Abstract
Free full text

Mutation of gene-proximal regulatory elements disrupts human
 -, γ-, and β-globin expression in yeast artificial chromosome
transgenic
-, γ-, and β-globin expression in yeast artificial chromosome
transgenic mice
mice
Abstract
Previous studies have defined transcriptional control elements, in
addition to the promoters, that both lie near individual human
β-globin locus genes and have been implicated in their differential
stage-specific regulation during development (i.e., are believed to
directly participate in hemoglobin switching). We have reinvestigated
the activities during erythropoiesis that might be conferred by two of
the more intensively analyzed of these elements, the  -globin gene 5′
silencer and the β-globin gene 3′ enhancer, by deleting them from a
yeast artificial chromosome that spans the human β-globin locus, and
then analyzing transgenic mice for expression of all of the human
genes. These studies show that sequences within the
-globin gene 5′
silencer and the β-globin gene 3′ enhancer, by deleting them from a
yeast artificial chromosome that spans the human β-globin locus, and
then analyzing transgenic mice for expression of all of the human
genes. These studies show that sequences within the  -globin
“silencer” are not only required for silencing but are also
required for activation of
-globin
“silencer” are not only required for silencing but are also
required for activation of  -globin transcription; furthermore,
deletion of the silencer simultaneously reduced γ-globin
transcription during the yolk sac stage of erythroid development.
Analysis of the adult β-globin gene 3′ enhancer deletion showed that
its deletion affects only that gene.
-globin transcription; furthermore,
deletion of the silencer simultaneously reduced γ-globin
transcription during the yolk sac stage of erythroid development.
Analysis of the adult β-globin gene 3′ enhancer deletion showed that
its deletion affects only that gene.
 -globin silencer, β-globin 3′ enhancer
-globin silencer, β-globin 3′ enhancerThe five genes of the human β-globin locus are transcriptionally regulated by both gene-proximal and -distal control elements. The well characterized distal elements are the five 5′ DNase I hypersensitive sites (HS; refs. 1 and 2) comprising the β-globin locus control region (LCR; refs. 3 and 4); the LCR is generally acknowledged to be required for the regulation of all five genes of the locus during erythroid development. While the promoters of each gene play critical roles in their individual responses to LCR-mediated stimulation, other transcriptional control elements (lying in close physical proximity to the genes) have also been implicated, but their roles in developmental stage-specific control over gene expression in the locus are less clear.
The human β-globin LCR has been studied most extensively after dissection into its constituent components: the three central tissue-specific HS sites (HS2, HS3, and HS4) are thought to play a major role in elaborating the multiple activities attributed to the LCR, while HS5 and HS1 play currently less well defined roles. Early analysis of its activity represented the LCR as an integral organizational, and perhaps structural, unit (5, 6) that has since been referred to as a “holocomplex” (7, 8). A key experiment in the progressive dissection of LCR function showed that each of the three central HS elements was capable of conferring significant stage specificity to activation of the γ- and β-globin genes, and yet none of the individual HS elements was able to fully restore LCR activity (9). These and other studies have led to general acceptance of the hypothesis that the LCR functions as a single, cooperative unit for β-globin locus gene regulation.
Human β-globin locus transcriptional regulation has also been
described as arising from either competitive or autonomous gene
control, based principally on the chicken β/ -globin gene
enhancer/promoter regulatory paradigm (10). Autonomous control
implies that a gene is not influenced by placing other transcriptional
elements nearby, whereas competitive control implies that when other
regulatory elements are placed in cis, or alternatively
removed from a particular construct, normal tissue-specific or temporal
control is lost (10, 11). Early studies indicated that while the human
-globin gene
enhancer/promoter regulatory paradigm (10). Autonomous control
implies that a gene is not influenced by placing other transcriptional
elements nearby, whereas competitive control implies that when other
regulatory elements are placed in cis, or alternatively
removed from a particular construct, normal tissue-specific or temporal
control is lost (10, 11). Early studies indicated that while the human
 -globin gene is regulated autonomously (12) and that the fetal γ-
and adult β-globin genes are regulated through competition (13), the
mode of regulation for the γ-globin genes was disputed (14, 15). An
important caveat in the interpretation of these experiments was that
each of the transgenes was analyzed in the context of a DNA
construction with arbitrary endpoints, where other participating
transcriptional control elements may have been inadvertently omitted.
Experiments analyzing gene regulatory activity in the murine locus
first implicated the LCR as a direct participant in competition (16).
Competitive regulatory mechanisms have more recently been proposed to
explain regulation over allele-specific imprinting of the
IGF2 and h19 genes (17–19), control over HOM-C
complex temporal regulation (20) and control of allele-specific
transcription of the olfactory receptor genes
(21).
-globin gene is regulated autonomously (12) and that the fetal γ-
and adult β-globin genes are regulated through competition (13), the
mode of regulation for the γ-globin genes was disputed (14, 15). An
important caveat in the interpretation of these experiments was that
each of the transgenes was analyzed in the context of a DNA
construction with arbitrary endpoints, where other participating
transcriptional control elements may have been inadvertently omitted.
Experiments analyzing gene regulatory activity in the murine locus
first implicated the LCR as a direct participant in competition (16).
Competitive regulatory mechanisms have more recently been proposed to
explain regulation over allele-specific imprinting of the
IGF2 and h19 genes (17–19), control over HOM-C
complex temporal regulation (20) and control of allele-specific
transcription of the olfactory receptor genes
(21).
Studies on the transcriptional regulation of the human β-globin locus
genes, and dissection of the regulatory mechanisms that are employed
during this well characterized developmental program, have recently
been advanced by incorporating large DNAs that span the human
β-globin locus [linked cosmids and yeast artificial chromosomes
(YACs); refs. 22 and 23] into the germ line of transgenic mice, as
well as through gene targeting experiments in the murine LCR. Two well
characterized YACs have been shown to contain all currently
characterized distal and gene-proximal regulatory elements required for
human β-globin locus gene control, and both of these, examined as
transgenes in mice, have been shown to faithfully recapitulate the
activation and developmental stage-specific inactivation of the human
 - and γ-globin genes at paralogous hematopoietic sites and
developmental stages during murine embryogenesis to finally result in
appropriate temporal activation of the adult β-globin gene (24, 25).
