Abstract
Free full text

Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin.
Abstract
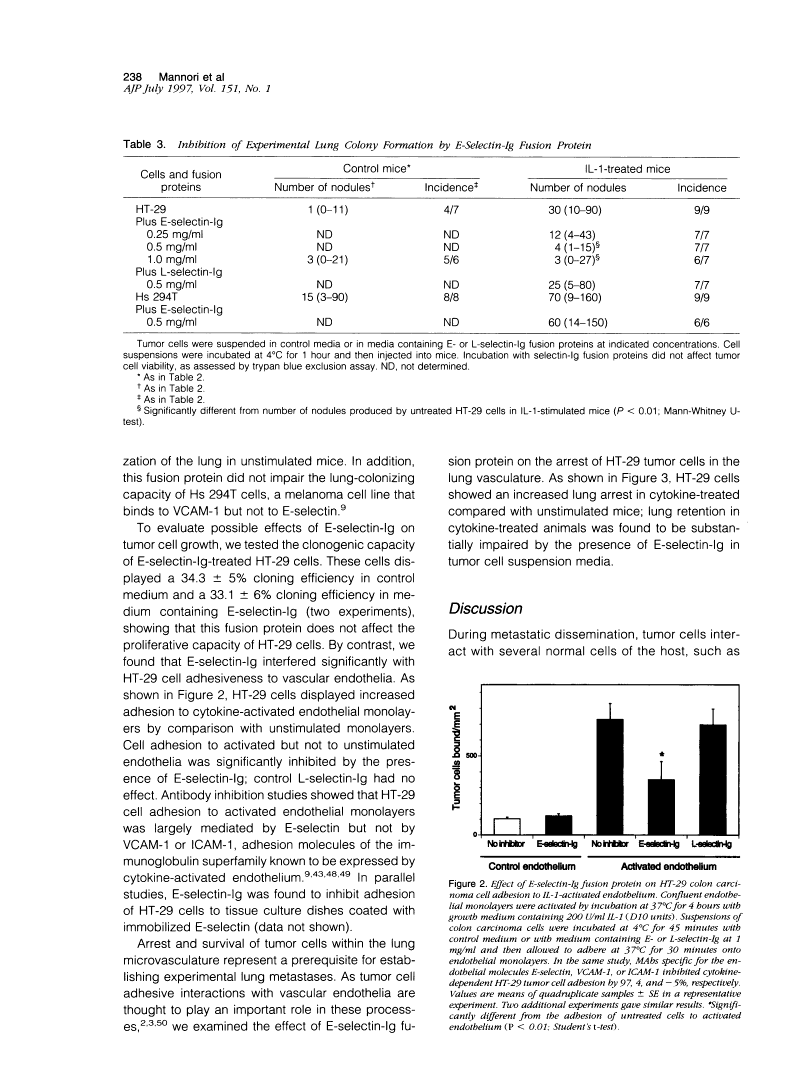
During metastasis, tumor cells adhere to vascular endothelia. E-selectin is an adhesive protein expressed by cytokine-activated endothelium that can support adhesion of colon cancer cells through the recognition of specific carbohydrate ligands. Using a series of colon carcinoma cell lines that displayed E-selectin adhesiveness and an increased metastatic capacity in cytokine-treated mice, we examined possible inhibition of cytokine-dependent experimental lung metastasis by a soluble form of E-selectin, the recombinant fusion protein E-selectin-immunoglobulin. We found that E-selectin-immunoglobulin bound to the surfaces of HT-29 colon carcinoma cells and blocked the formation of cytokine-inducible experimental lung metastases; control L-selectin-immunoglobulin also bound to HT-29 cells but had no effect on tumor cell lung colonization. E-selectin-immunoglobulin was found to interfere with E-selectin-dependent adhesion of HT-29 cells to activated vascular endothelium and to block the retention of these cells in the lung, a process that implies tumor cell adhesive interactions with the host vasculature. Our results demonstrate that E-selectin-immunoglobulin inhibits adhesion and formation of lung metastases by colon carcinoma cells and suggest that impairment of tumor cell-endothelium adhesion might represent a therapeutic approach to the metastatic diffusion of tumors.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Weiss L, Orr FW, Honn KV. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin Exp Metastasis. 1989 Mar-Apr;7(2):127–167. [Abstract] [Google Scholar]
- Honn KV, Tang DG. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992 Nov;11(3-4):353–375. [Abstract] [Google Scholar]
- Pauli BU, Augustin-Voss HG, el-Sabban ME, Johnson RC, Hammer DA. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev. 1990 Nov;9(3):175–189. [Abstract] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. [Abstract] [Google Scholar]
- Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. [Abstract] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7390–7397. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994 Oct;6(5):663–673. [Abstract] [Google Scholar]
- McEver RP. Selectins. Curr Opin Immunol. 1994 Feb;6(1):75–84. [Abstract] [Google Scholar]
- Rice GE, Bevilacqua MP. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. [Abstract] [Google Scholar]
- Lauri D, Needham L, Martin-Padura I, Dejana E. Tumor cell adhesion to endothelial cells: endothelial leukocyte adhesion molecule-1 as an inducible adhesive receptor specific for colon carcinoma cells. J Natl Cancer Inst. 1991 Sep 18;83(18):1321–1324. [Abstract] [Google Scholar]
- Kojima N, Handa K, Newman W, Hakomori S. Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1288–1295. [Abstract] [Google Scholar]
- Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993 Jan 15;53(2):354–361. [Abstract] [Google Scholar]
- Giavazzi R, Foppolo M, Dossi R, Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993 Dec;92(6):3038–3044. [Europe PMC free article] [Abstract] [Google Scholar]
- Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995 Oct 1;55(19):4425–4431. [Abstract] [Google Scholar]
- Sawada R, Tsuboi S, Fukuda M. Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1994 Jan 14;269(2):1425–1431. [Abstract] [Google Scholar]
- Sawada R, Lowe JB, Fukuda M. E-selectin-dependent adhesion efficiency of colonic carcinoma cells is increased by genetic manipulation of their cell surface lysosomal membrane glycoprotein-1 expression levels. J Biol Chem. 1993 Jun 15;268(17):12675–12681. [Abstract] [Google Scholar]
- Stone JP, Wagner DD. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest. 1993 Aug;92(2):804–813. [Europe PMC free article] [Abstract] [Google Scholar]
- Fukushima K, Hirota M, Terasaki PI, Wakisaka A, Togashi H, Chia D, Suyama N, Fukushi Y, Nudelman E, Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984 Nov;44(11):5279–5285. [Abstract] [Google Scholar]
- Itzkowitz SH, Yuan M, Fukushi Y, Palekar A, Phelps PC, Shamsuddin AM, Trump BF, Hakomori S, Kim YS. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Res. 1986 May;46(5):2627–2632. [Abstract] [Google Scholar]
- Magnani JL, Nilsson B, Brockhaus M, Zopf D, Steplewski Z, Koprowski H, Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [Abstract] [Google Scholar]
- Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [Abstract] [Google Scholar]
- Berg EL, Magnani J, Warnock RA, Robinson MK, Butcher EC. Comparison of L-selectin and E-selectin ligand specificities: the L-selectin can bind the E-selectin ligands sialyl Le(x) and sialyl Le(a). Biochem Biophys Res Commun. 1992 Apr 30;184(2):1048–1055. [Abstract] [Google Scholar]
- Tyrrell D, James P, Rao N, Foxall C, Abbas S, Dasgupta F, Nashed M, Hasegawa A, Kiso M, Asa D, et al. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10372–10376. [Europe PMC free article] [Abstract] [Google Scholar]
- Handa K, Nudelman ED, Stroud MR, Shiozawa T, Hakomori S. Selectin GMP-140 (CD62; PADGEM) binds to sialosyl-Le(a) and sialosyl-Le(x), and sulfated glycans modulate this binding. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1223–1230. [Abstract] [Google Scholar]
- Nelson RM, Dolich S, Aruffo A, Cecconi O, Bevilacqua MP. Higher-affinity oligosaccharide ligands for E-selectin. J Clin Invest. 1993 Mar;91(3):1157–1166. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson RM, Aruffo A, Dolich S, Cecconi O, Mannori G, Bevilacqua MP. Quantitative determination of selectin-carbohydrate interactions. Cold Spring Harb Symp Quant Biol. 1992;57:271–279. [Abstract] [Google Scholar]
- Polley MJ, Phillips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, Paulson JC. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou Q, Moore KL, Smith DF, Varki A, McEver RP, Cummings RD. The selectin GMP-140 binds to sialylated, fucosylated lactosaminoglycans on both myeloid and nonmyeloid cells. J Cell Biol. 1991 Oct;115(2):557–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Foxall C, Watson SR, Dowbenko D, Fennie C, Lasky LA, Kiso M, Hasegawa A, Asa D, Brandley BK. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992 May;117(4):895–902. [Europe PMC free article] [Abstract] [Google Scholar]
