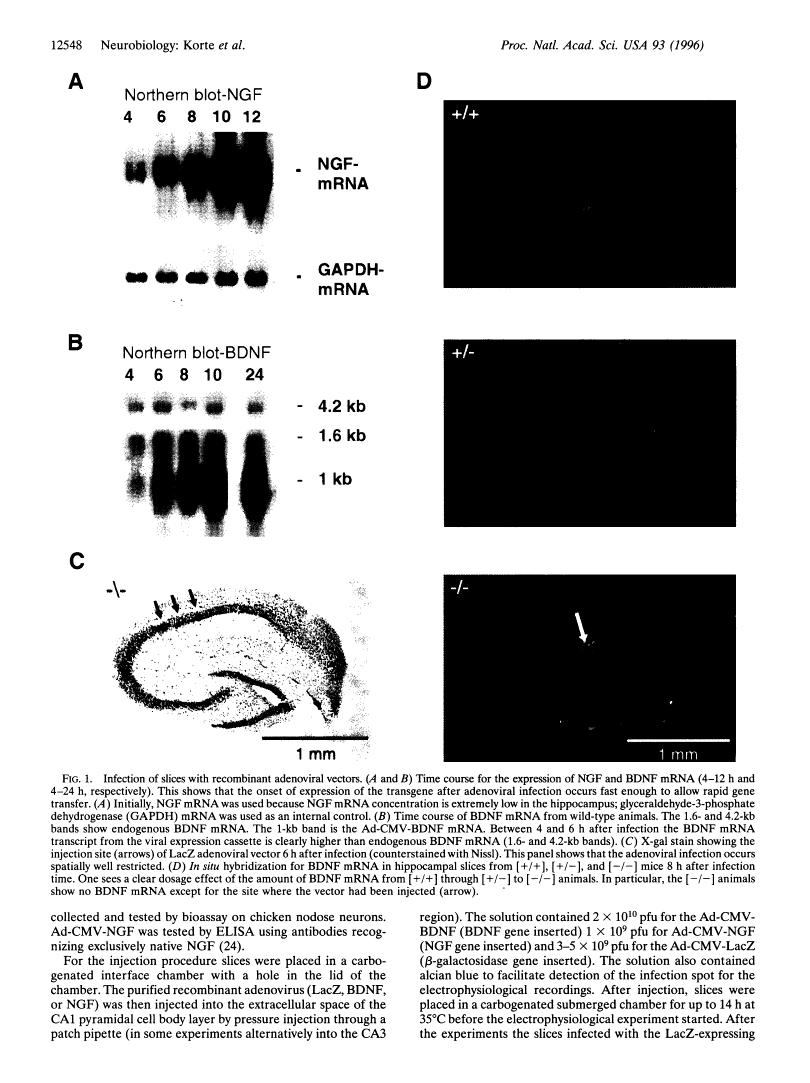
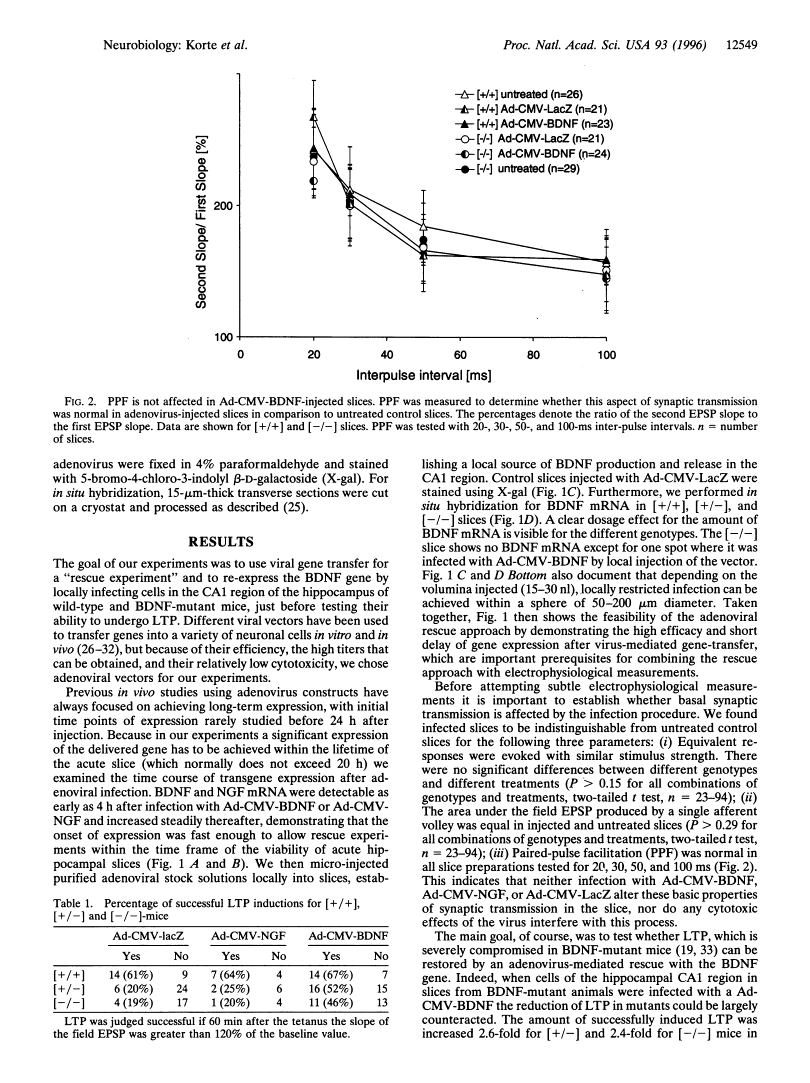