The YACs can be precisely modified using homologous recombination in
yeast, and analysis of the functional consequences of such mutations
has led to novel insights into the role the LCR may play in hemoglobin
switching. Based on the analysis of several LCR “core” element
mutant YACs incorporated as transgenes in mice, we concluded that all
of the human locus genes are controlled by competition between an
integral functional complex (the LCR holocomplex) and other (as yet
undefined) transcriptional control elements that probably lie near the
genes, and further that the individual core elements could be
substituted for one another to partially or fully restore mutant LCR
function (26).
- and γ-globin genes at paralogous hematopoietic sites and
developmental stages during murine embryogenesis to finally result in
appropriate temporal activation of the adult β-globin gene (24, 25).
The YACs can be precisely modified using homologous recombination in
yeast, and analysis of the functional consequences of such mutations
has led to novel insights into the role the LCR may play in hemoglobin
switching. Based on the analysis of several LCR “core” element
mutant YACs incorporated as transgenes in mice, we concluded that all
of the human locus genes are controlled by competition between an
integral functional complex (the LCR holocomplex) and other (as yet
undefined) transcriptional control elements that probably lie near the
genes, and further that the individual core elements could be
substituted for one another to partially or fully restore mutant LCR
function (26).
If the LCR is controlling human β-globin transcription by regulatory
sequence competition, which and where are the competing elements that
interact with the LCR? At least 11 distinct hypothetical or
demonstrable transcriptional control sequences lie in close physical
proximity to the five genes. These include all five gene promoters; an
embryonic  -globin gene silencer, required for suppression of
-globin gene silencer, required for suppression of
 -globin transcription during the fetal stage (27); 5′ γ-globin
gene elements (28–32); a 3′ γ-globin gene enhancer (33); and both
gene internal and 3′ β-globin gene enhancers (34, 35). Positive and
negative regulation of
-globin transcription during the fetal stage (27); 5′ γ-globin
gene elements (28–32); a 3′ γ-globin gene enhancer (33); and both
gene internal and 3′ β-globin gene enhancers (34, 35). Positive and
negative regulation of  -globin gene transcription has been the focus
of particularly intensive study (36–44).
-globin gene transcription has been the focus
of particularly intensive study (36–44).
As the initial step in efforts to more precisely define elements that
may contribute to temporally specific transcription in the human
β-globin gene locus, we describe here the effects of deletion of two
of these elements in a YAC, and their expression as mutant YAC
transgenes: the embryonic  -globin 5′ silencer and the adult
β-globin 3′ enhancer. Analysis of transgenic lines bearing these
mutants shows that the adult β-globin 3′ enhancer affects expression
from only that gene, indicating that this element does not directly
participate in competition for LCR-directed activity. Analysis of
sequences that were originally characterized as the
-globin 5′ silencer and the adult
β-globin 3′ enhancer. Analysis of transgenic lines bearing these
mutants shows that the adult β-globin 3′ enhancer affects expression
from only that gene, indicating that this element does not directly
participate in competition for LCR-directed activity. Analysis of
sequences that were originally characterized as the  -globin gene
“silencer” shows that mutation of this element confers unexpected
properties: sequence elements within the silencer region are required
for activation of both the embryonic
-globin gene
“silencer” shows that mutation of this element confers unexpected
properties: sequence elements within the silencer region are required
for activation of both the embryonic  - and fetal γ-globin genes.
- and fetal γ-globin genes.
MATERIALS AND METHODS
Generation of Element-Specific YAC Deletion Mutants by Homologous Recombination in Yeast.
To generate the targeting construct for
deleting the  -globin 5′ silencer using homologous recombination in
yeast, a 645-bp fragment immediately flanking, and 5′ to, position
−304 of the silencer was amplified from the parental β-globin locus
YAC using PCR, where primers incorporated new ClaI and
XhoI (italic lettering) sites on the 5′ and 3′ ends,
respectively. The 5′ fragment primers were: E5FF (sense strand
5′-
-globin 5′ silencer using homologous recombination in
yeast, a 645-bp fragment immediately flanking, and 5′ to, position
−304 of the silencer was amplified from the parental β-globin locus
YAC using PCR, where primers incorporated new ClaI and
XhoI (italic lettering) sites on the 5′ and 3′ ends,
respectively. The 5′ fragment primers were: E5FF (sense strand
5′- …GACTATCGATTCACTTTTTTCTCTGTTTGA)
and E5FR (antisense strand
5′-
…GACTATCGATTCACTTTTTTCTCTGTTTGA)
and E5FR (antisense strand
5′- …AGCTCTCGAGCCTCATATAAAGGAGCAAAT),
where the underlined nucleotides correspond to positions 18,637 and
19,282, respectively, of the human β-globin locus sequence (web site:
globin.cse.psu.edu). A 1900-bp 3′ flanking sequence (incorporating
XhoI and SacI sites at the 5′ and 3′ ends) was
also amplified by PCR to generate the 3′ flanking sequence to the
silencer. The 3′ fragment primers were E3FF (sense strand 5′-
…GTACCTCGAGGGATCCAGCACACATTATCA) and E3FR
(antisense strand
5′-…GCATGAGCTCCTTAATTAACCATTTTCCCA), where
the two underlined nucleotides correspond to positions 19,407 and
21,307, respectively, of the locus. These two PCR fragments were then
digested with the appropriate restriction enzymes and ligated together
into yeast shuttle vector pRS306, which had been previously digested
with ClaI and SacI, to generate the final
…AGCTCTCGAGCCTCATATAAAGGAGCAAAT),
where the underlined nucleotides correspond to positions 18,637 and
19,282, respectively, of the human β-globin locus sequence (web site:
globin.cse.psu.edu). A 1900-bp 3′ flanking sequence (incorporating
XhoI and SacI sites at the 5′ and 3′ ends) was
also amplified by PCR to generate the 3′ flanking sequence to the
silencer. The 3′ fragment primers were E3FF (sense strand 5′-
…GTACCTCGAGGGATCCAGCACACATTATCA) and E3FR
(antisense strand
5′-…GCATGAGCTCCTTAATTAACCATTTTCCCA), where
the two underlined nucleotides correspond to positions 19,407 and
21,307, respectively, of the locus. These two PCR fragments were then
digested with the appropriate restriction enzymes and ligated together
into yeast shuttle vector pRS306, which had been previously digested
with ClaI and SacI, to generate the final
 -silencer deletion targeting construct. The joining points of the
two fragments (as well as ≈1 kbp of the
-silencer deletion targeting construct. The joining points of the
two fragments (as well as ≈1 kbp of the  -globin gene) were
resequenced to verify that no mutations had been introduced during PCR.
