Abstract
Free full text

This article has been retracted. Retraction in:
Role of Interleukin 12 and Costimulators in T Cell Anergy In Vivo
Abstract
The induction of T cell anergy in vivo is thought to result from antigen recognition in the absence of co-stimulation and inflammation, and is associated with a block in T cell proliferation and Th1 differentiation. Here we have examined the role of interleukin (IL)-12, a potent inducer of Th1 responses, in regulating this process. T cell tolerance was induced by the administration of protein antigen without adjuvant in normal mice, and in recipients of adoptively transferred T cells from T cell receptor transgenic mice. The administration of IL-12 at the time of tolerance induction stimulates Th1 differentiation, but does not promote antigen-specific T cell proliferation. Conversely, inhibiting CTLA-4 engagement during anergy induction reverses the block in T cell proliferation, but does not promote full Th1 differentiation. T cells exposed to tolerogenic antigen in the presence of both IL-12 and anti–CTLA-4 antibody are not anergized, and behave identically to T cells which have encountered immunogenic antigen. These results suggest that two processes contribute to the induction of anergy in vivo; CTLA-4 engagement, which leads to a block in the ability of T cells to proliferate to antigen, and the absence of a prototypic inflammatory cytokine, IL-12, which prevents the differentiation of T cells into Th1 effector cells. The combination of IL-12 and anti–CTLA-4 antibody is sufficient to convert a normally tolerogenic stimulus to an immunogenic one.
Clonal anergy is an important mechanism of peripheral T cell tolerance (1, 2). The development of anergy was first analyzed in cloned lines of mouse Th1 cells. These studies have led to the widely accepted view that anergy is induced when antigen is recognized in the absence of second signals (3–5), the best defined of which are costimulators of the B7 family (6). Thus classical studies, showing that antigens administered with strong adjuvants elicit T cell responses and antigens without adjuvants induce tolerance, are now interpreted to suggest that an important function of adjuvants is to enhance the expression of the costimulators that determine whether antigen recognition will lead to activation or anergy (3). Based on these ideas, many attempts have been made to prevent immune reactions, e.g., against organ allografts, and to induce tolerance by blocking B7 molecules in vivo (7–10). Conversely, the ectopic expression of B7 proteins in tissues results in an increased predisposition to tissue-specific autoimmune injury, presumably by breaking T cell tolerance to self antigens in the tissues (11–14).
Two sets of findings suggest that costimulators alone may not dictate the choice between T cell activation and tolerance. First, expression of B7-1 as a transgene in tissues does not, by itself, lead to autoimmune disease. In most of these studies, an additional local stimulus, such as the proinflammatory cytokine TNF-α, has to be provided (13). Second, we have recently shown that completely blocking costimulation during exposure to antigen leads to a state of clonal ignorance and not to anergy in vivo (15). In fact, anergy develops when antigen-specific T cells use the inhibitory receptor for B7 molecules, CTLA-4, to interact with costimulators at the time of antigen recognition. These results provide an explanation for the severe autoimmune disease that develops in mice in which CTLA-4 is deleted by targeted gene disruption (16, 17). Thus, the choice between T cell activation versus anergy may be determined partly by whether CD28 or CTLA-4 engages B7 molecules on APCs (18). Although the mechanisms that influence this choice are not yet defined, the findings do suggest that simply the absence of costimulation may not be the mechanism of T cell anergy in vivo.
It is well known that adjuvants induce the production of numerous cytokines from APCs. One of these cytokines, IL-12, is a potent, and obligatory, inducer of Th1 differentiation (19). Since T cell anergy preferentially affects Th1 cells (3, 20–22), we postulated that a deficiency of local IL-12 production may play an important role in the induction of anergy. We have tested this hypothesis in two experimental models of T cell anergy induced in vivo, i.e., normal mice and recipients of T cells from TCR transgenic mice treated with high doses of antigen in the absence of adjuvant. We show that in both models of anergy, IL-12 by itself promotes Th1 differentiation, but not T cell proliferation or expansion, in response to tolerogenic antigen. Blocking CTLA-4 during anergy induction overcomes the block in T cell expansion, but does not lead to full Th1 differentiation. Exposure to tolerogenic antigen in the presence of both IL-12 and an anti–CTLA-4 antibody prevents the induction of anergy, and induces a full T cell response. Thus, anergy induction in vivo involves both a block in Th1 differentiation and in T cell proliferation. These different aspects of T cell anergy are regulated by IL-12 and CTLA-4, respectively. Local inflammation also prevents the induction of T cell anergy, presumably by stimulating IL-12 secretion and changing the expression and recognition of costimulators. Our results support the potential importance of IL-12 in breaking T cell tolerance, e.g., in autoimmune diseases, and conversely, the potential of IL-12 antagonists in inducing tolerance, e.g., to allografts.
