Abstract
Free full text

Interleukin-6 cDNA transfected Lewis lung carcinoma cells show unaltered net tumour growth rate but cause weight loss and shortened survival in syngeneic mice.
Abstract
HuIL-6 cDNA, cloned into a neomycin resistant conferring expression vector, BMGNeo, was transfected into Lewis Lung Carcinoma (LLC) cells. LLC cells (5 x 10(6) ml-1) transfected with IL-6 cDNA (LLC-IL6) secreted IL-6 into the culture supernatant at a concentration of 9.9 ng ml-1 within 48 h. When 1,000,000 of untransfected LLC, BMGNeo vector transfected LLC (LLC-Neo) or LLC-IL6 cells were transplanted into C57BL/6 mice subcutaneously, the mean +/- s.d. of survival times of these mice were 33.3 +/- 9.7, 34.3 +/- 7.1 and 17.0 +/- 3.1 days, respectively. The survival time of LLC-IL6 cells transplanted mice was significantly shorter than that of LLC (P < 0.01) or LLC-Neo (P < 0.01) cells transplanted mice without a measurable difference of tumour size. Plasma concentration of IL-6 steadily increased in LLC-IL6 transplanted mice. Body weight and serum albumin were significantly lower in LLC-IL6 transplanted mice than in LLC transplanted mice. Mouse IL-1 alpha and mouse TNF-alpha were not detected in the plasma of LLC-IL6 transplanted mice. These data suggested that secretion of IL-6 from LLC cells was unable to alter net tumour growth rate but rather caused a state similar to cachexia without detectable increase of IL-1 alpha and TNF-alpha in the plasma. This state may be responsible for the shortened survival of LLC-IL6 tumour-bearing mice.
Full text
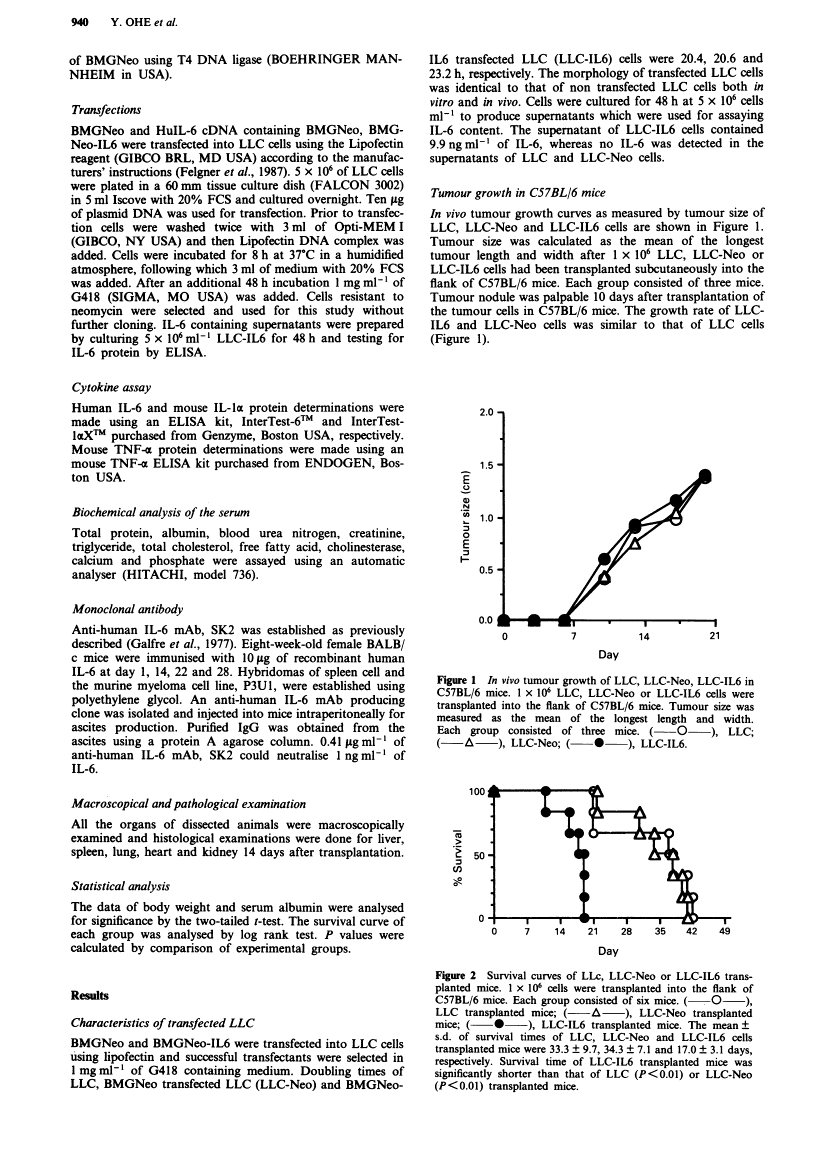
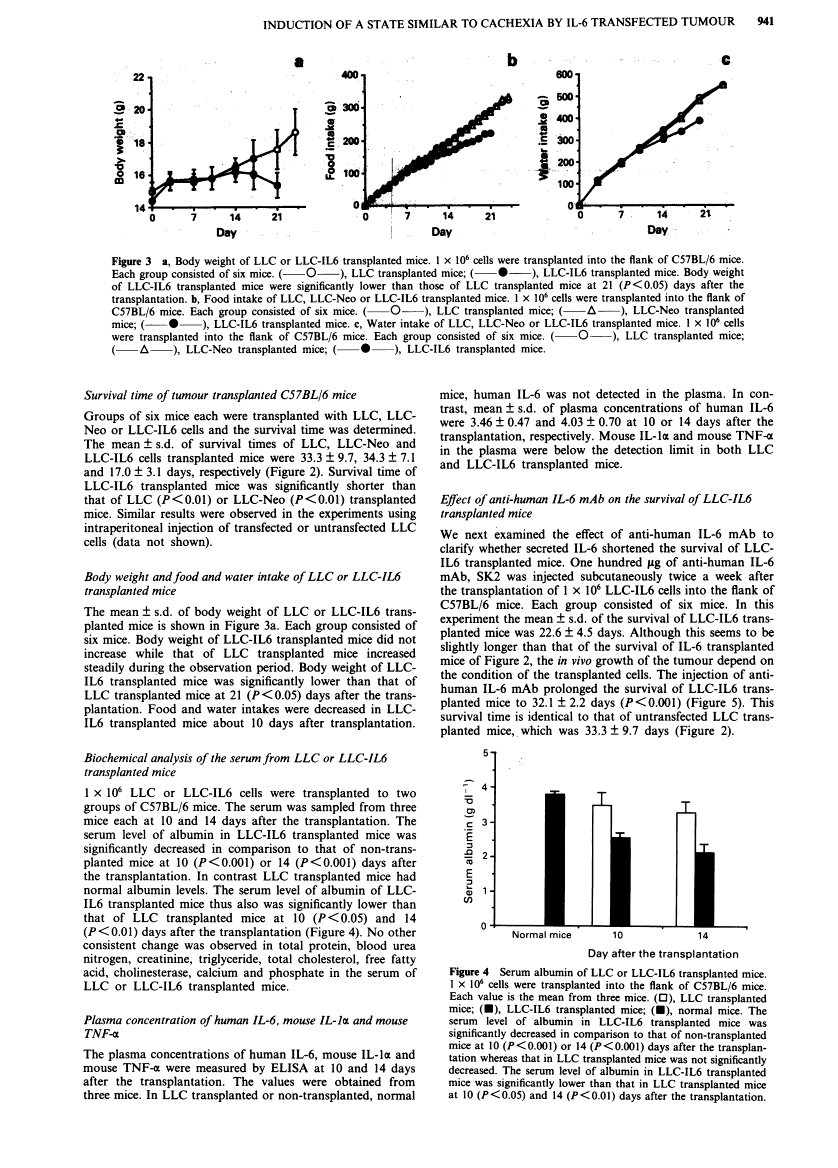
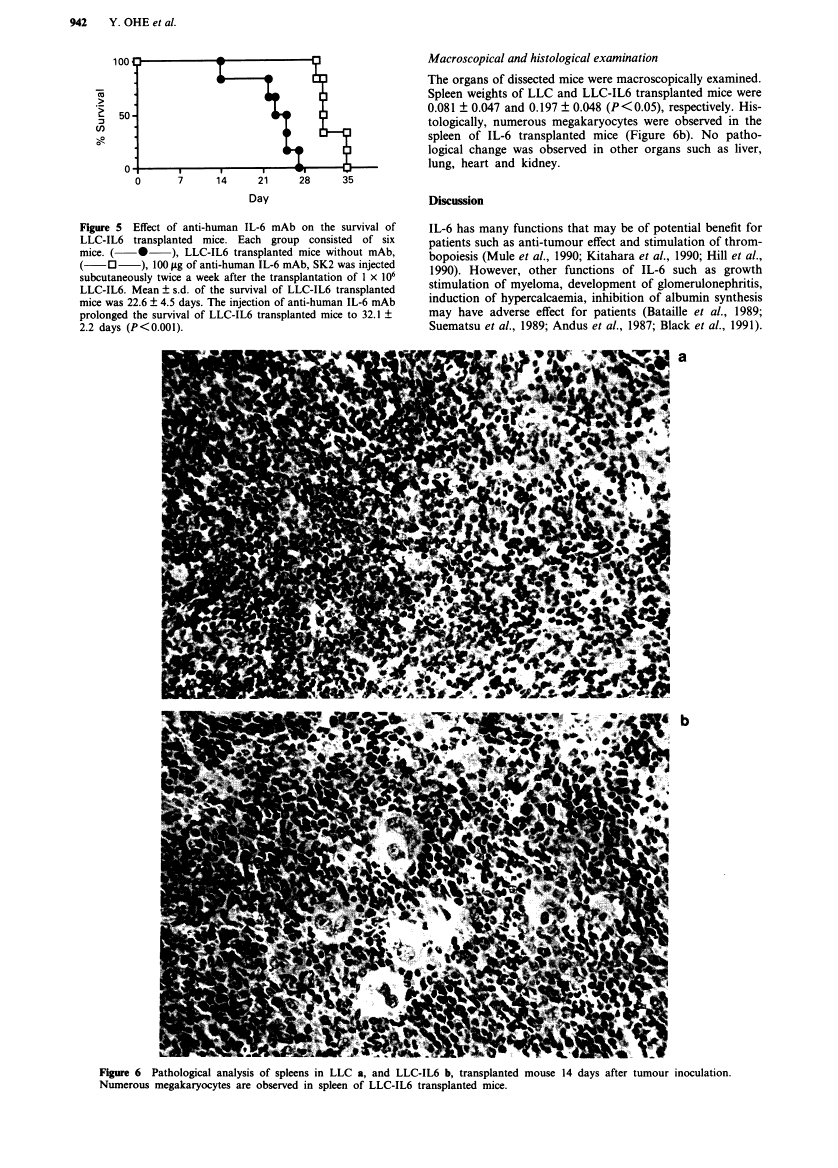
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T, Geiger T, Hirano T, Northoff H, Ganter U, Bauer J, Kishimoto T, Heinrich PC. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987 Aug 31;221(1):18–22. [Abstract] [Google Scholar]
- Asher AL, Mulé JJ, Kasid A, Restifo NP, Salo JC, Reichert CM, Jaffe G, Fendly B, Kriegler M, Rosenberg SA. Murine tumor cells transduced with the gene for tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991 May 1;146(9):3227–3234. [Europe PMC free article] [Abstract] [Google Scholar]
- Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989 Dec;84(6):2008–2011. [Europe PMC free article] [Abstract] [Google Scholar]
- Black K, Garrett IR, Mundy GR. Chinese hamster ovarian cells transfected with the murine interleukin-6 gene cause hypercalcemia as well as cachexia, leukocytosis and thrombocytosis in tumor-bearing nude mice. Endocrinology. 1991 May;128(5):2657–2659. [Abstract] [Google Scholar]
- Blankenstein T, Qin ZH, Uberla K, Müller W, Rosen H, Volk HD, Diamantstein T. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991 May 1;173(5):1047–1052. [Europe PMC free article] [Abstract] [Google Scholar]
- Colombo MP, Ferrari G, Stoppacciaro A, Parenza M, Rodolfo M, Mavilio F, Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991 Apr 1;173(4):889–897. [Europe PMC free article] [Abstract] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. [Europe PMC free article] [Abstract] [Google Scholar]
- Galfre G, Howe SC, Milstein C, Butcher GW, Howard JC. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. [Abstract] [Google Scholar]
- Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990 Dec 15;50(24):7820–7825. [Abstract] [Google Scholar]
- Gelin J, Moldawer LL, Lönnroth C, Sherry B, Chizzonite R, Lundholm K. Role of endogenous tumor necrosis factor alpha and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991 Jan 1;51(1):415–421. [Abstract] [Google Scholar]
- Hill RJ, Warren MK, Levin J. Stimulation of thrombopoiesis in mice by human recombinant interleukin 6. J Clin Invest. 1990 Apr;85(4):1242–1247. [Europe PMC free article] [Abstract] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. [Abstract] [Google Scholar]
- Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. [Abstract] [Google Scholar]
- Kitahara M, Kishimoto S, Hirano T, Kishimoto T, Okada M. The in vivo anti-tumor effect of human recombinant interleukin-6. Jpn J Cancer Res. 1990 Oct;81(10):1032–1038. [Europe PMC free article] [Abstract] [Google Scholar]