-globin gene) were
resequenced to verify that no mutations had been introduced during PCR.
A λ phage clone containing the 3′ end of the human adult β-globin gene was used to generate the plasmid employed for deleting the β-globin 3′ enhancer (26). A 4.65-kbp BamHI/SmaI adult β-globin gene fragment was isolated from a parental λ genomic clone (26) and subcloned into pGEM7. The 250-bp enhancer was removed by PstI digestion and the flanking sequences were religated. This fragment, now missing the enhancer, was then transferred into pRS306 to generate the final β-globin enhancer deletion targeting construct.
Most of the yeast methods used here were derived from a single source (45). Yeast integrative plasmid pRS306 contains the URA3 selectable marker used to monitor homologous integration into, and excision from, the YAC genome (46), as previously detailed (26). To take advantage of this selection, the YAC right vector arm in the original clone A201F4 (22) was modified to replace the resident URA3 gene with the LYS2 gene (called A201F4.2; ref. 26). The size and integrity of the two final mutated YACs examined in this study were assayed by restriction digestion of the yeast DNA and subsequent Southern blotting with probes spanning the locus (22).
YAC DNA Isolation/Transgenic Mice.
YAC DNA was isolated from pulse-field gels according to standard protocols (47, 48) with minor modifications (26). The DNA was injected into fertilized mouse eggs (CD1; Charles River Breeding Laboratory) before transfer to foster mothers (B6D2F1/J) as described (49). Tail DNAs from offspring were analyzed for the presence of the left and right YAC vector arms by PCR, and then for integrity of the transgenic β-globin locus by Southern blot hybridization of SfiI/SalI digests of DNA from transgenic organs (see below; ref. 22).
Transgene Copy Number and YAC Integrity.
To assess the integrated copy number of human β-globin YACs, genomic DNA recovered from transgenics was digested with PstI, which has two sites in the pYAC4-derived left vector arm. A 1.7-kbp EarI fragment spanning the 3′ PstI site was used for Southern blot hybridization, where a 1.6-kbp common band, as well as others that vary in size, would be detected, depending on the transgene copy number and YAC integration pattern(s).
To assess the integrity of the integrated YACs, hematopoietic cells from 0.5 g of transgenic spleen were suspended in 1 ml of cell suspension buffer (10 mM Tris·HCl, pH 7.5/20 mM NaCl/50 mM EDTA, pH 8.0). This solution was then diluted 1:10 in 0.4% agarose solution and poured into clamped homogeneous electric field (CHEF) plug molds. Solidified plugs were then treated at 50°C in Proteinase K buffer (100 mM EDTA, pH 8.0/0.2% sodium deoxycholate/1% Sarcosyl/10 mg/ml Proteinase K) for 48 h, followed by several 1-h washes with 10 mM Tris·HCl (pH 7.4) and 1 mM EDTA buffer. The plugs were digested with SfiI at 50°C for 12 h, and then with SalI for 12 h before pulse field gel electrophoresis and Southern blot hybridization. Two radiolabeled probes were employed (corresponding to HS4 or the Aγ-globin gene) that would be expected to hybridize to 10-kbp (SfiI/SfiI) or 52-kbp (SfiI/SalI) bands, respectively, in the event that the integrated human β-globin YAC transgenes were intact.
Semiquantitative Multiplex Reverse Transcriptase—PCR Assay.
The assay was developed and described in detail elsewhere (11, 26). A titration for cycle number was performed on each cDNA sample to verify that the amplification of all globin templates was within the linear range. An aliquot of each PCR reaction was electrophoresed on 8% polyacrylamide gels, dried, and subjected to autoradiography. Individual band intensities were quantified on a Molecular Dynamics PhosphorImager (11); expression per gene copy was calculated based on normalization to the four genomic copies of the murine α-globin gene (26). Multiple samples representing each developmental stage in erythropoiesis for each transgenic line were analyzed in like manner using different initial RNA preparations as well as different cDNA synthesis reactions.
RESULTS AND DISCUSSION
Generation of Gene-Proximal Regulatory Element huβYAC (human β-globin YAC) Mutants.
The human  -globin gene 5′ silencer was
originally identified as a 215-bp fragment (−392 to −177 relative to
the
-globin gene 5′ silencer was
originally identified as a 215-bp fragment (−392 to −177 relative to
the  -globin mRNA cap site; ref. 27) that was later found to possibly
overlap a positive regulatory element (41). We therefore deleted only
125 bp of the
-globin mRNA cap site; ref. 27) that was later found to possibly
overlap a positive regulatory element (41). We therefore deleted only
125 bp of the  -globin upstream sequence (from −304 to −179),
containing one YY1 and two GATA factor binding sites that had
previously been shown to participate in
-globin upstream sequence (from −304 to −179),
containing one YY1 and two GATA factor binding sites that had
previously been shown to participate in  -globin negative
transcriptional control (36, 44, 50). To generate a transplacement
targeting construct for deleting this fragment from an otherwise intact
human β-globin locus YAC, a 645-bp 5′ flanking sequence homology and
the 1.9 kbp of 3′ flanking sequence were amplified from the parental
A201F4 YAC clone DNA (Fig. (Fig.11A).