MATERIALS AND METHODS
Mice.
BALB/c mice, 6–8 wk old, were purchased from the Jackson Laboratory (Bar Harbor, ME). Transgenic mice expressing the DO.11.10 TCR (hereafter called DO.11) specific for the chicken OVA peptide, OVA323–339, in association with I-Ad were obtained from Dr. D. Loh (Hoffmann-La Roche, Tokyo, Japan). The mice were bred in our pathogen- and viral antibody–free facility in accordance with the guidelines of the Committee on Animals of the Harvard Medical School (Cambridge, MA) and those prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (Washington, DC). The mice were typed for the DO.11 by staining peripheral blood cells with antibodies against CD4 and Vβ8.
Adoptive Transfers and In Vivo Tolerance Induction.
To induce tolerance in normal BALB/c mice, animals were treated with 0.3–1 mg i.p. of OVA or PBS. 7–10 d later, mice were immunized with 100 μg OVA in CFA (Difco, Detroit, MI) and s.c. Draining lymph nodes were harvested 7 d later and assayed as described below. To induce tolerance in TCR transgenic T cells, we used the protocol described by Kearney et al. (23). Lymph node and spleen cells pooled from DO.11 transgenic mice were prepared as previously described (15) and injected intravenously into unirradiated syngeneic BALB/c recipients, such that each received 5 × 106 KJ-126+ CD4+ T cells. The recipients were either not immunized (naive), or 2 d after cell transfer were treated subcutaneously in the footpad with 50 μg of the OVA323–339 peptide emulsified in IFA (immunized) or intravenously with 300 μg of the peptide in PBS (tolerized). The peripheral lymph nodes (submandibular, axillary, brachial, inguinal, and popliteal) were harvested 3 d after treatment in all the groups except for the immunized recipients, from which only the draining lymph nodes (inguinal and popliteal) were harvested. For in vivo challenge experiments, mice treated as described above were immunized subcutaneously in the footpad with 50 μg of the peptide emulsified in IFA 10 d after the initial treatment, and the draining lymph nodes (inguinal and popliteal) were harvested 3 d later. To study the effects of cytokines and costimulation on the induction of tolerance, mice were treated either with 0.3–3 μg of murine IL-12 (gift from Dr. S. Wolf, Genetics Institute, Andover, MA) or 100 μg of anti–CTLA-4 antibody (reference 18; gift from Dr. J. Bluestone, University of Chicago, Chicago, IL) on days −1, 0, and +1 of the initial antigen exposure. To study the effects of local inflammation on the induction of tolerance, tolerized mice were given PBS emulsified in CFA subcutaneously in the footpad on day 0. After the harvest, the lymph node cells from each group were stained with CyC-labeled anti-CD4 mAb (PharMingen, San Diego, CA) and biotinylated KJ-126 clonotypic antibody specific for the DO.11 followed by streptavidin-FITC for FACS® analysis. Analyses were performed on a FACScan® flow cytometer.
In Vitro Proliferation and Cytokine Assays.
Harvested cells were assayed as previously described (15). In brief, 5 × 105 lymph node cells from each of the groups were cultured in 0.2 ml of RPMI 1640 supplemented with 1 mM l-glutamine, penicillin, streptomycin, nonessential amino acids, sodium pyruvate, Hepes (all from Life Technologies, Grand Island, NY), 5 × 10−5 M 2-ME, and 10% fetal bovine serum (Sigma Chemical Co., St. Louis, MO). Cells were restimulated with 0–1 μg/ml of OVA323–339. After 48 h, cultures were pulsed for 6 h with 1 μCi [3H]TdR (New England Nuclear, Boston, MA), and incorporated radioactivity was measured in a Betaplate scintillation counter (LBK Pharmacia, Piscataway, NJ). To determine cytokine production, 2 × 106 lymphocytes were cultured in 1 ml of medium in the presence of 0, 0.1, or 1 μg/ml of OVA323–339. Supernatants were collected after 24, 48, and 72 h, and levels of IL-2, IL-4, and IFN-γ were assayed by ELISA as described previously (15). In some experiments, CD4+ T cells were purified from the lymph nodes of experimental mice using CD4+ Dynalbeads® (Dynal A.S., Oslo, Norway), and cultured with mitomycin C–treated BALB/c splenocytes as APCs. Typically, T cell preparations were >98% CD4+ as determined by staining and flow cytometry.
Intracellular Staining for IFN-γ.