- Hoff SD, Irimura T, Matsushita Y, Ota DM, Cleary KR, Hakomori S. Metastatic potential of colon carcinoma. Expression of ABO/Lewis-related antigens. Arch Surg. 1990 Feb;125(2):206–209. [Abstract] [Google Scholar]
- Saitoh O, Wang WC, Lotan R, Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992 Mar 15;267(8):5700–5711. [Abstract] [Google Scholar]
- Dejana E, Martin-Padura I, Lauri D, Bernasconi S, Bani MR, Garofalo A, Giavazzi R, Magnani J, Mantovani A, Menard S. Endothelial leukocyte adhesion molecule-1-dependent adhesion of colon carcinoma cells to vascular endothelium is inhibited by an antibody to Lewis fucosylated type I carbohydrate chain. Lab Invest. 1992 Mar;66(3):324–330. [Abstract] [Google Scholar]
- Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [Abstract] [Google Scholar]
- Mulligan MS, Watson SR, Fennie C, Ward PA. Protective effects of selectin chimeras in neutrophil-mediated lung injury. J Immunol. 1993 Dec 1;151(11):6410–6417. [Abstract] [Google Scholar]
- Lee WP, Gribling P, De Guzman L, Ehsani N, Watson SR. A P-selectin-immunoglobulin G chimera is protective in a rabbit ear model of ischemia-reperfusion. Surgery. 1995 Apr;117(4):458–465. [Abstract] [Google Scholar]
- Watson SR, Fennie C, Lasky LA. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 1991 Jan 10;349(6305):164–167. [Abstract] [Google Scholar]
- Watson SR. L-selectin-IgG chimera--in vitro and in vivo. Agents Actions Suppl. 1993;41:103–109. [Abstract] [Google Scholar]
- Becker-André M, Hooft van Huijsduijnen R, Losberger C, Whelan J, Delamarter JF. Murine endothelial leukocyte-adhesion molecule 1 is a close structural and functional homologue of the human protein. Eur J Biochem. 1992 Jun 1;206(2):401–411. [Abstract] [Google Scholar]
- Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. [Europe PMC free article] [Abstract] [Google Scholar]
- Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [Abstract] [Google Scholar]
- Rice GE, Munro JM, Bevilacqua MP. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. [Europe PMC free article] [Abstract] [Google Scholar]
- Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. [Abstract] [Google Scholar]
- Aruffo A, Kolanus W, Walz G, Fredman P, Seed B. CD62/P-selectin recognition of myeloid and tumor cell sulfatides. Cell. 1991 Oct 4;67(1):35–44. [Abstract] [Google Scholar]
- Palmiter RD, Behringer RR, Quaife CJ, Maxwell F, Maxwell IH, Brinster RL. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. [Abstract] [Google Scholar]
- Mannori G, Cecconi O, Mugnai G, Ruggieri S. Role of glycolipids in the metastatic process: characteristics of neutral glycolipids in clones with different metastatic potentials isolated from a murine fibrosarcoma cell line. Int J Cancer. 1990 May 15;45(5):984–988. [Abstract] [Google Scholar]
- Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. [Abstract] [Google Scholar]
- Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [Abstract] [Google Scholar]
- Zetter BR. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990 Mar 1;322(9):605–612. [Abstract] [Google Scholar]
- Honn KV, Tang DG, Chen YQ. Platelets and cancer metastasis: more than an epiphenomenon. Semin Thromb Hemost. 1992;18(4):392–415. [Abstract] [Google Scholar]