Similarly, a 250-bp PstI fragment originally identified as
the adult β-globin gene 3′ enhancer was removed by PstI
digestion of a 4.65-kbp BamHI/SmaI genomic
fragment of the β-globin gene (34). The flanking sequences were
religated and the fragment, now missing the 250-bp enhancer sequence,
was excised and subcloned into pRS306. These two yeast targeting
subclones were then linearized and used to transform the
(LYS2-retrofitted) A201F4.2 YAC (26).
-globin negative
transcriptional control (36, 44, 50). To generate a transplacement
targeting construct for deleting this fragment from an otherwise intact
human β-globin locus YAC, a 645-bp 5′ flanking sequence homology and
the 1.9 kbp of 3′ flanking sequence were amplified from the parental
A201F4 YAC clone DNA (Fig. (Fig.11A).
Similarly, a 250-bp PstI fragment originally identified as
the adult β-globin gene 3′ enhancer was removed by PstI
digestion of a 4.65-kbp BamHI/SmaI genomic
fragment of the β-globin gene (34). The flanking sequences were
religated and the fragment, now missing the 250-bp enhancer sequence,
was excised and subcloned into pRS306. These two yeast targeting
subclones were then linearized and used to transform the
(LYS2-retrofitted) A201F4.2 YAC (26).
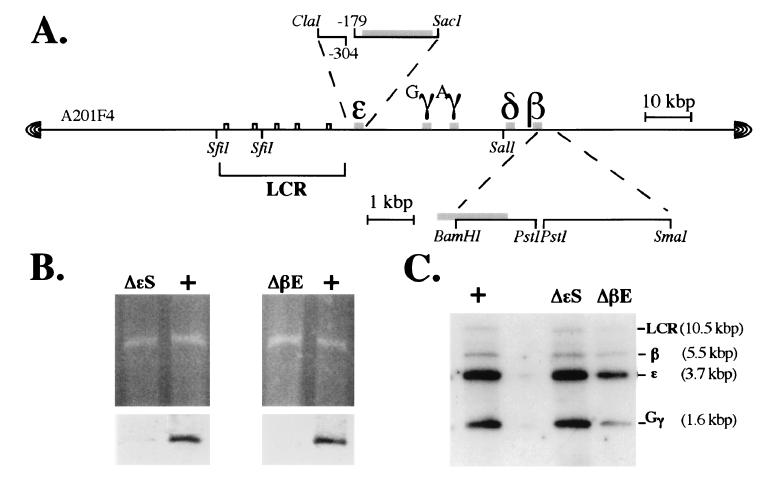
Structure of the wild-type and mutant human
β-globin YACs. (A) YAC clone A201F4 (22) was modified as
described (26) to permit ease of manipulation for targeted mutagenesis
in yeast. Expanded diagrams depicting the two targeting constructs
generated here are shown above (the  -globin silencer deletion
mutant) or below (the β-globin enhancer deletion mutant) the diagram
of the YAC clone (the position of the deleted elements is indicated by
a break in the contiguity of the subclone). Note the position of the
SfiI and SalI sites in the locus that
were used in the mapping experiments described in Fig. Fig.2.2. (B
Upper) An ethidium bromide-stained pulse field gel of the
wild-type,
-globin silencer deletion
mutant) or below (the β-globin enhancer deletion mutant) the diagram
of the YAC clone (the position of the deleted elements is indicated by
a break in the contiguity of the subclone). Note the position of the
SfiI and SalI sites in the locus that
were used in the mapping experiments described in Fig. Fig.2.2. (B
Upper) An ethidium bromide-stained pulse field gel of the
wild-type,  -silencer, and β-enhancer deletion mutant YACs.
(B Lower) Autoradiographic exposure of the blots of the
gels shown in the Upper panel after hybridization to
probes corresponding to the
-silencer, and β-enhancer deletion mutant YACs.
(B Lower) Autoradiographic exposure of the blots of the
gels shown in the Upper panel after hybridization to
probes corresponding to the  -globin gene silencer
(Left) or β-globin gene enhancer
(Right). (C) Southern blots of both wild-type
and mutant YAC DNAs depicting the size and integrity of the
EcoRI fragments bearing the LCR and the
-globin gene silencer
(Left) or β-globin gene enhancer
(Right). (C) Southern blots of both wild-type
and mutant YAC DNAs depicting the size and integrity of the
EcoRI fragments bearing the LCR and the  -, γ-, and
β-globin genes.
-, γ-, and
β-globin genes.
After transformation of yeast clone A201F4.2 with either of the two
targeting plasmids, a majority of colonies growing on the selective
medium (Trp−Lys−Ura−) were found
to have plasmid integrated at the homologous human sequence. This
initial targeting event creates a duplication of the homologous
sequences separated by the vector, pRS306. Selective excision of the
targeting plasmid was performed on medium containing uracil, followed
by counterselection on 5-fluroorotic acid-containing plates (lethal to
cells retaining the URA3 gene). Excision of pRS306 results
in reversion to either the parental YAC structure or replacement of
parental sequences by the mutant, now missing either the  -globin
silencer or the globin enhancer. The structures of the mutant and
wild-type YACs were verified by Southern blot analysis (Fig. (Fig.1
1
B and C, respectively).
-globin
silencer or the globin enhancer. The structures of the mutant and
wild-type YACs were verified by Southern blot analysis (Fig. (Fig.1
1
B and C, respectively).
Transgenic Mice/Transgene Structure.
