Abstract
Free full text

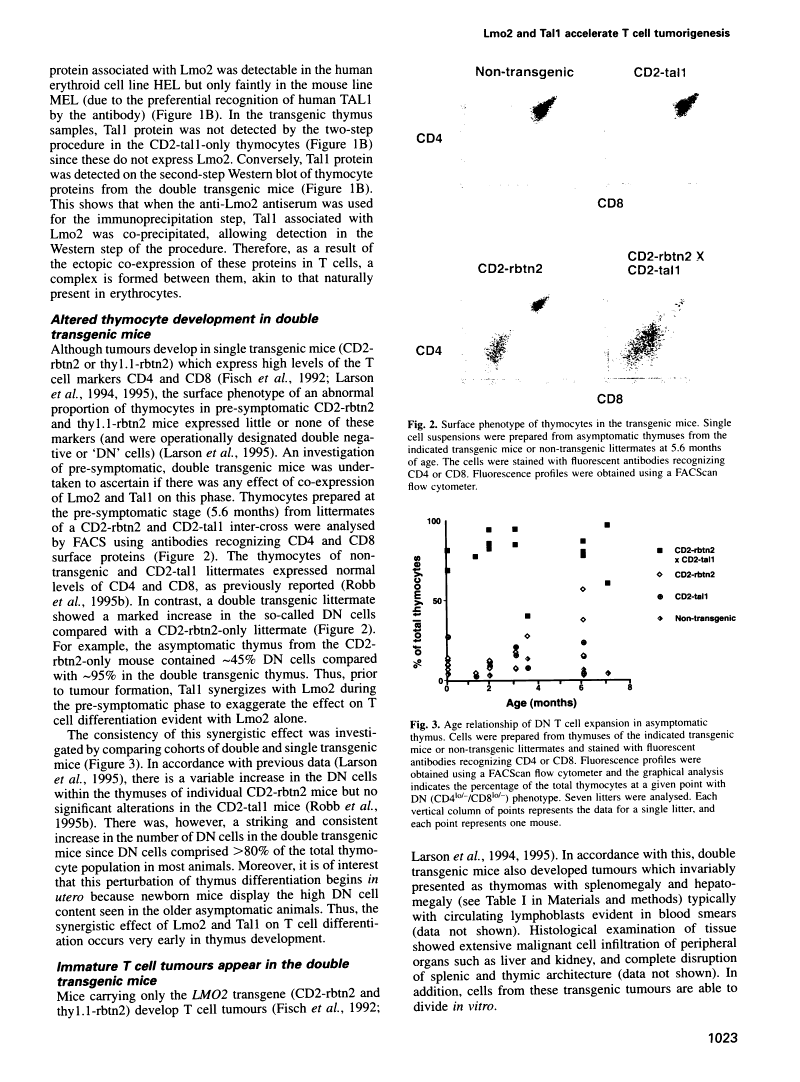
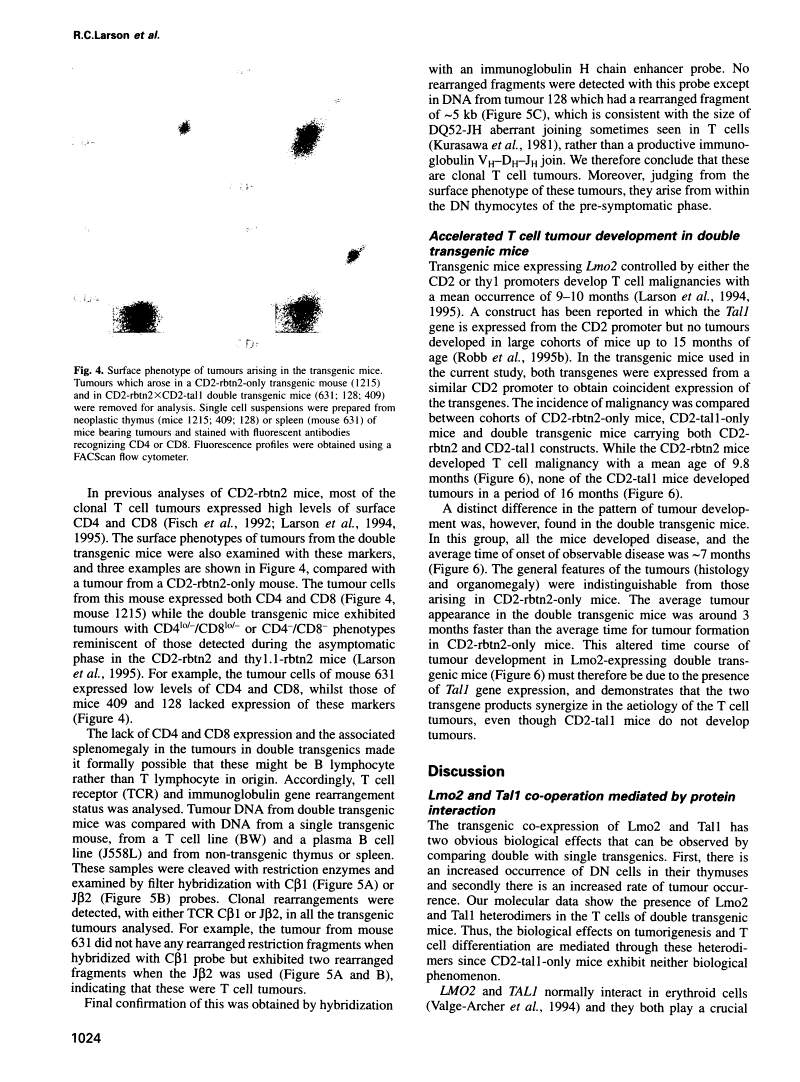
