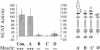Abstract
Free full text

Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain.
Abstract
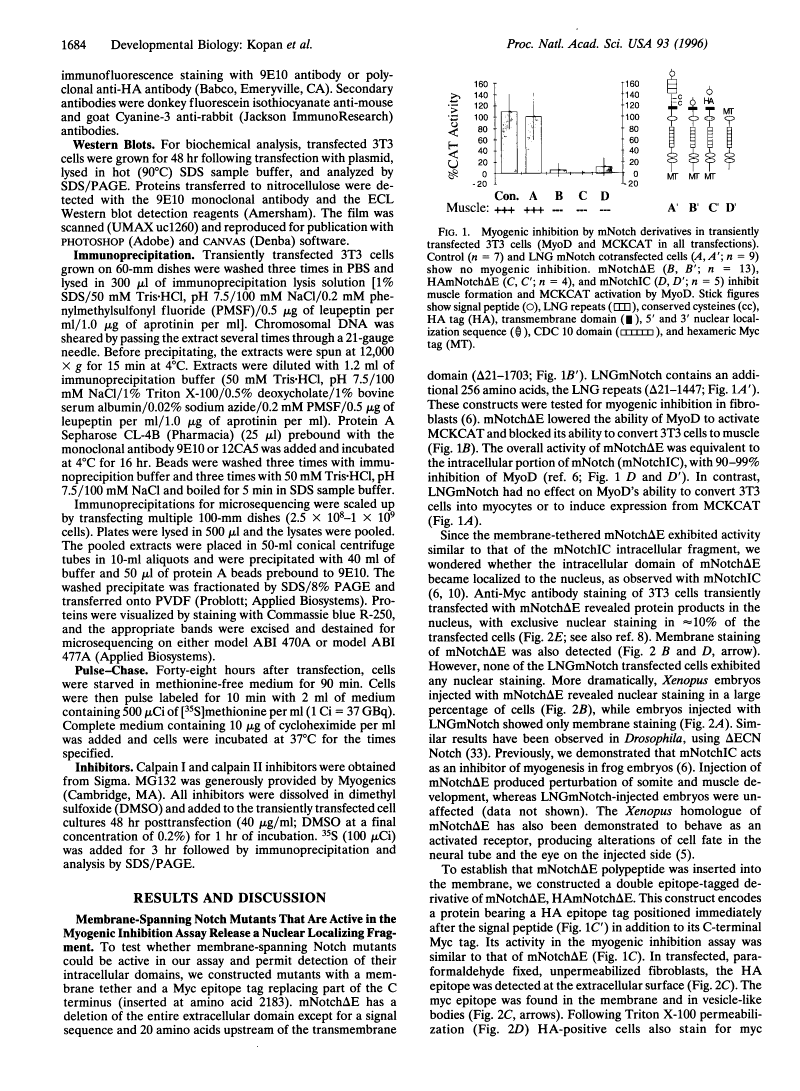
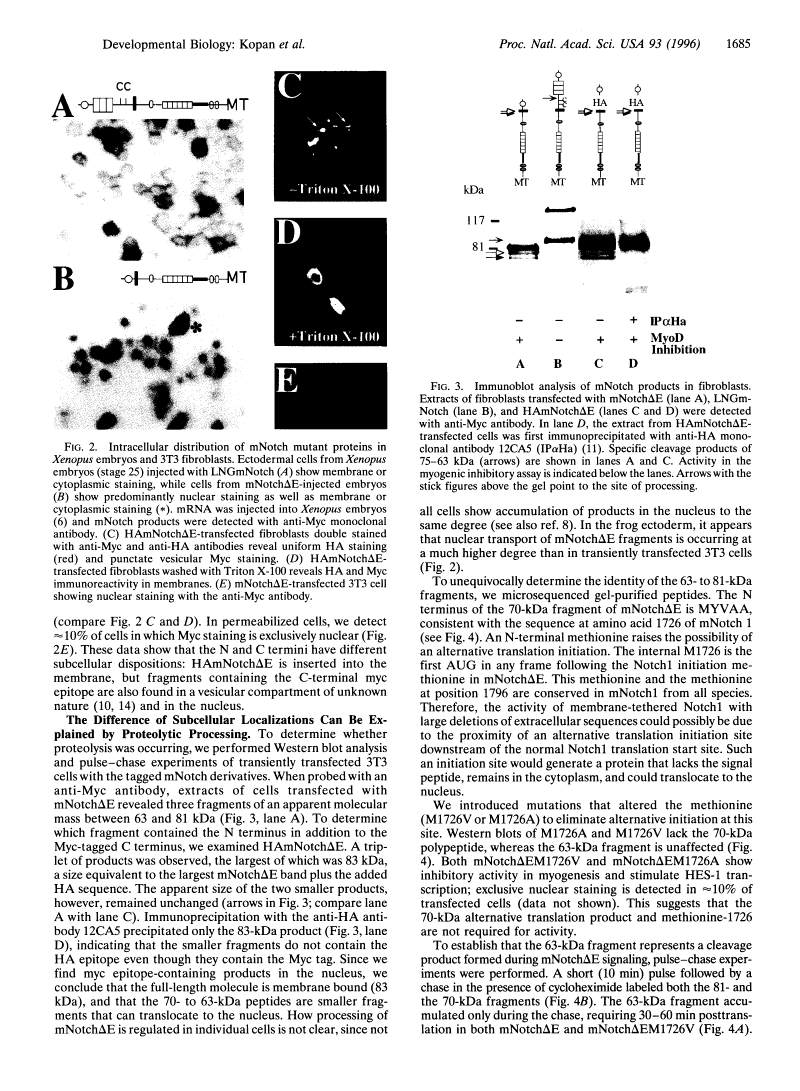
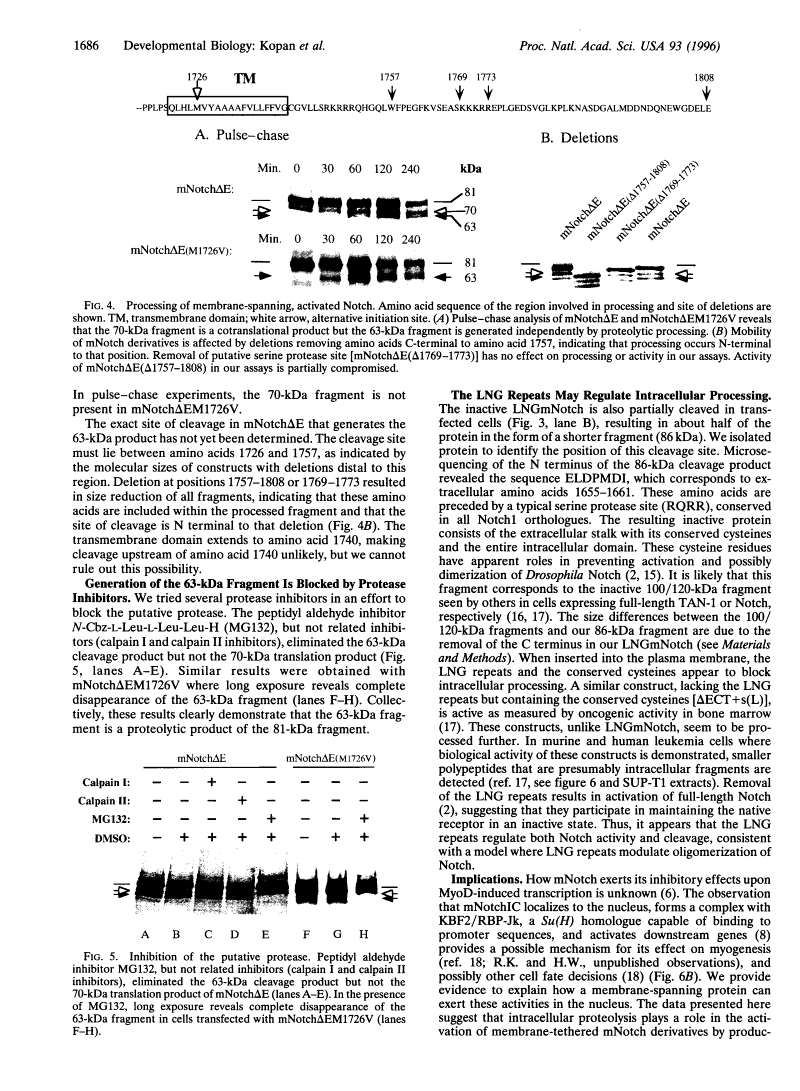
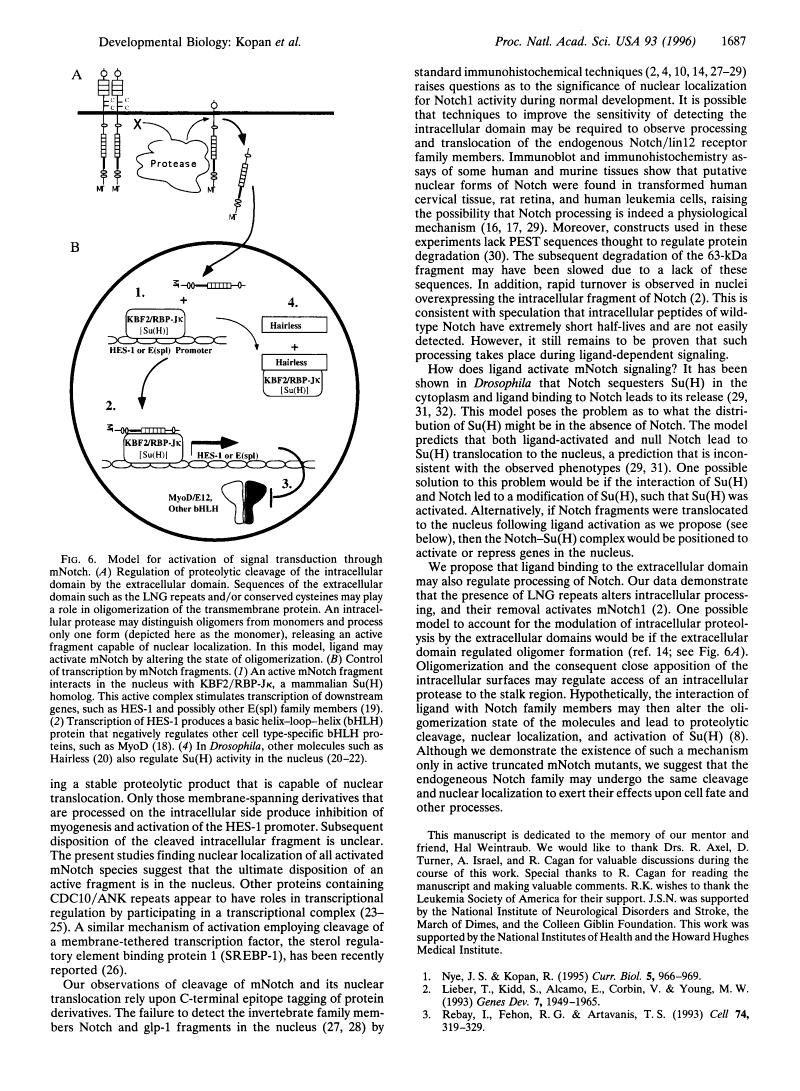
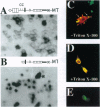
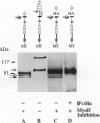
Previous studies imply that the intracellular domain of Notch1 must translocate to the nucleus for its activity. In this study, we demonstrate that a mNotch1 mutant protein that lacks its extracellular domain but retains its membrane-spanning region becomes proteolytically processed on its intracellular surface and, as a result, the activated intracellular domain (mNotchIC) is released and can move to the nucleus. Proteolytic cleavage at an intracellular site is blocked by protease inhibitors. Intracellular cleavage is not seen in cells transfected with an inactive variant, which includes the extracellular lin-Notch-glp repeats. Collectively, the studies presented here support the model that mNotch1 is proteolytically processed and the cleavage product is translocated to the nucleus for mNotch1 signal transduction.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Nye JS, Kopan R. Developmental signaling. Vertebrate ligands for Notch. Curr Biol. 1995 Sep 1;5(9):966–969. [Abstract] [Google Scholar]
- Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993 Oct;7(10):1949–1965. [Abstract] [Google Scholar]
- Rebay I, Fehon RG, Artavanis-Tsakonas S. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993 Jul 30;74(2):319–329. [Abstract] [Google Scholar]
- Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993 Oct 7;365(6446):555–557. [Abstract] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993 May 21;73(4):659–671. [Abstract] [Google Scholar]
- Kopan R, Nye JS, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994 Sep;120(9):2385–2396. [Abstract] [Google Scholar]
- Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993 Jul 30;74(2):331–345. [Abstract] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995 Sep 28;377(6547):355–358. [Abstract] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994 Jun 15;8(12):1434–1447. [Abstract] [Google Scholar]
- Nye JS, Kopan R, Axel R. An activated Notch suppresses neurogenesis and myogenesis but not gliogenesis in mammalian cells. Development. 1994 Sep;120(9):2421–2430. [Abstract] [Google Scholar]
- Pati UK. Novel vectors for expression of cDNA encoding epitope-tagged proteins in mammalian cells. Gene. 1992 May 15;114(2):285–288. [Abstract] [Google Scholar]
- Del Amo FF, Smith DE, Swiatek PJ, Gendron-Maguire M, Greenspan RJ, McMahon AP, Gridley T. Expression pattern of Motch, a mouse homolog of Drosophila Notch, suggests an important role in early postimplantation mouse development. Development. 1992 Jul;115(3):737–744. [Abstract] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. [Europe PMC free article] [Abstract] [Google Scholar]
- Fehon RG, Johansen K, Rebay I, Artavanis-Tsakonas S. Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J Cell Biol. 1991 May;113(3):657–669. [Europe PMC free article] [Abstract] [Google Scholar]
- Kidd S, Baylies MK, Gasic GP, Young MW. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 1989 Aug;3(8):1113–1129. [Abstract] [Google Scholar]