5 × 105 lymph node cells were restimulated with 1 μg/ml OVA323–339 for 24 h. During the last 6 h, 10 μg/ml brefeldin A (Sigma Chemical Co.) was added to cultures. The lymph node cells were then washed in PBS/1% BSA containing 10 μg/ml brefeldin A, stained with antibodies to CD4 and the DO.11 (with the monoclonal antibody KJ-126; reference 24 ), and fixed overnight with 4% paraformaldehyde (Sigma Chemical Co.). The fixed cells were permeabilized by incubation in PBS with 1% BSA and 2% saponin (Sigma Chemical Co.) at room temperature for 10 min. A phycoerythrin-conjugated anti– IFN-γ antibody (PharMingen), diluted to 20 μg/ml in PBS with 1% BSA and 2% saponin was then added, and the cells were incubated for a further 30 min. Finally, the cells were washed once with PBS with 1% BSA and 2% saponin and once with PBS with 1% BSA, and analyzed on a FACScan® flow cytometer.
), and fixed overnight with 4% paraformaldehyde (Sigma Chemical Co.). The fixed cells were permeabilized by incubation in PBS with 1% BSA and 2% saponin (Sigma Chemical Co.) at room temperature for 10 min. A phycoerythrin-conjugated anti– IFN-γ antibody (PharMingen), diluted to 20 μg/ml in PBS with 1% BSA and 2% saponin was then added, and the cells were incubated for a further 30 min. Finally, the cells were washed once with PBS with 1% BSA and 2% saponin and once with PBS with 1% BSA, and analyzed on a FACScan® flow cytometer.
RESULTS
IL-12 Promotes Th1 Differentiation in Response to Tolerogenic Antigen, but Does not Rescue T Cell Proliferation.
T cell responses to antigen are induced by administering a foreign antigen subcutaneously with an adjuvant. In contrast, T cells are rendered unresponsive when high doses of antigen are given systemically in the absence of adjuvant (20–23). A vigorous response to OVA can be detected in the peripheral lymph node cells of BALB/c mice immunized with OVA in CFA subcutaneously (Fig. (Fig.1).1). If these mice are pretreated with 1 mg of OVA in saline i.v., they fail to generate Th1 responses when they are subsequently immunized with antigen in CFA (Fig. (Fig.1).1). Noticeably, Th2 responses to OVA are not abrogated in tolerized mice. The observation that tolerance is associated with a selective Th1 block led us to examine whether anergy induction was a result of antigen recognition in the absence of the major Th1-inducing cytokine, IL-12 (19). Moreover, the production of IL-12 is enhanced by inflammatory stimuli, and it is likely to be poorly expressed under the conditions where anergy is induced. When mice are tolerized and given three daily injections of recombinant IL-12 (1 μg i.p.), the T cells secrete IFN-γ after a challenge with antigen in adjuvant (Fig. (Fig.1).1). However, IL-12 is not able to prevent the proliferative block associated with anergy.

IL-12 promotes Th1 differentiation in response to tolerogenic antigen in vivo. BALB/c mice were pretreated on day 0 with saline (immunized) or 1 mg of OVA i.v. (tolerized), with or without daily treatments (days −1, 0, +1) of 1 μg of IL-12 (tolerized + IL-12). All mice were immunized with OVA emulsified in CFA in the footpad on day 10. The popliteal and inguinal lymph nodes were harvested 7 d later, and lymph node cells were restimulated in vitro with OVA. Proliferative responses (A) were assessed after 72 h by measuring [3H]thymidine incorporation, and cytokine secretion (B) was assayed by ELISA. Results are from one representative experiment of three. The error bars represent standard deviations calculated from duplicate wells.
The limitation of studying T cell responses to immunogenic and tolerogenic antigen in normal mice is that the fate of antigen-specific T cells can not be followed. Therefore, it is difficult to determine if the inability of T cells to respond to antigen is due to the deletion, migration, or functional inactivation of antigen-specific T cells. To study the regulation of T cell anergy by IL-12 in antigen-specific T cells, we used the method described by Kearney et al. (23), in which small numbers of DO.11 transgenic T cells (specific for OVA323–339+ I–Ad) are adoptively transferred into syngeneic BALB/c mice and exposed to antigen in immunogenic or tolerogenic form. Approximately 1–2% of the peripheral lymph node cells in these adoptive transfer recipients are OVA-specific DO.11 T cells, and these can be readily followed using a clonotypic mAb (KJ-126; reference 24). DO.11 T cells can be tolerized in vivo by treating adoptive transfer recipients with high doses of aqueous OVA323–339 peptide intraveneously (see below, and references 15 and 23).
Two important features of the adoptive transfer system are that responses of naive T cells can be measured, and that it is possible to detect changes in functional response patterns within 3 d of antigen exposure. At this time, one can readily distinguish naive, activated (immunized), and tolerized T cells (Fig. (Fig.2).2). Naive cells produce IL-2 with a peak at ~day 3 of in vitro stimulation with antigen, proliferate weakly, and do not produce IFN-γ. T cells from immunized mice produce IL-2 rapidly, show strong proliferation, and secrete IFN-γ. (The decline in supernatant IL-2 levels with increasing duration of culture may be due to absorption of secreted IL-2 by the proliferating T cells.) Tolerized cells do not proliferate or secrete IL-2 or IFN-γ. Similar to the results obtained with normal BALB/c mice (Fig. (Fig.1),1), administration of IL-12 (1 μg/d) at the time of tolerance induction markedly enhances IFN-γ secretion, but has no effect on IL-2 production and proliferation in this adoptive transfer tolerance system (Fig. (Fig.2).2).