- Whitworth PW, Pak CC, Esgro J, Kleinerman ES, Fidler IJ. Macrophages and cancer. Cancer Metastasis Rev. 1990 Feb;8(4):319–351. [Abstract] [Google Scholar]
- Tözeren A, Kleinman HK, Grant DS, Morales D, Mercurio AM, Byers SW. E-selectin-mediated dynamic interactions of breast- and colon-cancer cells with endothelial-cell monolayers. Int J Cancer. 1995 Jan 27;60(3):426–431. [Abstract] [Google Scholar]
- Kishimoto T, Ishikura H, Kimura C, Takahashi T, Kato H, Yoshiki T. Phenotypes correlating to metastatic properties of pancreas adenocarcinoma in vivo: the importance of surface sialyl Lewis(a) antigen. Int J Cancer. 1996 Aug 22;69(4):290–294. [Abstract] [Google Scholar]
- Bani MR, Garofalo A, Scanziani E, Giavazzi R. Effect of interleukin-1-beta on metastasis formation in different tumor systems. J Natl Cancer Inst. 1991 Jan 16;83(2):119–123. [Abstract] [Google Scholar]
- Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, Mantovani A, Dejana E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990 Aug 1;50(15):4771–4775. [Abstract] [Google Scholar]
- Arguello F, Baggs RB, Graves BT, Harwell SE, Cohen HJ, Frantz CN. Effect of IL-1 on experimental bone/bone-marrow metastases. Int J Cancer. 1992 Nov 11;52(5):802–807. [Abstract] [Google Scholar]
- Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA. Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst. 1996 Feb 21;88(3-4):198–205. [Abstract] [Google Scholar]
- Orosz P, Echtenacher B, Falk W, Rüschoff J, Weber D, Männel DN. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med. 1993 May 1;177(5):1391–1398. [Europe PMC free article] [Abstract] [Google Scholar]
- Bennicelli JL, Elias J, Kern J, Guerry D., 4th Production of interleukin 1 activity by cultured human melanoma cells. Cancer Res. 1989 Feb 15;49(4):930–935. [Abstract] [Google Scholar]
- Köck A, Schwarz T, Urbanski A, Peng Z, Vetterlein M, Micksche M, Ansel JC, Kung HF, Luger TA. Expression and release of interleukin-1 by different human melanoma cell lines. J Natl Cancer Inst. 1989 Jan 4;81(1):36–42. [Abstract] [Google Scholar]
- Burrows FJ, Haskard DO, Hart IR, Marshall JF, Selkirk S, Poole S, Thorpe PE. Influence of tumor-derived interleukin 1 on melanoma-endothelial cell interactions in vitro. Cancer Res. 1991 Sep 15;51(18):4768–4775. [Abstract] [Google Scholar]
- Spriggs D, Imamura K, Rodriguez C, Horiguchi J, Kufe DW. Induction of tumor necrosis factor expression and resistance in a human breast tumor cell line. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6563–6566. [Europe PMC free article] [Abstract] [Google Scholar]
- Old LJ. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. [Abstract] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. [Abstract] [Google Scholar]
- Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–378. [Abstract] [Google Scholar]
- Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez MJ, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986 Nov;150(3):195–203. [Abstract] [Google Scholar]
- Biancone L, Araki M, Araki K, Vassalli P, Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. J Exp Med. 1996 Feb 1;183(2):581–587. [Europe PMC free article] [Abstract] [Google Scholar]
- Dennis JW, Laferté S, Waghorne C, Breitman ML, Kerbel RS. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. [Abstract] [Google Scholar]