- Zagouras P, Stifani S, Blaumueller CM, Carcangiu ML, Artavanis-Tsakonas S. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6414–6418. [Europe PMC free article] [Abstract] [Google Scholar]
- Aster J, Pear W, Hasserjian R, Erba H, Davi F, Luo B, Scott M, Baltimore D, Sklar J. Functional analysis of the TAN-1 gene, a human homolog of Drosophila notch. Cold Spring Harb Symp Quant Biol. 1994;59:125–136. [Abstract] [Google Scholar]
- Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992 Dec;6(12B):2620–2634. [Abstract] [Google Scholar]
- Jennings B, Preiss A, Delidakis C, Bray S. The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development. 1994 Dec;120(12):3537–3548. [Abstract] [Google Scholar]
- Brou C, Logeat F, Lecourtois M, Vandekerckhove J, Kourilsky P, Schweisguth F, Israël A. Inhibition of the DNA-binding activity of Drosophila suppressor of hairless and of its human homolog, KBF2/RBP-J kappa, by direct protein-protein interaction with Drosophila hairless. Genes Dev. 1994 Oct 15;8(20):2491–2503. [Abstract] [Google Scholar]
- Schweisguth F, Posakony JW. Antagonistic activities of Suppressor of Hairless and Hairless control alternative cell fates in the Drosophila adult epidermis. Development. 1994 Jun;120(6):1433–1441. [Abstract] [Google Scholar]
- Schweisguth F. Suppressor of Hairless is required for signal reception during lateral inhibition in the Drosophila pupal notum. Development. 1995 Jun;121(6):1875–1884. [Abstract] [Google Scholar]
- Thompson CC, Brown TA, McKnight SL. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science. 1991 Aug 16;253(5021):762–768. [Abstract] [Google Scholar]
- LaMarco K, Thompson CC, Byers BP, Walton EM, McKnight SL. Identification of Ets- and notch-related subunits in GA binding protein. Science. 1991 Aug 16;253(5021):789–792. [Abstract] [Google Scholar]