IL-12 promotes early Th1 differentiation, but not T cell proliferation, in antigen-specific T cells exposed to tolerogenic antigen. BALB/c recipients of DO.11 T cells were left untreated (A); immunized with OVA323–339 peptide in IFA in the footpad on day 0 (B); tolerized with OVA323–339 peptide intravenously on day 0 (C); or tolerized and treated daily (days −1, 0, +1) with 1 μg of IL-12 (D). The submandibular, axillary, brachial, popliteal, and inguinal lymph nodes (only popliteal and inguinal lymph nodes in B) were harvested 3 d later. Lymph node cells were restimulated in vitro with OVA323–339 peptide. Proliferative responses were assessed after 72 h by measuring [3H]-thymidine incorporation, and are corrected for the numbers of KJ-126+ CD4+ cells per well. Cytokine secretion was assayed daily by ELISA. Results are from one representative experiment of three. The error bars represent standard deviations calculated from duplicate wells.
To exclude a contribution of differential T cell expansion in vivo on responses to antigen restimulation, CD4+ T cells were purified from the lymph nodes of naive, immunized, and tolerized mice that either had been treated or had not been treated with IL-12. By determining the percentage of KJ-126+ cells present in these purified CD4+ T cell populations, we were able to set up restimulation assays with the same number of DO.11 T cells from each group of mice (4,000–5,000 KJ-126+ T cells/well for proliferation assays and 20,000–25,000 KJ-126+ T cells/well for cytokine assays), and use syngeneic spleen cells from untreated mice as APCs. As shown in Fig. Fig.3,3, IFN-γ is detected in cultures of purified T cells from tolerized mice given IL-12, demonstrating that they are able to secrete this cytokine when restimulated with antigen in vitro. These T cells do not produce IL-2 or proliferate when restimulated with OVA323–339 peptide, confirming that IL-12 does not prevent the block in T cell proliferation associated with anergy induction. The results obtained in this set of experiments, using equivalent numbers of purified antigen-specific T cells from different experimental groups of mice, were identical to those obtained with bulk lymph node cultures (Fig. (Fig.2).2). We therefore used unfractionated lymph node cells in all subsequent experiments.

Purified, anergic antigen-specific T cells treated with IL-12 in vivo produce IFN-γ, but do not proliferate. BALB/c recipients of DO.11 T cells were treated as in Fig. Fig.2.2. CD4+ T cells were purified from lymph node cells. An aliquot of the purified CD4+ T cells was stained with antibodies to CD4 and the DO.11 (KJ-126) to determine the percentage of KJ-126+ T cells present. Equal numbers of antigen-specific T cells were restimulated in vitro with OVA323–339 peptide and syngeneic spleen cells from BALB/c mice as APCs. Proliferative responses of 5,000 DO.11 T cells/well, and cytokine secretion from 25,000 DO.11 T cells/well, were assayed as in Fig. Fig.2.2. Results are from one representative experiment of two. The error bars represent standard deviations calculated from duplicate wells.
Since IL-12 is known to stimulate both T cells and NK cells to produce IFN-γ, an important question is, what cells produce IFN-γ in mice given tolerogenic antigen + IL-12. When lymph node cells from tolerized mice treated with IL-12 are restimulated in vitro, the secretion of IFN-γ is antigen dose–dependent, suggesting that OVA323–339-specific DO.11 T cells are the source of this cytokine. The production of IFN-γ can be directly visualized by intracellular staining and flow cytometry. As shown in Fig. Fig.4,4, antigen-specific T cells exposed in vivo to immunogenic antigen or tolerogenic antigen and IL-12 synthesize detectable levels of IFN-γ when restimulated with antigen in culture. In contrast, naive or tolerized T cells do not make IFN-γ, and under these conditions KJ-126− cells also do not synthesize IFN-γ. Therefore, in lymph node cells stimulated with antigen in vitro, the major source of IFN-γ is the antigen-specific T cells.

Intracellular staining for IFN-γ in immunized and tolerized DO.11 T cells. BALB/c recipients of DO.11 T cells were treated as in Fig. Fig.2.2. CD4+ T cells were purified from lymph node cells. Lymph node cells were restimulated in vitro with 1 μg/ml OVA323–339 peptide for 24 h, stained for expression of CD4, the DO.11, and intracellular IFN-γ as described in Materials and Methods, and analyzed by flow cytometry. The dot plots show expression of KJ-126 and IFN-γ in CD4+ T cells, and the histograms show the expression of IFN-γ in KJ-126+ CD4+ T cells (continuous black lines). Control stains are represented by the discontinuous grey lines.