- Mannori G, Mugnai G, Ruggieri S. Lectin reactivity of murine fibrosarcoma lines with a different metastatic potential. Cancer Lett. 1991 Aug;59(2):133–138. [Abstract] [Google Scholar]
- Mulligan MS, Varani J, Dame MK, Lane CL, Smith CW, Anderson DC, Ward PA. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. [Europe PMC free article] [Abstract] [Google Scholar]
- Gundel RH, Wegner CD, Torcellini CA, Clarke CC, Haynes N, Rothlein R, Smith CW, Letts LG. Endothelial leukocyte adhesion molecule-1 mediates antigen-induced acute airway inflammation and late-phase airway obstruction in monkeys. J Clin Invest. 1991 Oct;88(4):1407–1411. [Europe PMC free article] [Abstract] [Google Scholar]
- Pilewski JM, Yan HC, Juhasz I, Christofidou-Solomidou M, Williams J, Murphy GF, Albelda SM. Modulation of adhesion molecules by cytokines in vivo using human/severe combined immunodeficient (SCID) mouse chimeras. J Clin Immunol. 1995 Nov;15(6 Suppl):122S–129S. [Abstract] [Google Scholar]
- Martens CL, Cwirla SE, Lee RY, Whitehorn E, Chen EY, Bakker A, Martin EL, Wagstrom C, Gopalan P, Smith CW, et al. Peptides which bind to E-selectin and block neutrophil adhesion. J Biol Chem. 1995 Sep 8;270(36):21129–21136. [Abstract] [Google Scholar]
Associated Data
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/151/1/233
Citations & impact
Impact metrics
Citations of article over time
Article citations
Nucleolin-Sle A Glycoforms as E-Selectin Ligands and Potentially Targetable Biomarkers at the Cell Surface of Gastric Cancer Cells.
Cancers (Basel), 12(4):E861, 02 Apr 2020
Cited by: 16 articles | PMID: 32252346 | PMCID: PMC7226152
Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation.
Mol Oncol, 12(5):579-593, 30 Mar 2018
Cited by: 38 articles | PMID: 29215790 | PMCID: PMC5928367
The Interaction of Selectins and PSGL-1 as a Key Component in Thrombus Formation and Cancer Progression.
Biomed Res Int, 2017:6138145, 07 Jun 2017
Cited by: 47 articles | PMID: 28680883 | PMCID: PMC5478826
Review Free full text in Europe PMC
Sialic acids in cancer biology and immunity.
Glycobiology, 26(2):111-128, 30 Oct 2015
Cited by: 206 articles | PMID: 26518624
Review
A potential role for 6-sulfo sialyl Lewis X in metastasis of bladder urothelial carcinoma.
Urol Oncol, 33(11):496.e1-9, 29 Jun 2015
Cited by: 16 articles | PMID: 26137907
Go to all (45) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
E-selectin modulates the malignant properties of T84 colon carcinoma cells.
Biochem Biophys Res Commun, 293(3):1099-1106, 01 May 2002
Cited by: 14 articles | PMID: 12051773
Endothelial leukocyte adhesion molecule-1-dependent adhesion of colon carcinoma cells to vascular endothelium is inhibited by an antibody to Lewis fucosylated type I carbohydrate chain.
Lab Invest, 66(3):324-330, 01 Mar 1992
Cited by: 51 articles | PMID: 1371572
A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells.
Cancer Res, 60(2):450-456, 01 Jan 2000
Cited by: 82 articles | PMID: 10667600
[Clinical significance of circulating soluble E-selectin (ELAM-1) in patients with cancers].
Nihon Rinsho, 53(7):1770-1775, 01 Jul 1995
Cited by: 1 article | PMID: 7543167
Review