater than 70 microns in length were seen extending from rickettsiae. Treatment of infected cells with chloramphenicol eliminated rickettsia-associated F-actin tails, suggesting that de novo protein synthesis of one or more rickettsial proteins is required for tail formation. Rickettsiae were coated with F-actin as early as 15 min postinfection, and tail formation was detected by 30 min. A survey of virulent and avirulent species within the SFG rickettsiae demonstrated that all formed actin tails. Typhus group rickettsiae, which do not spread directly from cell to cell, lacked F-actin tails entirely or exhibited only very short tails. Transmission electron microscopy demonstrated fibrillar material in close association with R. rickettsii but not Rickettsia prowazekii. Biochemical evidence that actin polymerization plays a role in movement was provided by showing that transit of R. rickettsii from infected cells into the cell culture medium was inhibited by treatment of host cells with cytochalasin D. These data suggest that the cell-to-cell transmission of SFG rickettsiae may be aided by induction of actin polymerization in a fashion similar to that described for Shigella flexneri and Listeria monocytogenes.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker RL, Mann RE, Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987 Jan;25(1):167–171. [Europe PMC free article] [Abstract] [Google Scholar]
- Anacker RL, Philip RN, Williams JC, List RH, Mann RE. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Anderson BE, McDonald GA, Jones DC, Regnery RL. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. [Europe PMC free article] [Abstract] [Google Scholar]
- Bernardini ML, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. [Europe PMC free article] [Abstract] [Google Scholar]
- Bremer JW, Busch H, Yeoman LC. Evidence for a species of nuclear actin distinct from cytoplasmic and muscles actins. Biochemistry. 1981 Mar 31;20(7):2013–2017. [Abstract] [Google Scholar]
- Burgdorfer W, Sexton DJ, Gerloff RK, Anacker RL, Philip RN, Thomas LA. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975 Jul;12(1):205–210. [Europe PMC free article] [Abstract] [Google Scholar]
- Cox HR. CULTIVATION OF RICKETTSIAE OF THE ROCKY MOUNTAIN SPOTTED FEVER, TYPHUS AND Q FEVER GROUPS IN THE EMBRYONIC TISSUES OF DEVELOPING CHICKS. Science. 1941 Oct 31;94(2444):399–403. [Abstract] [Google Scholar]
- Dabiri GA, Sanger JM, Portnoy DA, Southwick FS. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. [Europe PMC free article] [Abstract] [Google Scholar]
- Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wächter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. [Europe PMC free article] [Abstract] [Google Scholar]
- Ewing EP, Jr, Takeuchi A, Shirai A, Osterman JV. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. [Europe PMC free article] [Abstract] [Google Scholar]
- Faulstich H, Zobeley S, Rinnerthaler G, Small JV. Fluorescent phallotoxins as probes for filamentous actin. J Muscle Res Cell Motil. 1988 Oct;9(5):370–383. [Abstract] [Google Scholar]
- Gilmore RD, Jr, Hackstadt T. DNA polymorphism in the conserved 190 kDa antigen gene repeat region among spotted fever group Rickettsiae. Biochim Biophys Acta. 1991 Jul 26;1097(1):77–80. [Abstract] [Google Scholar]
- Hackstadt T, Messer R, Cieplak W, Peacock MG. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect Immun. 1992 Jan;60(1):159–165. [Europe PMC free article] [Abstract] [Google Scholar]
- Havell EA. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. [Europe PMC free article] [Abstract] [Google Scholar]
- Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. [Abstract] [Google Scholar]
- Kokorin IN. Biological peculiarities of the development of rickettsiae. Acta Virol. 1968 Jan;12(1):31–35. [Abstract] [Google Scholar]
- Kuhn M, Prévost MC, Mounier J, Sansonetti PJ. A nonvirulent mutant of Listeria monocytogenes does not move intracellularly but still induces polymerization of actin. Infect Immun. 1990 Nov;58(11):3477–3486. [Europe PMC free article] [Abstract] [Google Scholar]
- Lett MC, Sasakawa C, Okada N, Sakai T, Makino S, Yamada M, Komatsu K, Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989 Jan;171(1):353–359. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991 Mar;16(3):87–92. [Abstract] [Google Scholar]
- Mounier J, Ryter A, Coquis-Rondon M, Sansonetti PJ. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. [Europe PMC free article] [Abstract] [Google Scholar]
- Oaks EV, Wingfield ME, Formal SB. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985 Apr;48(1):124–129. [Europe PMC free article] [Abstract] [Google Scholar]
- Philip RN, Casper EA, Burgdorfer W, Gerloff RK, Hughes LE, Bell EJ. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [Abstract] [Google Scholar]
- Prévost MC, Lesourd M, Arpin M, Vernel F, Mounier J, Hellio R, Sansonetti PJ. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992 Oct;60(10):4088–4099. [Europe PMC free article] [Abstract] [Google Scholar]
- Robertson MN, Miyazawa M, Mori S, Caughey B, Evans LH, Hayes SF, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991 Oct;34(3):255–271. [Abstract] [Google Scholar]
- Robinson EN, Jr, McGee ZA, Kaplan J, Hammond ME, Larson JK, Buchanan TM, Schoolnik GK. Ultrastructural localization of specific gonococcal macromolecules with antibody-gold sphere immunological probes. Infect Immun. 1984 Nov;46(2):361–366. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanger JM, Sanger JW, Southwick FS. Host cell actin assembly is necessary and likely to provide the propulsive force for intracellular movement of Listeria monocytogenes. Infect Immun. 1992 Sep;60(9):3609–3619. [Europe PMC free article] [Abstract] [Google Scholar]