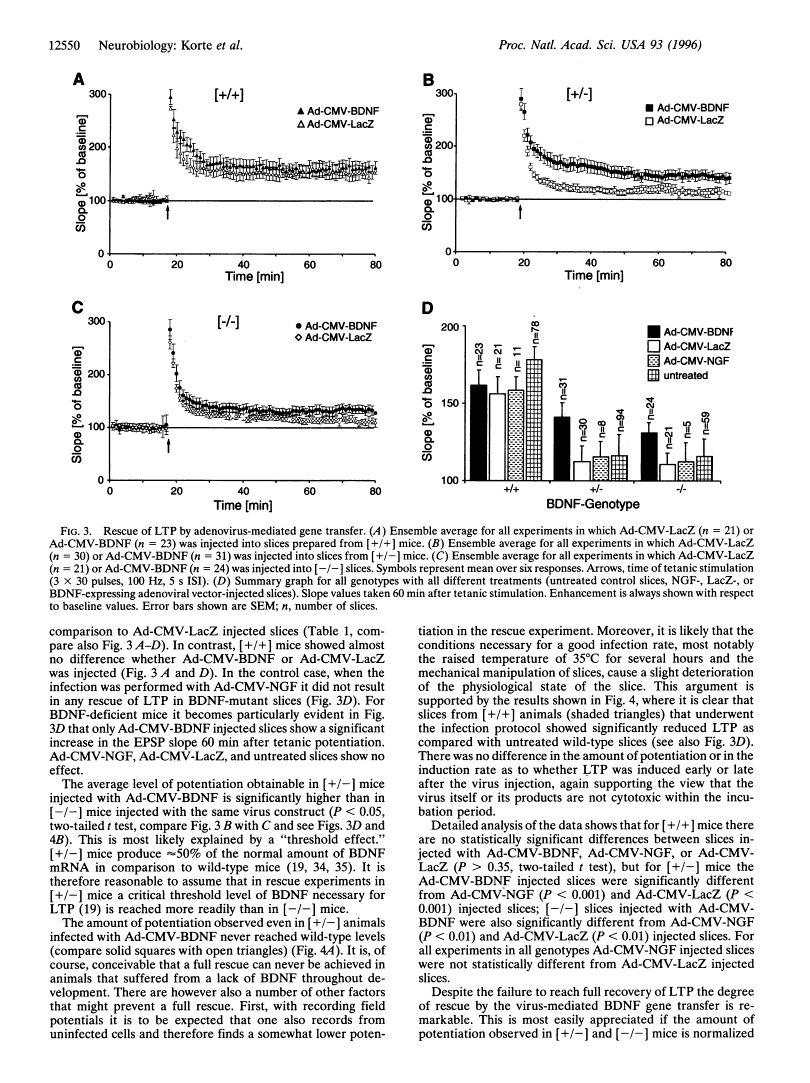
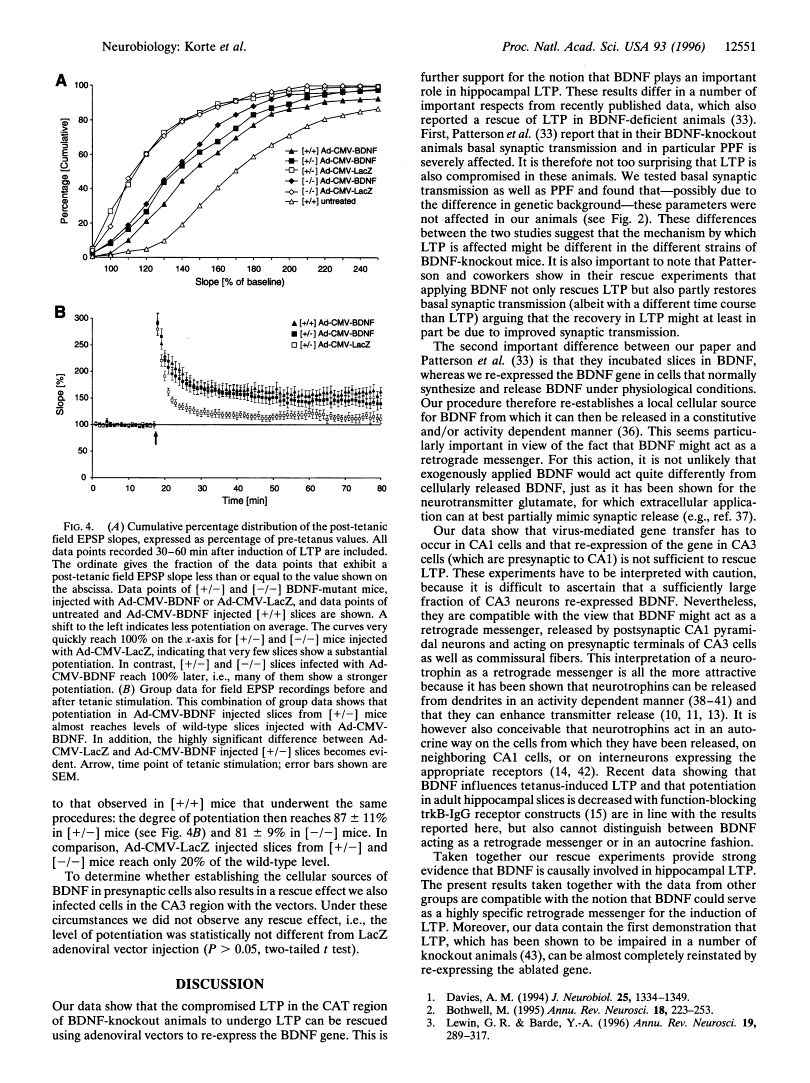
Abstract
Free full text

Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice.