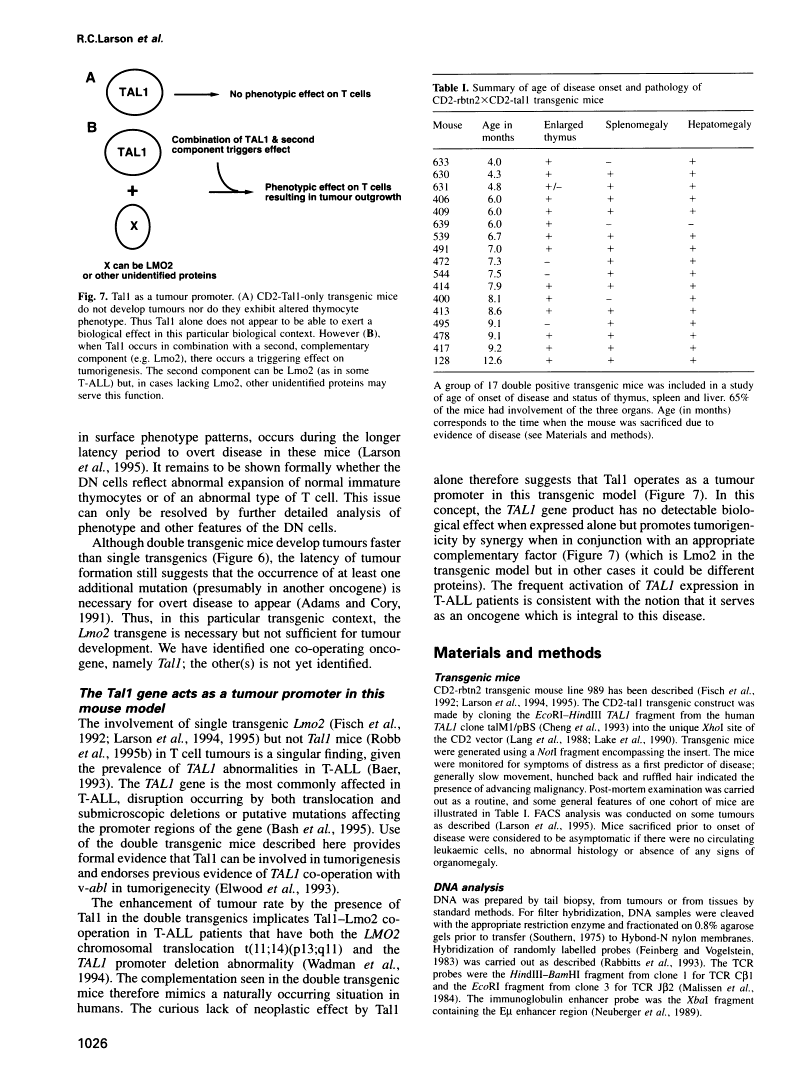
Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice.