Finally, we have also examined the effects of different doses of IL-12 on T cell expansion and functional responses. The injection of tolerogenic antigen (aqueous peptide intravenously) results in a significant expansion of antigen-specific T cells, although this is not as great as the expansion seen in draining lymph nodes after priming with peptide in adjuvant (Table (Table1).1). Administration of up to 1 μg/d of IL-12 together with tolerogenic antigen has a small effect on T cell numbers. This effect is more pronounced at higher concentrations of IL-12 (Table (Table1),1), and is probably due to an antigen-independent polyclonal stimulation induced by IL-12 (24a). If adoptive transfer recipients are treated with IL-12 alone, without antigen, the numbers of lymph node cells recovered, as well as the numbers of KJ-126+ cells, increase about two- to threefold (Table (Table1).1). Lower doses of IL-12 do not induce this polyclonal response, but at these doses the Th1 response is also much less. Interestingly, despite the T cell expansion seen with tolerogenic antigen, and even more with tolerogenic antigen + IL-12, the cells do not proliferate upon in vitro restimulation. This suggests that IL-12 may augment the abortive T cell response to tolerogenic antigen, but is not able to prevent the proliferative block associated with anergy.
Table 1
Effects of Increasing Doses of IL-12 on T Cell Responses In Vivo
| IL-12 | Naive | Immunized | Tolerized | |||
|---|---|---|---|---|---|---|
| T cell expansion in vivo | ||||||
| No. KJ-126+ T cells (× 10−4) per lymph node | ||||||
| μg/d | ||||||
 0 0 | 1.7 ± 0.8 | 31 ± 6.5 | 16.9 ± 5.3 | |||
 0.3 0.3 | ND | ND | 17.3 ± 2.4 | |||
 1 1 | 4.1 ± 1.1 | 44.5 ± 9.1 | 22.3 ± 8.6 | |||
 3 3 | 6.6 ± 1.8 | ND | 63.1 ± 18.2 | |||
| In vitro proliferation after restimulation with peptide | ||||||
| Proliferation (CPM/KJ-126+ cell) after 3 d of restimulation | ||||||
| μg/d | ||||||
 0 0 | 12.4 ± 2.4 | 22.8 ± 1.0 | 10.3 ± 0.9 | |||
 0.3 0.3 | ND | ND | 8.9 ± 0.6 | |||
 1 1 | 12.4 ± 1.3 | 30.5 ± 2.4 | 8.3 ± 0.1 | |||
 3 3 | 10.5 ± 2.6 | ND | 12.9 ± 3.2 | |||
| In vitro IFN-γ production following restimulation with peptide | ||||||
| IFN-γ (U/ml) production after 3 d of restimulation | ||||||
| μg/d | ||||||
 0 0 | 0 | 169 ± 9 | 0 | |||
 0.3 0.3 | ND | ND | 52 ± 13 | |||
 1 1 | 24 ± 4 | 212 ± 49 | 157 ± 41 | |||
 3 3 | 78 ± 3 | ND | 199 ± 18 | |||
Peripheral lymph nodes (axillary, brachial, inguinal, and popliteal for D3 naive and tolerized mice, inguinal and popliteal only for D3 immunized) were harvested from adoptive transfer recipients which were treated as described in the text. The percentage of KJ-126+ CD4+ cells present was determined by flow cytometry. To obtain the total numbers of KJ-126+ cells present per lymph node, the percent of KJ-126+ cells in lymph nodes was multiplied by the total number of cells recovered in each group and divided by the number of lymph nodes harvested. In vitro proliferation and IFN-γ production was assessed by restimulating lymph node cells with 1 μg/ml OVA 323–339 peptide for 3 d. Every treatment group consisted of two to three mice. The numbers shown are the averages of two to four experiments.
The Combination of IL-12 and Anti-CTLA-4 Antibody Prevents the Induction of Anergy In Vivo.
We have recently shown that tolerance induction in vivo is due to an active inhibition that requires the recognition of B7 molecules by the inhibitory T cell receptor for B7, CTLA-4 (15). This raises the possibility that blocking CTLA-4 may enable T cells to proliferate in response to tolerogenic antigen and, together with IL-12, may convert a normally tolerogenic stimulus to an immunogenic one. To test this, DO.11 transfer recipients were tolerized by injection of aqueous peptide antigen, and treated with anti–CTLA-4 mAb, IL-12, or both. As shown in Fig. Fig.5,5, in mice given tolerogenic antigen, anti–CTLA-4 mAb significantly enhances IL-2 production and antigen-specific T cell proliferation, IL-12 alone promotes IFN-γ production, and the two together stimulate responses to tolerogenic antigen that are comparable to those induced in draining lymph node cells after immunization with antigen in adjuvant. Furthermore, blocking CTLA-4 enables T cells to expand in vivo after administration of tolerogenic antigen (Table (Table2).2).