The mutated YAC DNAs were isolated from pulse-field gels and purified as described (see Materials and Methods and ref. 26). These DNAs were then microinjected into pronuclei of fertilized mouse ova. Founder tail DNAs were initially screened by PCR, using primers specific for both right and left YAC vector arms, and then verified by Southern blot hybridization. Southern blotting of each of the F1 or F2 lines, using probes that span the locus (22) showed that each contained restriction fragments of the expected size (e.g., Fig. Fig.11C), and that no other bands (indicative of breakpoints in the YACs) were present. Fragmented YAC transgenes detected through this analysis were excluded from further consideration.
The YAC transgene copy number in the lines chosen for final expression
analysis was determined using a YAC end assay, employing a probe that
would detect a common 1.6-kbp band as well as other characteristic
fragments in each digest. For example, a head-to-head tandem
integration was predicted from the map of pYAC4 to yield an additional
fragment 4.52 kbp in size (Fig. (Fig.22A,
lanes 3 and 6), whereas a head-to-tail tandem integration would result
in the generation of a fragment of 5.36 kbp (Fig.
(Fig.22A, lane 5); if the YAC end was embedded in mouse
chromosomal DNA (as either a single copy transgene or as the flanking
copy of a multi-YAC integration), the size of the fragment would be
dependent on the next random PstI site encountered in the
mouse genome. From these data, combined with the data obtained from
standard Southern blots and comparison of internal fragment
intensities, we estimate that the three  -silencer deletion lines
contain three, two, and two transgene copies (Fig.
(Fig.22A, lanes 1–3), respectively, whereas the two
β-globin enhancer deletion lines both harbor four tandemly integrated
YAC copies (Fig. (Fig.22A, lanes 5 and 6). Furthermore,
the analysis shows that, as previously stated, the wild-type YAC
transgenic line HS4321b (26) is single copy (Fig.
(Fig.22A, lane 4).
-silencer deletion lines
contain three, two, and two transgene copies (Fig.
(Fig.22A, lanes 1–3), respectively, whereas the two
β-globin enhancer deletion lines both harbor four tandemly integrated
YAC copies (Fig. (Fig.22A, lanes 5 and 6). Furthermore,
the analysis shows that, as previously stated, the wild-type YAC
transgenic line HS4321b (26) is single copy (Fig.
(Fig.22A, lane 4).
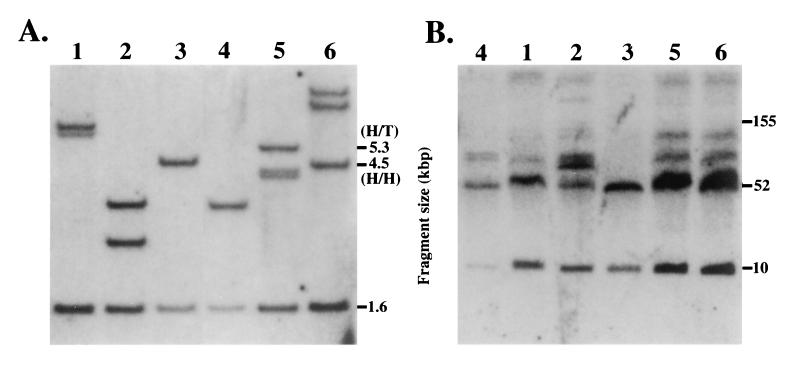
Copy number and integrity of YAC transgenes.
Lanes: 1–3,  -globin silencer deletion mutant transgenic lines 1–3,
respectively; 4, wild type β-globin YAC transgenic line HS4321b (26);
5 and 6, β-globin enhancer deletion mutant lines 1 and 2,
respectively (numbering at the top of each panel). (A) Tail
DNA from F1 or F2 pups representing each of the
transgenic lines was digested with PstI and
electrophoresed and blotted normally. A 1.7-kbp EarI
fragment isolated from plasmid vector pYAC4 was radiolabeled and then
hybridized to the Southern blot, and finally exposed for
autoradiography. Each lane displays a (common) internal fragment band
from the pYAC4 vector (1.58 kbp) as well as other bands, depending on
the transgene copy number and integration site. Head-to-head tandem
integrations would be expected to yield a 4.5-kbp band, whereas
head-to-tail tandem integrations are predicted to yield a 5.3-kbp band.
(B) Spleen cells were isolated from each transgenic line and
digested with SfiI plus SalI as described
prior to electrophoresis on pulse field gels. The gel was then blotted
to nylon and hybridized to probes corresponding to either LCR HS4 or
the Gγ-globin gene.
-globin silencer deletion mutant transgenic lines 1–3,
respectively; 4, wild type β-globin YAC transgenic line HS4321b (26);
5 and 6, β-globin enhancer deletion mutant lines 1 and 2,
respectively (numbering at the top of each panel). (A) Tail
DNA from F1 or F2 pups representing each of the
transgenic lines was digested with PstI and
electrophoresed and blotted normally. A 1.7-kbp EarI
fragment isolated from plasmid vector pYAC4 was radiolabeled and then
hybridized to the Southern blot, and finally exposed for
autoradiography. Each lane displays a (common) internal fragment band
from the pYAC4 vector (1.58 kbp) as well as other bands, depending on
the transgene copy number and integration site. Head-to-head tandem
integrations would be expected to yield a 4.5-kbp band, whereas
head-to-tail tandem integrations are predicted to yield a 5.3-kbp band.
(B) Spleen cells were isolated from each transgenic line and
digested with SfiI plus SalI as described
prior to electrophoresis on pulse field gels. The gel was then blotted
to nylon and hybridized to probes corresponding to either LCR HS4 or
the Gγ-globin gene.