Abstract
The LMO2 and TAL1 genes were first identified via chromosomal translocations and later found to encode proteins that interact during normal erythroid development. Some T cell leukaemia patients have chromosomal abnormalities involving both genes, implying that LMO2 and TAL1 act synergistically to promote tumorigenesis after their inappropriate co-expression. To test this hypothesis, transgenic mice were made which co-express Lmo2 and Tal1 genes in T cells. Dimers of Lmo2 and Tal1 proteins were formed in thymocytes of double but not single transgenic mice. Furthermore, thymuses of double transgenic mice were almost completely populated by immature T cells from birth, and these mice develop T cell tumours approximately 3 months earlier than those with only the Lmo2 transgene. Thus interaction between these two proteins can alter T cell development and potentiate tumorigenesis. The data also provide formal proof that TAL1 is an oncogene, apparently acting as a tumour promoter in this system.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams JM, Cory S. Transgenic models of tumor development. Science. 1991 Nov 22;254(5035):1161–1167. [Abstract] [Google Scholar]
- Aplan PD, Lombardi DP, Kirsch IR. Structural characterization of SIL, a gene frequently disrupted in T-cell acute lymphoblastic leukemia. Mol Cell Biol. 1991 Nov;11(11):5462–5469. [Europe PMC free article] [Abstract] [Google Scholar]
- Aplan PD, Lombardi DP, Reaman GH, Sather HN, Hammond GD, Kirsch IR. Involvement of the putative hematopoietic transcription factor SCL in T-cell acute lymphoblastic leukemia. Blood. 1992 Mar 1;79(5):1327–1333. [Abstract] [Google Scholar]
- Baer R. TAL1, TAL2 and LYL1: a family of basic helix-loop-helix proteins implicated in T cell acute leukaemia. Semin Cancer Biol. 1993 Dec;4(6):341–347. [Abstract] [Google Scholar]
- Bash RO, Hall S, Timmons CF, Crist WM, Amylon M, Smith RG, Baer R. Does activation of the TAL1 gene occur in a majority of patients with T-cell acute lymphoblastic leukemia? A pediatric oncology group study. Blood. 1995 Jul 15;86(2):666–676. [Abstract] [Google Scholar]
- Begley CG, Aplan PD, Denning SM, Haynes BF, Waldmann TA, Kirsch IR. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. [Europe PMC free article] [Abstract] [Google Scholar]
- Bernard O, Guglielmi P, Jonveaux P, Cherif D, Gisselbrecht S, Mauchauffe M, Berger R, Larsen CJ, Mathieu-Mahul D. Two distinct mechanisms for the SCL gene activation in the t(1;14) translocation of T-cell leukemias. Genes Chromosomes Cancer. 1990 Jan;1(3):194–208. [Abstract] [Google Scholar]
- Bernard O, Lecointe N, Jonveaux P, Souyri M, Mauchauffé M, Berger R, Larsen CJ, Mathieu-Mahul D. Two site-specific deletions and t(1;14) translocation restricted to human T-cell acute leukemias disrupt the 5' part of the tal-1 gene. Oncogene. 1991 Aug;6(8):1477–1488. [Abstract] [Google Scholar]
- Boehm T, Foroni L, Kennedy M, Rabbitts TH. The rhombotin gene belongs to a class of transcriptional regulators with a potential novel protein dimerisation motif. Oncogene. 1990 Jul;5(7):1103–1105. [Abstract] [Google Scholar]
- Boehm T, Foroni L, Kaneko Y, Perutz MF, Rabbitts TH. The rhombotin family of cysteine-rich LIM-domain oncogenes: distinct members are involved in T-cell translocations to human chromosomes 11p15 and 11p13. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4367–4371. [Europe PMC free article] [Abstract] [Google Scholar]
- Breit TM, Mol EJ, Wolvers-Tettero IL, Ludwig WD, van Wering ER, van Dongen JJ. Site-specific deletions involving the tal-1 and sil genes are restricted to cells of the T cell receptor alpha/beta lineage: T cell receptor delta gene deletion mechanism affects multiple genes. J Exp Med. 1993 Apr 1;177(4):965–977. [Europe PMC free article] [Abstract] [Google Scholar]
- Brown L, Cheng JT, Chen Q, Siciliano MJ, Crist W, Buchanan G, Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. [Europe PMC free article] [Abstract] [Google Scholar]
