Abstract
Free full text

Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo
vivo
Abstract
Gcn5p is a transcriptional coactivator required for correct expression of various genes in yeast. Several transcriptional regulators, including Gcn5p, possess intrinsic histone acetyltransferase (HAT) activity in vitro. However, whether the HAT activity of any of these proteins is required for gene activation remains unclear. Here, we demonstrate that the HAT activity of Gcn5p is critical for transcriptional activation of target genes in vivo. Core histones are hyperacetylated in cells overproducing functional Gcn5p, and promoters of Gcn5p-regulated genes are associated with hyperacetylated histones upon activation by low-copy Gcn5p. Point mutations within the Gcn5p catalytic domain abolish both promoter-directed histone acetylation and Gcn5p-mediated transcriptional activation. These data provide the first in vivo evidence that promoter-specific histone acetylation, catalyzed by functional Gcn5p, plays a critical role in gene activation.
Eukaryotic transcription occurs on DNA templates that exist in a repressive chromatin environment. As revealed by both in vitro and in vivo studies, binding of transcriptional activators and basal transcription machinery to this template is often, but not always, hampered by barriers imposed by nucleosomal arrays (for review, see Owen-Hughes and Workman 1994; Wolffe 1994; Felsenfeld 1996). One solution to this problem is the existence of chromatin remodeling factors/activities that can alter or restructure nucleosomes near promoter elements, often in an ATP-dependent fashion, thus facilitating transcriptional initiation (for review, see Kingston et al. 1996; Pazin and Kadonaga 1997a; Tsukiyama and Wu 1997). Another solution may be provided by the post-translational modification of the core histones themselves (Bradbury 1992; Turner and O’Neill 1995; Wolffe and Pruss 1996).
Among the known covalent modifications of core histones, the reversible acetylation of internal, often invariant, lysine residues in the amino-terminal domains has long been linked positively to transcriptional activation (Brownell and Allis 1996). Although transcriptional regulation and histone acetylation appear to be correlated in several biological systems (e.g., Jeppesen and Turner 1993; Bone et al. 1994; Hebbes et al. 1994), experimental data demonstrating a causative role of histone acetylation in gene activation have remained elusive (however, see Ura et al. 1997). Recent discoveries of enzymes responsible for influencing the steady-state balance of histone acetylation (for review, see Hampsey 1997; Wade and Wolffe 1997) provide new opportunities to address this and related issues.
The Saccharomyces cerevisiae GCN5 gene encodes a transcription adaptor (or coactivator) that is required for full-level transcription of various genes (Georgakopoulos and Thireos 1992; Brandl et al. 1996; Martens et al. 1996; Saleh et al. 1997; Welihinda et al. 1997). Recently, yeast Gcn5p and homologous GCN5 family members from organisms ranging from Tetrahymena (p55; Brownell et al. 1996; Kuo et al. 1996) to humans [hGcn5p; human p300/CBP-associated factor (hPCAF); Yang et al. 1996; Wang et al. 1997], have been shown to possess intrinsic histone acetyltransferase (HAT) activity with strong preference for specific histone substrates and internal acetylation sites (e.g., Lys-14 of H3 and Lys-8 and Lys-16 of H4 with yGcn5p; Kuo et al. 1996). Intrinsic HAT activity has also been observed in several other proteins involved in transcriptional activation, such as PCAF (Yang et al. 1996), TATA-binding protein-associated factorII250 (TAFII250) and its homologs (Mizzen et al. 1996), p300/CBP (CREB-binding protein) (Bannister and Kouzarides 1996; Ogryzko et al. 1996), ACTR/SRC-1 (activator of retinoid receptor/steroid recptor coactivator) (Chen et al. 1997; Spencer et al. 1997), and ESA1 (essential SAS family acetyl transferase) (E.R. Smith, A. Eisen, J.C. Lucchesi, and C.D. Allis, in prep).