The integrity of the YAC transgenes was assayed by examining spleen cell DNA that had been separated on pulse field gels for the presence of 10- and 52-kbp diagnostic bands on Southern blots after digestion with SfiI and SalI (Fig. (Fig.22B). The two β-globin locus fragments detected in this assay extend from beyond LCR HS5 at the 5′ end of the locus to a position near the start of the δ-globin gene (22). The data are shown in Fig. Fig.22B, where the two expected diagnostic bands are clearly the most prominent in each of the transgenic lines; in particular, it should be noted that there are no bands detected below 60 kbp other than the two that are expected, thus adding credence to the contention that the YACs examined here are unbroken within the 62-kbp interval surveyed in this assay. It should also be noted that other bands of higher molecular weight are clearly detected in the same blot, but other experiments show that these are primarily due to partial digestion of the spleen cell plugs by SalI, a vexing problem we have not yet fully surmounted. We assume that this partial digestion is due to methylation of some SalI CpG sites.
Sequences Within the  -Globin Gene Silencer Are Important for
Embryonic Stage-Specific Activation of Both the
-Globin Gene Silencer Are Important for
Embryonic Stage-Specific Activation of Both the  - and γ-Globin
Genes.
- and γ-Globin
Genes.
In transgenic mice, the human  -globin gene is expressed
in the embryonic yolk sac beginning between day 8.5 and 9.5 days
postcoitus of gestation and switches off by day 12.5 (23–25); this
repression is postulated to result from the binding of negative
regulatory proteins to the
-globin gene is expressed
in the embryonic yolk sac beginning between day 8.5 and 9.5 days
postcoitus of gestation and switches off by day 12.5 (23–25); this
repression is postulated to result from the binding of negative
regulatory proteins to the  -globin gene silencer. Multiple
regulatory elements have been described in the
-globin gene silencer. Multiple
regulatory elements have been described in the  -globin gene upstream
region (41), and the most prominent of these is a silencer located from
−304 to −179 relative to the
-globin gene upstream
region (41), and the most prominent of these is a silencer located from
−304 to −179 relative to the  -globin gene transcription initiation
site (27). This silencer element contains consensus binding sites both
for GATA factors and for YY1. If the silencer harbors only the expected
negative regulatory activity, one would predict that deletion of this
element from an otherwise intact human β-globin gene locus would
result in the continued expression of the
-globin gene transcription initiation
site (27). This silencer element contains consensus binding sites both
for GATA factors and for YY1. If the silencer harbors only the expected
negative regulatory activity, one would predict that deletion of this
element from an otherwise intact human β-globin gene locus would
result in the continued expression of the  -globin gene into the
fetal stage of erythropoiesis. We therefore directly tested the
hypothesis that the silencer acts to restrict expression of the
-globin gene into the
fetal stage of erythropoiesis. We therefore directly tested the
hypothesis that the silencer acts to restrict expression of the
 -globin gene to the embryonic yolk sac stage.
-globin gene to the embryonic yolk sac stage.
To our surprise, deletion of the silencer did not lead to high level
 -globin gene transcription in the fetal liver period, as
anticipated, but rather caused a significant decline in
-globin gene transcription in the fetal liver period, as
anticipated, but rather caused a significant decline in  -globin gene
transcription in the yolk sac, thus demonstrating that this narrowly
defined silencer sequence element of 125 bp still harbors cryptic
activity that is required for stimulation of
-globin gene
transcription in the yolk sac, thus demonstrating that this narrowly
defined silencer sequence element of 125 bp still harbors cryptic
activity that is required for stimulation of  -globin RNA synthesis
(Fig. (Fig.3).3). An even more unexpected observation
was that deletion of the
-globin RNA synthesis
(Fig. (Fig.3).3). An even more unexpected observation
was that deletion of the  -globin gene silencer not only diminished
-globin gene silencer not only diminished
 -globin gene transcription, but also resulted in a significant
decline in γ-globin RNA synthesis in the yolk sac (Fig. (Fig.3).
3).
Transcription of the γ- and β-globin genes during both the fetal
liver and adult stages was not as significantly affected in these
animals, thereby implying that these
-globin gene transcription, but also resulted in a significant
decline in γ-globin RNA synthesis in the yolk sac (Fig. (Fig.3).
3).
Transcription of the γ- and β-globin genes during both the fetal
liver and adult stages was not as significantly affected in these
animals, thereby implying that these  -globin 5′ sequences harbor a
more general function in regulatory control over globin genes that are
expressed in the embryonic yolk sac.
-globin 5′ sequences harbor a
more general function in regulatory control over globin genes that are
expressed in the embryonic yolk sac.
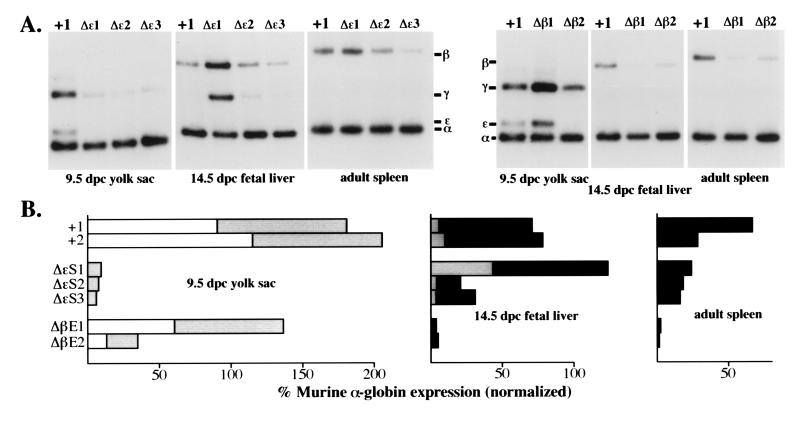
Expression of human β-globin YAC mutants during
erythropoiesis. (A Upper). Typical reverse
transcriptase–PCR expression data for the human  -, γ-, and
β-globin genes as compared with the murine α-globin used as the
internal control for each RNA sample. The transgenic line examined is
abbreviated at the top of each lane, while the developmental stage and
sites are shown below each panel. A +1 refers to RNA isolated from the
single copy wild-type YAC transgenic line, HS4321b (26). (B)
Quantification of the expression data shown in A after
PhosphorImager analysis and correction for gene copy number (Fig. (Fig.2).