- Carroll AJ, Crist WM, Link MP, Amylon MD, Pullen DJ, Ragab AH, Buchanan GR, Wimmer RS, Vietti TJ. The t(1;14)(p34;q11) is nonrandom and restricted to T-cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1990 Sep 15;76(6):1220–1224. [Abstract] [Google Scholar]
- Cheng JT, Hsu HL, Hwang LY, Baer R. Products of the TAL1 oncogene: basic helix-loop-helix proteins phosphorylated at serine residues. Oncogene. 1993 Mar;8(3):677–683. [Abstract] [Google Scholar]
- Elwood NJ, Cook WD, Metcalf D, Begley CG. SCL, the gene implicated in human T-cell leukaemia, is oncogenic in a murine T-lymphocyte cell line. Oncogene. 1993 Nov;8(11):3093–3101. [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. [Abstract] [Google Scholar]
- Finger LR, Kagan J, Christopher G, Kurtzberg J, Hershfield MS, Nowell PC, Croce CM. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5039–5043. [Europe PMC free article] [Abstract] [Google Scholar]
- Fisch P, Boehm T, Lavenir I, Larson T, Arno J, Forster A, Rabbitts TH. T-cell acute lymphoblastic lymphoma induced in transgenic mice by the RBTN1 and RBTN2 LIM-domain genes. Oncogene. 1992 Dec;7(12):2389–2397. [Abstract] [Google Scholar]
- Hsu HL, Cheng JT, Chen Q, Baer R. Enhancer-binding activity of the tal-1 oncoprotein in association with the E47/E12 helix-loop-helix proteins. Mol Cell Biol. 1991 Jun;11(6):3037–3042. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu HL, Huang L, Tsan JT, Funk W, Wright WE, Hu JS, Kingston RE, Baer R. Preferred sequences for DNA recognition by the TAL1 helix-loop-helix proteins. Mol Cell Biol. 1994 Feb;14(2):1256–1265. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu HL, Wadman I, Baer R. Formation of in vivo complexes between the TAL1 and E2A polypeptides of leukemic T cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3181–3185. [Europe PMC free article] [Abstract] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. [Abstract] [Google Scholar]
- Kurosawa Y, von Boehmer H, Haas W, Sakano H, Trauneker A, Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. [Abstract] [Google Scholar]
- Lake RA, Wotton D, Owen MJ. A 3' transcriptional enhancer regulates tissue-specific expression of the human CD2 gene. EMBO J. 1990 Oct;9(10):3129–3136. [Europe PMC free article] [Abstract] [Google Scholar]
- Lang G, Wotton D, Owen MJ, Sewell WA, Brown MH, Mason DY, Crumpton MJ, Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 1988 Jun;7(6):1675–1682. [Europe PMC free article] [Abstract] [Google Scholar]
- Larson RC, Fisch P, Larson TA, Lavenir I, Langford T, King G, Rabbitts TH. T cell tumours of disparate phenotype in mice transgenic for Rbtn-2. Oncogene. 1994 Dec;9(12):3675–3681. [Abstract] [Google Scholar]
- Larson RC, Osada H, Larson TA, Lavenir I, Rabbitts TH. The oncogenic LIM protein Rbtn2 causes thymic developmental aberrations that precede malignancy in transgenic mice. Oncogene. 1995 Sep 7;11(5):853–862. [Abstract] [Google Scholar]
- Malissen M, Minard K, Mjolsness S, Kronenberg M, Goverman J, Hunkapiller T, Prystowsky MB, Yoshikai Y, Fitch F, Mak TW, et al. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. [Abstract] [Google Scholar]
- Neuberger MS, Caskey HM, Pettersson S, Williams GT, Surani MA. Isotype exclusion and transgene down-regulation in immunoglobulin-lambda transgenic mice. Nature. 1989 Mar 23;338(6213):350–352. [Abstract] [Google Scholar]
- Omichinski JG, Clore GM, Schaad O, Felsenfeld G, Trainor C, Appella E, Stahl SJ, Gronenborn AM. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993 Jul 23;261(5120):438–446. [Abstract] [Google Scholar]
- Pérez-Alvarado GC, Miles C, Michelsen JW, Louis HA, Winge DR, Beckerle MC, Summers MF. Structure of the carboxy-terminal LIM domain from the cysteine rich protein CRP. Nat Struct Biol. 1994 Jun;1(6):388–398. [Abstract] [Google Scholar]