Abstract
Long-term potentiation (LTP) has been shown to be impaired in mice deficient in the brain-derived neurotrophic factor (BDNF) gene, as well as in a number of other knockout animals. Despite its power the gene-targeting approach is always fraught with the danger of looking at the cumulative direct and indirect effects of the absence of a particular gene rather than its immediate function. The re-expression of a specific gene at a selective time point and at a specific site in gene-defective mutants presents a potent procedure to overcome this limitation and to evaluate the causal relationship between the absence of a particular gene and the impairment of a function in gene-defective animals. Here we demonstrate that the re-expression of the BDNF gene in the CA1 region almost completely restores the severely impaired LTP in hippocampal slices of BDNF-deficient mice. The results therefore provide strong evidence for the direct involvement of BDNF in the process of LTP.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies AM. The role of neurotrophins in the developing nervous system. J Neurobiol. 1994 Nov;25(11):1334–1348. [Abstract] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. [Abstract] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. [Abstract] [Google Scholar]
- Berardi N, Domenici L, Parisi V, Pizzorusso T, Cellerino A, Maffei L. Monocular deprivation effects in the rat visual cortex and lateral geniculate nucleus are prevented by nerve growth factor (NGF). I. Visual cortex. Proc Biol Sci. 1993 Jan 22;251(1330):17–23. [Abstract] [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995 Mar 17;267(5204):1662–1666. [Abstract] [Google Scholar]
- Riddle DR, Lo DC, Katz LC. NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature. 1995 Nov 9;378(6553):189–191. [Abstract] [Google Scholar]
- Fox K, Zahs K. Critical period control in sensory cortex. Curr Opin Neurobiol. 1994 Feb;4(1):112–119. [Abstract] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995 Oct 27;270(5236):593–598. [Abstract] [Google Scholar]
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996 Feb;6(1):119–126. [Abstract] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993 May 27;363(6427):350–353. [Abstract] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995 Mar 17;267(5204):1658–1662. [Abstract] [Google Scholar]
- Berninger B, García DE, Inagaki N, Hahnel C, Lindholm D. BDNF and NT-3 induce intracellular Ca2+ elevation in hippocampal neurones. Neuroreport. 1993 Sep 30;4(12):1303–1306. [Abstract] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport. 1994 Dec 30;6(1):21–25. [Abstract] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8074–8077. [Europe PMC free article] [Abstract] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996 Jun 20;381(6584):706–709. [Abstract] [Google Scholar]
- Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9444–9448. [Europe PMC free article] [Abstract] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992 Dec;9(6):1081–1088. [Abstract] [Google Scholar]
- Castrén E, Pitkänen M, Sirviö J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993 Jul;4(7):895–898. [Abstract] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8856–8860. [Europe PMC free article] [Abstract] [Google Scholar]
- Brewer GJ, Cotman CW. Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res. 1989 Aug 7;494(1):65–74. [Abstract] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. [Abstract] [Google Scholar]
- Vilquin JT, Guérette B, Kinoshita I, Roy B, Goulet M, Gravel C, Roy R, Tremblay JP. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum Gene Ther. 1995 Nov;6(11):1391–1401. [Abstract] [Google Scholar]
- Korsching S, Thoenen H. Two-site enzyme immunoassay for nerve growth factor. Methods Enzymol. 1987;147:167–185. [Abstract] [Google Scholar]
- Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res. 1995 Sep 1;42(1):21–33. [Abstract] [Google Scholar]
- Akli S, Caillaud C, Vigne E, Stratford-Perricaudet LD, Poenaru L, Perricaudet M, Kahn A, Peschanski MR. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993 Mar;3(3):224–228. [Abstract] [Google Scholar]
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993 Mar;3(3):229–234. [Abstract] [Google Scholar]