- Lönnroth C, Moldawer LL, Gelin J, Kindblom L, Sherry B, Lundholm K. Tumor necrosis factor-alpha and interleukin-1 alpha production in cachectic, tumor-bearing mice. Int J Cancer. 1990 Nov 15;46(5):889–896. [Abstract] [Google Scholar]
- Luger TA, Krutmann J, Kirnbauer R, Urbanski A, Schwarz T, Klappacher G, Köck A, Micksche M, Malejczyk J, Schauer E, et al. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J Immunol. 1989 Aug 15;143(4):1206–1209. [Abstract] [Google Scholar]
- Merriman RL, Shackelford KA, Tanzer LR, Campbell JB, Bemis KG, Matsumoto K. Drug treatments for metastasis of the Lewis lung carcinoma: lack of correlation between inhibition of lung metastasis and survival. Cancer Res. 1989 Aug 15;49(16):4509–4516. [Abstract] [Google Scholar]
- Mizuno M, Yoshida J, Sugita K, Inoue I, Seo H, Hayashi Y, Koshizaka T, Yagi K. Growth inhibition of glioma cells transfected with the human beta-interferon gene by liposomes coupled with a monoclonal antibody. Cancer Res. 1990 Dec 15;50(24):7826–7829. [Abstract] [Google Scholar]
- Mulé JJ, McIntosh JK, Jablons DM, Rosenberg SA. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990 Mar 1;171(3):629–636. [Europe PMC free article] [Abstract] [Google Scholar]
- Oliff A, Defeo-Jones D, Boyer M, Martinez D, Kiefer D, Vuocolo G, Wolfe A, Socher SH. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell. 1987 Aug 14;50(4):555–563. [Abstract] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. [Abstract] [Google Scholar]
- Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7547–7551. [Europe PMC free article] [Abstract] [Google Scholar]
- SUGIURA K, STOCK CC. Studies in a tumor spectrum. III. The effect of phosphoramides on the growth of a variety of mouse and rat tumors. Cancer Res. 1955 Jan;15(1):38–51. [Abstract] [Google Scholar]
- Teng MN, Park BH, Koeppen HK, Tracey KJ, Fendly BM, Schreiber H. Long-term inhibition of tumor growth by tumor necrosis factor in the absence of cachexia or T-cell immunity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3535–3539. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from British Journal of Cancer are provided here courtesy of Cancer Research UK