- Sansonetti PJ. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay systems to shigellosis. Curr Top Microbiol Immunol. 1992;180:1–19. [Abstract] [Google Scholar]
- SCHAECHTER M, BOZEMAN FM, SMADEL JE. Study on the growth of Rickettsiae. II. Morphologic observations of living Rickettsiae in tissue culture cells. Virology. 1957 Feb;3(1):160–172. [Abstract] [Google Scholar]
- Silverman DJ, Wisseman CL., Jr In vitro studies of rickettsia-host cell interactions: ultrastructural changes induced by Rickettsia rickettsii infection of chicken embryo fibroblasts. Infect Immun. 1979 Nov;26(2):714–727. [Europe PMC free article] [Abstract] [Google Scholar]
- Silverman DJ, Wisseman CL, Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. [Europe PMC free article] [Abstract] [Google Scholar]
- Silverman DJ, Wisseman CL, Jr, Waddell AD, Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. [Europe PMC free article] [Abstract] [Google Scholar]
- Maupin-Szamier P, Pollard TD. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. [Europe PMC free article] [Abstract] [Google Scholar]
- Theriot JA, Mitchison TJ, Tilney LG, Portnoy DA. The rate of actin-based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992 May 21;357(6375):257–260. [Abstract] [Google Scholar]
- Tilney LG, Connelly PS, Portnoy DA. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol. 1990 Dec;111(6 Pt 2):2979–2988. [Europe PMC free article] [Abstract] [Google Scholar]
- Tilney LG, DeRosier DJ, Tilney MS. How Listeria exploits host cell actin to form its own cytoskeleton. I. Formation of a tail and how that tail might be involved in movement. J Cell Biol. 1992 Jul;118(1):71–81. [Europe PMC free article] [Abstract] [Google Scholar]
- Tilney LG, DeRosier DJ, Weber A, Tilney MS. How Listeria exploits host cell actin to form its own cytoskeleton. II. Nucleation, actin filament polarity, filament assembly, and evidence for a pointed end capper. J Cell Biol. 1992 Jul;118(1):83–93. [Europe PMC free article] [Abstract] [Google Scholar]
- Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. [Europe PMC free article] [Abstract] [Google Scholar]
- Todd WJ, Burgdorfer W, Wray GP. Detection of fibrils associated with Rickettsia rickettsii. Infect Immun. 1983 Sep;41(3):1252–1260. [Europe PMC free article] [Abstract] [Google Scholar]
- Vasselon T, Mounier J, Hellio R, Sansonetti PJ. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992 Mar;60(3):1031–1040. [Europe PMC free article] [Abstract] [Google Scholar]
- Vasselon T, Mounier J, Prevost MC, Hellio R, Sansonetti PJ. Stress fiber-based movement of Shigella flexneri within cells. Infect Immun. 1991 May;59(5):1723–1732. [Europe PMC free article] [Abstract] [Google Scholar]
- Walker TS, Winkler HH. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. [Europe PMC free article] [Abstract] [Google Scholar]
- Wike DA, Tallent G, Peacock MG, Ormsbee RA. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. [Europe PMC free article] [Abstract] [Google Scholar]
- Winkler HH. Rickettsia species (as organisms). Annu Rev Microbiol. 1990;44:131–153. [Abstract] [Google Scholar]
- Wisseman CL, Jr, Edlinger EA, Waddell AD, Jones MR. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976 Oct;14(4):1052–1064. [Europe PMC free article] [Abstract] [Google Scholar]
- Wisseman CL, Jr, Waddell AD. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.61.5.1926-1935.1993
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/61/5/1926.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/61/5/1926
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/61/5/1926
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/134442033
Article citations
Metagenome diversity illuminates the origins of pathogen effectors.
mBio, 15(5):e0075923, 02 Apr 2024
Cited by: 4 articles | PMID: 38564675 | PMCID: PMC11077975
Metabolism and physiology of pathogenic bacterial obligate intracellular parasites.
Front Cell Infect Microbiol, 14:1284701, 22 Mar 2024
Cited by: 0 articles | PMID: 38585652 | PMCID: PMC10995303
Review Free full text in Europe PMC
Pathogenic <i>Rickettsia</i> spp. as emerging models for bacterial biology.
J Bacteriol, 206(2):e0040423, 05 Feb 2024
Cited by: 4 articles | PMID: 38315013 | PMCID: PMC10883807
Review Free full text in Europe PMC
Orientia tsutsugamushi: A life between escapes.
Microbiologyopen, 12(5):e1380, 01 Oct 2023
Cited by: 2 articles | PMID: 37877457 | PMCID: PMC10493369
Identification of an autotransporter peptidase of Rickettsia rickettsii responsible for maturation of surface exposed autotransporters.
PLoS Pathog, 19(7):e1011527, 31 Jul 2023
Cited by: 5 articles | PMID: 37523399 | PMCID: PMC10414592
Go to all (137) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins.
Infect Immun, 68(8):4706-4713, 01 Aug 2000
Cited by: 67 articles | PMID: 10899876 | PMCID: PMC98416
Rickettsial actin-based motility: behavior and involvement of cytoskeletal regulators.
Ann N Y Acad Sci, 990:535-547, 01 Jun 2003
Cited by: 39 articles | PMID: 12860687
Regulator of Actin-Based Motility (RoaM) Downregulates Actin Tail Formation by Rickettsia rickettsii and Is Negatively Selected in Mammalian Cell Culture.
mBio, 13(2):e0035322, 14 Mar 2022
Cited by: 16 articles | PMID: 35285700 | PMCID: PMC9040884
The biology of rickettsiae.
Infect Agents Dis, 5(3):127-143, 01 Jun 1996
Cited by: 92 articles | PMID: 8805076
Review