- Caillaud C, Akli S, Vigne E, Koulakoff A, Perricaudet M, Poenaru L, Kahn A, Berwald-Netter Y. Adenoviral vector as a gene delivery system into cultured rat neuronal and glial cells. Eur J Neurosci. 1993 Oct 1;5(10):1287–1291. [Abstract] [Google Scholar]
- Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. [Abstract] [Google Scholar]
- Casaccia-Bonnefil P, Benedikz E, Shen H, Stelzer A, Edelstein D, Geschwind M, Brownlee M, Federoff HJ, Bergold PJ. Localized gene transfer into organotypic hippocampal slice cultures and acute hippocampal slices. J Neurosci Methods. 1993 Dec;50(3):341–351. [Abstract] [Google Scholar]
- Pettit DL, Koothan T, Liao D, Malinow R. Vaccinia virus transfection of hippocampal slice neurons. Neuron. 1995 Apr;14(4):685–688. [Abstract] [Google Scholar]
- Wilkemeyer MF, Smith KL, Zarei MM, Benke TA, Swann JW, Angelides KJ, Eisensmith RC. Adenovirus-mediated gene transfer into dissociated and explant cultures of rat hippocampal neurons. J Neurosci Res. 1996 Jan 15;43(2):161–174. [Abstract] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996 Jun;16(6):1137–1145. [Abstract] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994 Mar 10;368(6467):147–150. [Abstract] [Google Scholar]
- Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994 Mar 25;76(6):989–999. [Europe PMC free article] [Abstract] [Google Scholar]
- Taschenberger H, Engert F, Grantyn R. Synaptic current kinetics in a solely AMPA-receptor-operated glutamatergic synapse formed by rat retinal ganglion neurons. J Neurophysiol. 1995 Sep;74(3):1123–1136. [Abstract] [Google Scholar]
- Blöchl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995 Jun 1;7(6):1220–1228. [Abstract] [Google Scholar]
- Blöchl A, Thoenen H. Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996 Mar;7(3):173–190. [Abstract] [Google Scholar]
- Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996 Mar;7(3):222–238. [Abstract] [Google Scholar]
- Kim HG, Wang T, Olafsson P, Lu B. Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12341–12345. [Europe PMC free article] [Abstract] [Google Scholar]
- Mayford M, Abel T, Kandel ER. Transgenic approaches to cognition. Curr Opin Neurobiol. 1995 Apr;5(2):141–148. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.93.22.12547
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/93/22/12547.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
The Neurotrophin System in the Postnatal Brain-An Introduction.
Biology (Basel), 13(8):558, 24 Jul 2024
Cited by: 0 articles | PMID: 39194496 | PMCID: PMC11352095
Review Free full text in Europe PMC
Synaptic plasticity via receptor tyrosine kinase/G-protein-coupled receptor crosstalk.
Cell Rep, 43(1):113595, 19 Dec 2023
Cited by: 1 article | PMID: 38117654 | PMCID: PMC10844890
Moderate intensity aerobic exercise may enhance neuroplasticity of the contralesional hemisphere after stroke: a randomised controlled study.
Sci Rep, 13(1):14440, 02 Sep 2023
Cited by: 3 articles | PMID: 37660093 | PMCID: PMC10475034
BDNF and Cognitive Function in Chilean Schizophrenic Patients.
Int J Mol Sci, 24(13):10569, 24 Jun 2023
Cited by: 2 articles | PMID: 37445746 | PMCID: PMC10341589
Actions of the TrkB Agonist Antibody ZEB85 in Regulating the Architecture and Synaptic Plasticity in Hippocampal Neurons.
Front Mol Neurosci, 15:945348, 30 Jun 2022
Cited by: 3 articles | PMID: 35845610 | PMCID: PMC9280622
Go to all (248) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The involvement of brain-derived neurotrophic factor in hippocampal long-term potentiation revealed by gene targeting experiments.
J Physiol Paris, 90(3-4):157-164, 01 Jan 1996
Cited by: 63 articles | PMID: 9116659
Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor.
Proc Natl Acad Sci U S A, 92(19):8856-8860, 01 Sep 1995
Cited by: 940 articles | PMID: 7568031 | PMCID: PMC41066
Role of activity-dependent BDNF expression in hippocampal-prefrontal cortical regulation of behavioral perseverance.
Proc Natl Acad Sci U S A, 110(37):15103-15108, 26 Aug 2013
Cited by: 83 articles | PMID: 23980178 | PMCID: PMC3773762
Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF.
Ageing Res Rev, 3(4):407-430, 01 Nov 2004
Cited by: 208 articles | PMID: 15541709
Review