- Watanabe H, Sawada J, Yano K, Yamaguchi K, Goto M, Handa H. cDNA cloning of transcription factor E4TF1 subunits with Ets and notch motifs. Mol Cell Biol. 1993 Mar;13(3):1385–1391. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994 Apr 8;77(1):53–62. [Abstract] [Google Scholar]
- Roehl H, Kimble J. Control of cell fate in C. elegans by a GLP-1 peptide consisting primarily of ankyrin repeats. Nature. 1993 Aug 12;364(6438):632–635. [Abstract] [Google Scholar]
- Crittenden SL, Troemel ER, Evans TC, Kimble J. GLP-1 is localized to the mitotic region of the C. elegans germ line. Development. 1994 Oct;120(10):2901–2911. [Abstract] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995 Apr 14;268(5208):225–232. [Abstract] [Google Scholar]
- Rechsteiner M. Regulation of enzyme levels by proteolysis: the role of pest regions. Adv Enzyme Regul. 1988;27:135–151. [Abstract] [Google Scholar]
- Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994 Oct 21;79(2):273–282. [Abstract] [Google Scholar]
- Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995 Aug;121(8):2633–2644. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.93.4.1683
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc40002?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Endolysosomal dysfunction in radial glia progenitor cells leads to defective cerebral angiogenesis and compromised blood-brain barrier integrity.
Nat Commun, 15(1):8158, 17 Sep 2024
Cited by: 0 articles | PMID: 39289367 | PMCID: PMC11408700
A familial Alzheimer's disease associated mutation in presenilin-1 mediates amyloid-beta independent cell specific neurodegeneration.