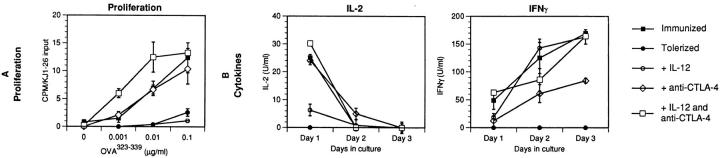
T cell anergy is prevented by a combination of IL-12 and anti-CTLA-4 antibody. BALB/c recipients of DO.11 T cells were immunized with OVA323–339 peptide in IFA in the footpad on day 0, or tolerized with 300 μg of OVA323–339 peptide intraveneously on day 0, with or without daily treatments (days −1, 0, +1) of 1 μg of IL-12 (tolerized + IL-12), 100 μg anti–CTLA-4 mAb (tolerized + anti–CTLA-4), or both IL-12 and anti– CTLA-4 (tolerized + IL-12 and anti–CTLA-4). The submandibular, axillary, brachial, popliteal, and inguinal lymph nodes from all tolerized mice, and popliteal and inguinal lymph nodes from immunized animals, were harvested 3 d later. Lymph node cells were restimulated in vitro with OVA323–339 peptide, and assayed as in Fig. Fig.2.2. Cytokine results are shown for cells restimulated with 1 μg/ml of OVA323–339 peptide; cells stimulated with no antigen showed no detectable cytokine production. Tolerized mice given a control antibody (isotype-matched with anti–CTLA-4 mAb) behaved identically to mice which were not given the antibody (reference 15 and data not shown). Results are pooled and averaged from two to four experiments with each treatment group. The error bars represent standard deviations calculated.
Table 2
T Cell Expansion in Response to Antigen In Vivo
| No. KJ-126+ cells (× 10−4) per lymph node | ||
|---|---|---|
| Group | Day 3 | |
| Naive | 1.8 ± 0.9 | |
| Immunized | 29.0 ± 3.6 | |
| Tolerized | 12.2 ± 2.4 | |
 + IL-12 + IL-12 | 17.2 ± 6.7 | |
 + anti–CTLA-4 + anti–CTLA-4 | 47.5 ± 6.4 | |
 + IL-12 and anti–CTLA-4 + IL-12 and anti–CTLA-4 | 58.7 ± 16.1 | |
Peripheral lymph nodes (axillary, brachial, inguinal, and popliteal for naive and tolerized mice, inguinal and popliteal only for immunized mice) were harvested from adoptive transfer recipients which were treated as described in the text. The percentage of KJ1-26+ CD4+ cells present was determined by flow cytometry. To obtain the total numbers of KJ-126+ cells present per lymph node, the percent of KJ-126+ cells in lymph nodes was multiplied by the total number of cells recovered in each group and divided by the number of lymph nodes harvested. Every treatment group consisted of two to three mice. The numbers shown are the averages of two to four experiments. All values are × 10−4, and the errors represent the standard deviations calculated.
The expression of IL-12 and costimulatory molecules by tissue APCs is enhanced by adjuvants and various microbial products (6, 19). We therefore asked if local inflammation provided by a strong adjuvant would also prevent the induction of T cell tolerance. To do this, DO.11 transfer recipients were given tolerogenic antigen (aqueous peptide intravenously) and treated with CFA, a potent adjuvant, subcutaneously. The expansion and functional responses of T cells were assayed in peripheral lymph nodes which did or did not drain the site of inflammation. As shown in Fig. Fig.6,6, anergy is induced in lymph nodes away from the site of CFA injection. However, T cells in lymph nodes which are near the sites of inflammation are not anergized, and respond as if they had encountered immunogenic antigen. Furthermore, the expansion of T cells in response to tolerogenic antigen is significantly enhanced in the lymph nodes near the source of inflammation. In two experiments, the numbers of KJ-126+ cells (× 10−4) in lymph nodes of different mice were: naive, 2.3 ± 0.6; immunized (draining nodes only), 27.1 ± 4.8; tolerized, 17.1 ± 5.9; tolerized + local CFA (draining nodes only), 45.6 ± 12.3; and tolerized + distant CFA (nondraining nodes), 15.8 ± 11.1. Thus, a potent inflammatory stimulus leads to local T cell expansion and Th1 differentiation even in response to the systemic administration of normally tolerogenic antigen.