Full text links
Read article at publisher's site: https://doi.org/10.1038/bjc.1993.174
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1968462?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Prognostic Significance of Serum Interleukin-6 Levels in Oral Squamous Cell Carcinoma.
Cureus, 16(2):e54439, 19 Feb 2024
Cited by: 0 articles | PMID: 38510850 | PMCID: PMC10951754
Platelet status in cancer cachexia progression in ApcMin/+ mice.
Front Immunol, 14:1253587, 28 Aug 2023
Cited by: 0 articles | PMID: 37701438 | PMCID: PMC10493779
Cachexia: A systemic consequence of progressive, unresolved disease.
Cell, 186(9):1824-1845, 01 Apr 2023
Cited by: 32 articles | PMID: 37116469
Review
LP07 and LLC preclinical models of lung cancer induce divergent anabolic deficits and expression of pro-inflammatory effectors of muscle wasting.
J Appl Physiol (1985), 133(6):1260-1272, 06 Oct 2022
Cited by: 6 articles | PMID: 36201324 | PMCID: PMC9678411
Cancer-Related Cachexia: The Vicious Circle between Inflammatory Cytokines, Skeletal Muscle, Lipid Metabolism and the Possible Role of Physical Training.
Int J Mol Sci, 23(6):3004, 10 Mar 2022
Cited by: 9 articles | PMID: 35328423 | PMCID: PMC8949960
Review Free full text in Europe PMC
Go to all (50) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Induction of tumor immunity by cytokine cDNA transfected Lewis lung carcinoma].
Nihon Kyobu Shikkan Gakkai Zasshi, 30 Suppl:48-51, 01 Dec 1992
Cited by: 2 articles | PMID: 1306238
Gene therapy for Lewis lung carcinoma with tumor necrosis factor and interleukin 2 cDNAs co-transfected subline.
Gene Ther, 1(4):269-275, 01 Jul 1994
Cited by: 7 articles | PMID: 7584091
Improvement by eicosanoids in cancer cachexia induced by LLC-IL6 transplantation.
J Cancer Res Clin Oncol, 122(12):711-715, 01 Jan 1996
Cited by: 11 articles | PMID: 8954167
Enhanced antitumor activities of TZT-1027 against TNF-alpha or IL-6 secreting Lewis lung carcinoma in vivo.
Cancer Chemother Pharmacol, 49(1):35-47, 01 Jan 2002
Cited by: 7 articles | PMID: 11855751