PLoS One, 19(9):e0289435, 06 Sep 2024
Cited by: 0 articles | PMID: 39240956 | PMCID: PMC11379242
Spatio-Temporal Regulation of Notch Activation in Asymmetrically Dividing Sensory Organ Precursor Cells in Drosophila melanogaster Epithelium.
Cells, 13(13):1133, 30 Jun 2024
Cited by: 0 articles | PMID: 38994985 | PMCID: PMC11240559
Review Free full text in Europe PMC
NOTCH localizes to mitochondria through the TBC1D15-FIS1 interaction and is stabilized via blockade of E3 ligase and CDK8 recruitment to reprogram tumor-initiating cells.
Exp Mol Med, 56(2):461-477, 27 Feb 2024
Cited by: 0 articles | PMID: 38409448 | PMCID: PMC10907578
The Notch-mediated circuitry in the evolution and generation of new cell lineages: the tooth model.
Cell Mol Life Sci, 80(7):182, 18 Jun 2023
Cited by: 0 articles | PMID: 37330998 | PMCID: PMC10277265
Go to all (323) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Notch receptor cleavage depends on but is not directly executed by presenilins.
Proc Natl Acad Sci U S A, 99(6):4014-4019, 12 Mar 2002
Cited by: 29 articles | PMID: 11891288 | PMCID: PMC122640
Conservation of the biochemical mechanisms of signal transduction among mammalian Notch family members.
Proc Natl Acad Sci U S A, 98(16):9026-9031, 17 Jul 2001
Cited by: 68 articles | PMID: 11459941 | PMCID: PMC55367
Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats.
Mol Cell Biol, 24(21):9265-9273, 01 Nov 2004
Cited by: 119 articles | PMID: 15485896 | PMCID: PMC522238
Biological function of nuclear receptor tyrosine kinase action.
Cold Spring Harb Perspect Biol, 5(7):a009001, 01 Jul 2013
Cited by: 20 articles | PMID: 23818495 | PMCID: PMC3685897
Review Free full text in Europe PMC