Local inflammation prevents the induction of T cell anergy in vivo. BALB/c recipients of DO.11 T cells were left untreated (A); immunized with OVA323–339 peptide in IFA in the footpad on day 0 (B); or tolerized with 300 μg of OVA323–339 peptide intravenously on day 0 (C). Some tolerized mice also received an injection of CFA in the footpad (D and E). The submandibular, axillary, brachial, popliteal, and inguinal lymph nodes (only popliteal and inguinal lymph nodes in B) were harvested 3 d later. From the CFA-treated mice, popliteal, and inguinal lymph node cells (D) and the submandibular, axillary, and brachial lymph node cells (E) were harvested and assayed separately. Lymph node cells were restimulated in vitro with OVA323–339 peptide and assayed as in Fig. Fig.2.2. Results are from one representative experiment of three. The error bars represent standard deviations calculated from duplicate wells.
DISCUSSION
The studies described in this paper were aimed at analyzing the mechanisms of peripheral T cell tolerance in vivo. Specifically, we examined the roles of a prototypic inflammatory cytokine, IL-12, and costimulatory molecules in this process. The experimental models we have chosen involve treating normal mice, or mice into which TCR transgenic T cells are transferred, with high doses of antigen without adjuvants. In the TCR transgenic system, it is possible to formally demonstrate that antigen-specific T cells are not killed when they are exposed to tolerogenic antigen, but are rendered functionally unresponsive, in that they become incapable of producing IL-2, proliferating, and differentiating into Th1 effectors (Figs. (Figs.22 and and3,3, and references 15 and 23). By these criteria, the form of tolerance induced by aqueous peptide antigen appears to be an in vivo counterpart of the phenomenon of clonal anergy first described in T cell clones (3, 4).
Our recent results have demonstrated that the role of B7-mediated costimulation in T cell anergy in vivo is complex, and somewhat unexpected. We have found that a B7 antagonist does not promote tolerance, as might have been expected based on the results of in vitro studies (3, 4), but rather it prevents the induction of anergy and keeps T cells in a naive but functionally competent state. The induction of tolerance depends on the engagement of B7 molecules, presumably on APCs, by the CTLA-4 counter receptor on antigen-responsive T cells (15). In striking contrast, T cell priming, as measured by proliferation and differentiation into effector cells, is dependent on B7 recognition by the activating counter-receptor, CD28 (15, 25). These results have led to the conclusion that a key determinant of T cell activation versus tolerance is which receptor, CD28 or CTLA-4, is used to engage B7 costimulators during T cell antigen recognition (15). During these studies, we realized that the block in Th1 differentiation associated with tolerance induction in vivo is unlikely to be solely due to CTLA-4–B7 interactions, because giving anti–CTLA-4 antibody at the time of tolerance induction led to variable amounts of IFN-γ produced by antigen-specific T cells (15). Moreover, in the earlier studies, tolerance was induced by intraperitoneal injection of peptide in IFA, and in subsequent experiments, when we induced tolerance with aqueous peptide without any adjuvant, anti–CTLA-4 alone was ineffective at allowing Th1 differentiation (Fig. (Fig.5).5). This is not surprising, because the development of Th1 effector cells requires IL-12, which is produced by macrophages and dendritic cells usually in response to microbes and other inflammatory stimuli (25). Tolerogenic antigens, such as aqueous peptides administered intraveneously, are unlikely to stimulate IL-12 production, since such antigens do not contain macrophage-activating substances. We therefore reasoned that to completely prevent T cell anergy and allow effector cell development, it might be necessary both to change the pattern of costimulator recognition and to provide IL-12. Our experimental results support this notion. A combination of anti–CTLA-4 antibody and recombinant IL-12 is sufficient to convert a tolerogenic form of peptide antigen to a fully immunogenic form, whereas neither alone is sufficient (Fig. (Fig.5).5). These conclusions are most convincingly demonstrated in the TCR transgenic adoptive transfer system, where the fates of antigen-specific TCR transgenic T cells can be followed quantitatively. However, it is likely that the same is true of tolerance induced in normal T cell populations, since IL-12 promotes Th1 differentiation but does not fully restore T cell expansion in normal mice (Fig. (Fig.1).1). Furthermore, a strong adjuvant, which induces local inflammation, has the same functional effects on tolerance induction as the combination of anti–CTLA-4 antibody and IL-12 (Fig. (Fig.6).6). Although this does not prove that the effects of the adjuvant are due to a change in costimulation and IL-12 production, the findings are consistent with this idea. Importantly, IL-12 alone stimulates production of the signature Th1 cytokine, IFN-γ, but does not prevent the proliferative block associated with tolerance induction (Figs. (Figs.22 and and3).3). Therefore, the inflammatory cytokine by itself is insufficient for preventing T cell tolerance.