- Pulford K, Lecointe N, Leroy-Viard K, Jones M, Mathieu-Mahul D, Mason DY. Expression of TAL-1 proteins in human tissues. Blood. 1995 Feb 1;85(3):675–684. [Abstract] [Google Scholar]
- Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994 Nov 10;372(6502):143–149. [Abstract] [Google Scholar]
- Rabbitts TH, Forster A, Larson R, Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet. 1993 Jun;4(2):175–180. [Abstract] [Google Scholar]
- Robb L, Lyons I, Li R, Hartley L, Köntgen F, Harvey RP, Metcalf D, Begley CG. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):7075–7079. [Europe PMC free article] [Abstract] [Google Scholar]
- Robb L, Rasko JE, Bath ML, Strasser A, Begley CG. scl, a gene frequently activated in human T cell leukaemia, does not induce lymphomas in transgenic mice. Oncogene. 1995 Jan 5;10(1):205–209. [Abstract] [Google Scholar]
- Royer-Pokora B, Loos U, Ludwig WD. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11). Oncogene. 1991 Oct;6(10):1887–1893. [Abstract] [Google Scholar]
- Sánchez-García I, Rabbitts TH. The LIM domain: a new structural motif found in zinc-finger-like proteins. Trends Genet. 1994 Sep;10(9):315–320. [Abstract] [Google Scholar]
- Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995 Feb 2;373(6513):432–434. [Abstract] [Google Scholar]
- Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. [Abstract] [Google Scholar]
- Valge-Archer VE, Osada H, Warren AJ, Forster A, Li J, Baer R, Rabbitts TH. The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8617–8621. [Europe PMC free article] [Abstract] [Google Scholar]
- Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH, Baer R. Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J. 1994 Oct 17;13(20):4831–4839. [Europe PMC free article] [Abstract] [Google Scholar]
- Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994 Jul 15;78(1):45–57. [Abstract] [Google Scholar]
- Chen Q, Cheng JT, Tasi LH, Schneider N, Buchanan G, Carroll A, Crist W, Ozanne B, Siciliano MJ, Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1996.tb00439.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc449997?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1996.tb00439.x
Article citations
LMO3 is a suppressor of the basal-like/squamous subtype and reduces disease aggressiveness of pancreatic cancer through glycerol 3-phosphate metabolism.
Carcinogenesis, 45(7):475-486, 01 Jul 2024
Cited by: 2 articles | PMID: 38366633
The role of quiescent thymic progenitors in TAL/LMO2-induced T-ALL chemotolerance.
Leukemia, 38(5):951-962, 29 Mar 2024
Cited by: 1 article | PMID: 38553571 | PMCID: PMC11073972
TAL1 cooperates with PI3K/AKT pathway activation in T-cell acute lymphoblastic leukemia.
Haematologica, 107(10):2304-2317, 01 Oct 2022
Cited by: 4 articles | PMID: 35354248
Overexpression of wild-type IL-7Rα promotes T-cell acute lymphoblastic leukemia/lymphoma.
Blood, 138(12):1040-1052, 01 Sep 2021
Cited by: 22 articles | PMID: 33970999 | PMCID: PMC8462360
T-Cell Lymphoblastic Lymphoma Arising in the Setting of Myeloid/Lymphoid Neoplasms with Eosinophilia: LMO2 Immunohistochemistry as a Potentially Useful Diagnostic Marker.
Cancers (Basel), 13(12):3102, 21 Jun 2021
Cited by: 5 articles | PMID: 34205834 | PMCID: PMC8234657
Go to all (120) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Chromosomal translocations and leukaemia: a role for LMO2 in T cell acute leukaemia, in transcription and in erythropoiesis.
Leukemia, 11 Suppl 3:271-272, 01 Apr 1997
Cited by: 10 articles | PMID: 9209362
A DNA-binding mutant of TAL1 cooperates with LMO2 to cause T cell leukemia in mice.
Oncogene, 30(10):1252-1260, 08 Nov 2010
Cited by: 26 articles | PMID: 21057528 | PMCID: PMC3691994
Structure of the leukemia oncogene LMO2: implications for the assembly of a hematopoietic transcription factor complex.
Blood, 117(7):2146-2156, 12 Nov 2010
Cited by: 52 articles | PMID: 21076045
LIM domain proteins in leukaemia and development.
Semin Cancer Biol, 4(6):349-358, 01 Dec 1993
Cited by: 35 articles | PMID: 8142620
Review