2).
Open rectangles represent
-, γ-, and
β-globin genes as compared with the murine α-globin used as the
internal control for each RNA sample. The transgenic line examined is
abbreviated at the top of each lane, while the developmental stage and
sites are shown below each panel. A +1 refers to RNA isolated from the
single copy wild-type YAC transgenic line, HS4321b (26). (B)
Quantification of the expression data shown in A after
PhosphorImager analysis and correction for gene copy number (Fig. (Fig.2).
2).
Open rectangles represent  -globin expression, shaded boxes represent
γ-globin expression, and filled boxes represent β-globin expression
levels.
-globin expression, shaded boxes represent
γ-globin expression, and filled boxes represent β-globin expression
levels.
We should note that the variation in expression levels is sporadically greater in all of the transgenic lines we have examined in this study than we had observed previously (26), where all the independent lines appeared to express the genes during different developmental stages with no greater than 10% variation. It may be that expression in our previous transgenic lines was fortuitously homogeneous for unknown reasons, or that the expression from single copy transgenes perturbs the erythroid cell transcriptional environment less than the presence of the two to four copies found in the animals under present analysis. Additionally, deletion of important regulatory DNA elements within the locus could (to some extent) affect the position-independent expression of the YAC (G. Li, J.B., and J.D.E., unpublished observations). For these reasons, we focused our interpretation and discussion on those results that display the most dramatic deviation(s) from the expression characteristics of the wild-type YAC transgenic lines and, more importantly, on those results that appeared to be consistent among the mutant lines.
It has been shown previously that binding sites for the ubiquitous
transcription factor YY1 and for GATA factors contribute to the
repressing activity of this element. However, Raich et al.
(44) also demonstrated that two GATA sites immediately downstream of
the YY1 site have a pronounced positive effect on  -globin gene
transcription. These results clearly demonstrate the presence of
additional, functionally important positive regulatory elements even
within this narrowly defined silencer region.
-globin gene
transcription. These results clearly demonstrate the presence of
additional, functionally important positive regulatory elements even
within this narrowly defined silencer region.
Because there are other positive regulatory elements both upstream and
downstream of the silencer, it could be argued that the mutant is
defective in activation because the missing sequence elements must
cooperate with these flanking positive elements to confer efficient
globin gene transcription (i.e., that the deleted sequences per
se have no positive regulatory effect). Alternatively, changing
the spacing between the more upstream and the more gene-proximal
positive regulatory elements (after deleting the 125-bp silencer) might
impair the formation of an active transcription complex on the
 -globin gene promoter. Finally, it is possible that the 3′ part of
the deleted silencer is additionally the extreme 5′ end of an extended
-globin gene promoter. Finally, it is possible that the 3′ part of
the deleted silencer is additionally the extreme 5′ end of an extended
 -globin gene promoter, thus affecting assembly of the basal
apparatus (but clearly this cannot be the only effect; next paragraph).
Further studies introducing specific mutations that do not alter the
spacing within this region should distinguish between these several
possibilities.
-globin gene promoter, thus affecting assembly of the basal
apparatus (but clearly this cannot be the only effect; next paragraph).
Further studies introducing specific mutations that do not alter the
spacing within this region should distinguish between these several
possibilities.
The more remarkable observation from analysis of the  -silencer
deletion mutant was that it also significantly reduced transcription of
the γ-globin genes in erythroid cells of the embryonic yolk sac (Fig.
(Fig.3).3). This effect was erythroid stage-specific, not significantly
affecting expression of either the γ- or β-globin genes during
either the fetal liver or adult stages. We interpret this observation
to mean that deletion of sequences within the upstream region of the
-silencer
deletion mutant was that it also significantly reduced transcription of
the γ-globin genes in erythroid cells of the embryonic yolk sac (Fig.
(Fig.3).3). This effect was erythroid stage-specific, not significantly
affecting expression of either the γ- or β-globin genes during
either the fetal liver or adult stages. We interpret this observation
to mean that deletion of sequences within the upstream region of the
 -globin gene generally affects embryonic stage-specific globin gene
expression. This is the second such mutation in the locus that appears
to quite specifically affect the activity of these same genes during
the embryonic stage of murine erythropoiesis. We previously reported
that duplication of HS4 of the LCR resulted in a decline in
-globin gene generally affects embryonic stage-specific globin gene
expression. This is the second such mutation in the locus that appears
to quite specifically affect the activity of these same genes during
the embryonic stage of murine erythropoiesis. We previously reported
that duplication of HS4 of the LCR resulted in a decline in  -, and a
simultaneous, reciprocal increase in γ-, globin mRNA accumulation in
the yolk sac (26). Taken together with the data presented here, both
mutations point to an effect which may best be explained by assuming
that the LCR as well as the
-, and a
simultaneous, reciprocal increase in γ-, globin mRNA accumulation in
the yolk sac (26). Taken together with the data presented here, both
mutations point to an effect which may best be explained by assuming
that the LCR as well as the  - and γ-globin genes are in a
different chromosomal domain in the yolk sac than in the fetal liver or
adult spleen. Under the assumption that an LCR holocomplex must
physically interact with other gene proximal protein/DNA complexes,
these observations could be further interpreted to infer that deletion
of the
- and γ-globin genes are in a
different chromosomal domain in the yolk sac than in the fetal liver or
adult spleen. Under the assumption that an LCR holocomplex must
physically interact with other gene proximal protein/DNA complexes,
these observations could be further interpreted to infer that deletion
of the  -globin silencer not only reduces the ability of the LCR to
productively interact with the
-globin silencer not only reduces the ability of the LCR to
productively interact with the  -globin gene promoter, but that these
sequences may be involved in establishing this specific chromatin
conformation that is required for proper γ-globin gene transcription
in the murine yolk sac.