The mechanisms by which anti–CTLA-4 antibody and IL-12 cooperate to convert a tolerogenic stimulus to an immunogenic one are not precisely defined. It is likely that tolerogenic antigens administered without adjuvants induce low levels of B7 costimulator expression on APCs. Such low levels may preferentially engage the high-affinity receptor for B7 molecules, which is CTLA-4, triggering inhibitory biochemical signals which block IL-2 production and T cell proliferation (26, 27). Antigen recognition by T cells in the absence of IL-2 and proliferation is known to lead to functional anergy (3, 5). Immunogenic forms of antigen, such as peptides administered with strong adjuvants, may induce higher levels and more prolonged expression of B7 molecules, resulting in the engagement of CD28 and T cell activation. Thus, changing B7 recognition from CTLA-4 to CD28 may be sufficient to release the block in IL-2 production and T cell proliferation. It appears unlikely that this alone would induce T cell differentiation into effectors, because differentiation requires another signal(s), which, for the Th1 pathway, is provided by IL-12. Thus, adjuvants may trigger two distinct reactions that contribute to preventing T cell tolerance and eliciting immune responses—they stimulate IL-12 production, and they alter costimulator expression in a way that promotes engagement by CD28.
The ability of IL-12 to function in preventing tolerance induction is consistent with the finding that IL-12 is produced locally in lesions associated with autoimmune diseases (28, 29). However, in these situations it is not possible to establish if IL-12 production is a cause of the autoimmune reactions or the consequence of local T cell activation and IFN-γ secretion. Moreover, it is not known if blocking IL-12 will promote tolerance induction. Clearly, this may not be an adequate treatment for inducing tolerance by itself, because if mice are immunized and treated with IL-12 antagonists, the result is a defect in Th1 differentiation, and not anergy (30). The same result is seen in mice lacking the p40 chain of IL-12, and in mice in which the major IL-12–triggered transcription factor, Stat4, is deleted (31–33). However, IL-12 antagonists administered with agents that favor CTLA-4-B7 interactions, or with forms of antigens that preferentially trigger such interactions, may enhance the induction of anergy. It remains to be determined if this will be useful for the thaerapeutic induction of tolerance to prevent allograft rejection, or to treat Th1-dependent autoimmune diseases.
Acknowledgments
We thank Dr. Jeff Bluestone for generous gifts of anti–CTLA-4 antibody and for his critical comments on the manuscript, and Dr. Stan Wolf for gift of recombinant IL-12. Supported by National Institutes of Health grants AI-35297 and AI-25022 (A.K. Abbas), T32 HL-07267 (V.L. Perez), and K01 RR-00121 (C.A. London), and a National Cancer Institute training grant (R.G. Maki).
Footnotes
1 Abbreviations used in this paper: DO.11, DO.11.10 TCR.
REFERENCES
Articles from The Journal of Experimental Medicine are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1084/jem.186.7.1119
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jem/article-pdf/186/7/1119/1112835/97-0639.pdf
Citations & impact
Impact metrics
Article citations
Reduced antigen presentation capability and modified inflammatory/immunosuppressive cytokine expression of induced monocyte-derived dendritic cells from peripheral blood of piglets infected with porcine circovirus type 2.
Arch Virol, 163(5):1231-1239, 03 Feb 2018
Cited by: 5 articles | PMID: 29397454
Reversal of New-Onset Type 1 Diabetes With an Agonistic TLR4/MD-2 Monoclonal Antibody.
Diabetes, 64(10):3614-3626, 30 Jun 2015
Cited by: 13 articles | PMID: 26130764 | PMCID: PMC9162148
Anti-diabetic actions of carbon monoxide-releasing molecule (CORM)-A1: Immunomodulation and regeneration of islet beta cells.
Immunol Lett, 165(1):39-46, 31 Mar 2015
Cited by: 12 articles | PMID: 25839127
Human Chorionic Villous Mesenchymal Stem Cells Modify the Functions of Human Dendritic Cells, and Induce an Anti-Inflammatory Phenotype in CD1+ Dendritic Cells.
Stem Cell Rev Rep, 11(3):423-441, 01 Jun 2015
Cited by: 46 articles | PMID: 25287760
The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis.
Ann N Y Acad Sci, 1281:16-35, 16 Jan 2013
Cited by: 168 articles | PMID: 23323860 | PMCID: PMC3715103
Review Free full text in Europe PMC
Go to all (54) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance.
Eur J Immunol, 32(4):972-981, 01 Apr 2002
Cited by: 73 articles | PMID: 11920563
Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement.
Immunity, 6(4):411-417, 01 Apr 1997
Cited by: 436 articles | PMID: 9133420
CTLA-4 regulates induction of anergy in vivo.
Immunity, 14(2):145-155, 01 Feb 2001
Cited by: 265 articles | PMID: 11239447
T cell anergy.
Annu Rev Immunol, 21:305-334, 19 Dec 2001
Cited by: 870 articles | PMID: 12471050
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: T32 HL-07267
NIAID NIH HHS (3)
Grant ID: P01 AI035297
Grant ID: AI-35297
Grant ID: AI-25022