-globin gene promoter, but that these
sequences may be involved in establishing this specific chromatin
conformation that is required for proper γ-globin gene transcription
in the murine yolk sac.
Previously, Wijgerde et al. (7) showed that two globin genes
can be transcribed inside a single erythroid cell within only a brief
time span. In terms of the competition model (10), these data were also
interpreted to suggest that an LCR holocomplex (26) somehow
distinguishes which gene to activate, presumably depending on the array
and concentration of transcription factors present in an erythroid cell
at different developmental stages. It has been shown experimentally
that the human γ-globin genes are more abundantly expressed than the
 -globin gene in the murine embryonic yolk sac, thus implying that in
a competitive regulatory environment, the human LCR interacts with the
more distant human γ-globin gene promoters either more frequently or
more stably than with the
-globin gene in the murine embryonic yolk sac, thus implying that in
a competitive regulatory environment, the human LCR interacts with the
more distant human γ-globin gene promoters either more frequently or
more stably than with the  -globin gene promoter at this stage of
murine erythropoiesis. However, the
-globin gene promoter at this stage of
murine erythropoiesis. However, the  -globin upstream region might be
important for recruiting or positioning the LCR, not only to enhance
-globin upstream region might be
important for recruiting or positioning the LCR, not only to enhance
 -globin gene transcription but also to allow efficient interaction
with the γ-globin gene promoters by decreasing the distance between
LCR and γ-globin genes. Deletion of such a hypothetical LCR
“positioning” element would then result in a decline in
expression of both the
-globin gene transcription but also to allow efficient interaction
with the γ-globin gene promoters by decreasing the distance between
LCR and γ-globin genes. Deletion of such a hypothetical LCR
“positioning” element would then result in a decline in
expression of both the  - and γ-globin genes.
- and γ-globin genes.
Competition Between γ- and β-Globin Genes Is Not Mediated by the β-Globin 3′ Enhancer.
Previous work has shown that the human fetal γ- and adult β-globin genes are regulated by competition (13), but the participating cis-regulatory elements have not yet been defined. We therefore tested the hypothesis that the adult β-globin gene enhancer might be playing a role in this competition by deleting it from the huβYAC (human β-globin YAC) and then analyzing expression of the globin locus genes.
Deletion of the β-globin 3′ enhancer resulted in a significant loss of β-globin expression both in the fetal liver and in the adult spleen (Fig. (Fig.3),3), indicating, as expected, that this enhancer activity is required for adult β-globin gene transcription (34, 35). However, γ-globin synthesis was not elevated in the fetal liver, nor did its expression persist into the adult stage of erythropoiesis, even though β-globin synthesis had dramatically declined, thereby demonstrating that the adult β-globin gene 3′ enhancer does not play a role in the competition between the γ- and β-globin genes in either the fetus or in adult animals. Furthermore, deletion of the β-globin gene 3′ enhancer had a quite specific effect, solely on the expression of the adult β-globin gene.
Acknowledgments
We thank Jie Fan for outstanding technical assistance. We gratefully acknowledge fellowship support from the Cooley’s Anemia Foundation (Q.L.), the Deutsche Forschungsgemeinschaft (Grant A2:Bu 895/1-1), and the American Heart Association (J. B.), and research support from the National Institutes of Health (HL 24415; J.D.E.).
Footnotes
Abbreviations: YAC, yeast artificial chromosome; HS, hypersensitive sites; LCR, locus control region.
References
 R. (1991) Methods Enzymol.
194. [Abstract]
R. (1991) Methods Enzymol.
194. [Abstract]Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.94.1.169
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/94/1/169.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Transvection-like interchromosomal interaction is not observed at the transcriptional level when tested in the Rosa26 locus in mouse.
PLoS One, 14(2):e0203099, 14 Feb 2019
Cited by: 1 article | PMID: 30763343 | PMCID: PMC6375575
Engineering Globin Gene Expression.
Mol Ther Methods Clin Dev, 12:102-110, 18 Dec 2018
Cited by: 8 articles | PMID: 30603654 | PMCID: PMC6310746
Review Free full text in Europe PMC
Genomic editing tools to model human diseases with isogenic pluripotent stem cells.
Stem Cells Dev, 23(22):2673-2686, 07 Oct 2014
Cited by: 31 articles | PMID: 25075441 | PMCID: PMC4216528
Review Free full text in Europe PMC
Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease.
Blood, 118(17):4599-4608, 31 Aug 2011
Cited by: 199 articles | PMID: 21881051 | PMCID: PMC3208277
Shadow enhancers: frequently asked questions about distributed cis-regulatory information and enhancer redundancy.
Bioessays, 34(2):135-141, 15 Nov 2011
Cited by: 107 articles | PMID: 22083793 | PMCID: PMC3517143
Go to all (32) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mutation of a transcriptional motif of a distant regulatory element reduces the expression of embryonic and fetal globin genes.
Hum Mol Genet, 12(22):2941-2948, 23 Sep 2003
Cited by: 7 articles | PMID: 14506128 | PMCID: PMC2808411
Investigations of a human embryonic globin gene silencing element using YAC transgenic mice.
Exp Biol Med (Maywood), 231(3):328-334, 01 Mar 2006
Cited by: 4 articles | PMID: 16514181 | PMCID: PMC2812921
Sequences located 3' to the breakpoint of the hereditary persistence of fetal hemoglobin-3 deletion exhibit enhancer activity and can modify the developmental expression of the human fetal A gamma-globin gene in transgenic mice.
J Biol Chem, 270(17):10256-10263, 01 Apr 1995
Cited by: 26 articles | PMID: 7537267
Developmental control of epsilon- and gamma-globin genes.
Ann N Y Acad Sci, 850:10-17, 01 Jun 1998
Cited by: 1 article | PMID: 9668523
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: HL 24415
Grant ID: R01 HL024415