As a transcriptional coactivator, yGcn5p functions within the context of a multisubunit complex that interacts with specific transcription activators as well as the basal transcription machinery (Marcus et al. 1994; Silverman et al. 1994; Georgakopoulos et al. 1995; Candau and Berger 1996; Chiang et al. 1996). Known components of this adaptor complex include Ada1p, Ada2p, Ada3p/Ngg1p, and Ada5p/Spt20p (Candau and Berger 1996; Marcus et al. 1996; Roberts and Winston 1996; Grant et al. 1997; Horiuchi et al. 1997; Saleh et al. 1997). Recent biochemical and genetic evidence suggests that other members of the TATA-binding protein (TBP)-related class of SPT (suppressor of Ty) gene products may function with Gcn5p in larger, potentially redundant, chromatin remodeling or modification complexes (Grant et al. 1997; Roberts and Winston 1997).
To date, the best evidence linking HAT activity to transcriptional activation is derived from analyses of serial deletion mutants of yGcn5p (Candau et al. 1997). All GCN5 truncation alleles that are able to support Gcn5p functions in vivo possess detectable HAT activity in vitro. In contrast, mutants that lose the capability to acetylate histones in vitro fail to complement gcn5 null mutants in either growth or transcriptional reporter assays. However, whether Gcn5p affects levels of histone acetylation in vivo has not yet been directly demonstrated. Whether other proteins, including known components of the transcription machinery, are also physiological substrates for these activities remains an open question (Gu and Roeder 1997; Imhof et al. 1997; for review, see Pazin and Kadonaga 1997b).
In this report, we sought to investigate further the relationship between histone acetylation and transcriptional activation. Point mutations were introduced to residues of the minimal catalytic domain of yGcn5p (Candau et al. 1997) that are conserved in all known GCN5 family members. We demonstrate that Gcn5p is likely to acetylate core histones in vivo and that the HAT activity of this enzyme is critical for enhancing the transcription of target genes. Cross-linking and coimmunoprecipitation experiments using antibodies directed at a known site of Gcn5p-mediated acetylation reveal that the promoter region of affected genes is enriched in acetylated histone isoforms only in the presence of functional Gcn5p for transcriptional activation. Together, these results are consistent with models of targeted histone acetylation, and provide strong evidence that HAT activity of yGcn5p is essential for its in vivo function.
Results
Identification of amino acid residues in yGcn5p important for HAT activity
A minimal catalytic domain supporting Gcn5p-mediated histone acetylation has been mapped recently to a region that, between amino acids 170 and 253, contains three of four highly conserved subdomains identified in the GCN5 family of acetyltransferases (II–IV; see Fig. Fig.1)1) (Brownell et al. 1996; Candau et al. 1997). Others have identified conserved sequence motifs in amino-acetyltransferases (Coon et al. 1995; Lu et al. 1996) and putative acetyltransferases (Lin et al. 1996; Reifsnyder et al. 1996; Neuwald and Landsman 1997); the functional significance of residues within these motifs is not well understood. However, in a small number of cases, mutagenesis of conserved residues in domains II and III implicate these regions in acetyl CoA binding (Tercero and Wickner 1992; Coleman et al. 1996; Lu et al. 1996).
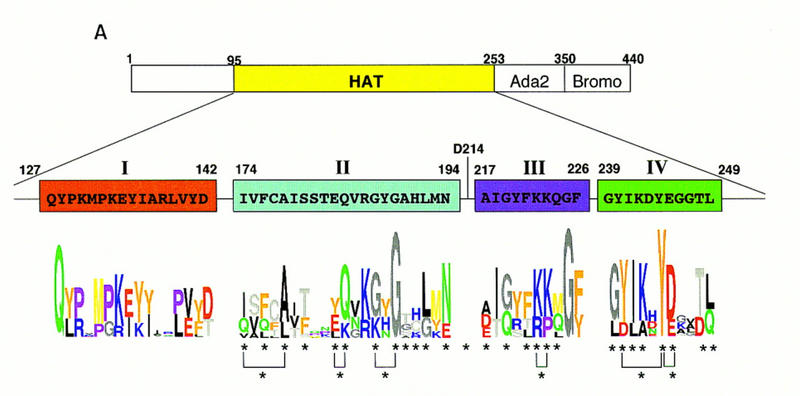

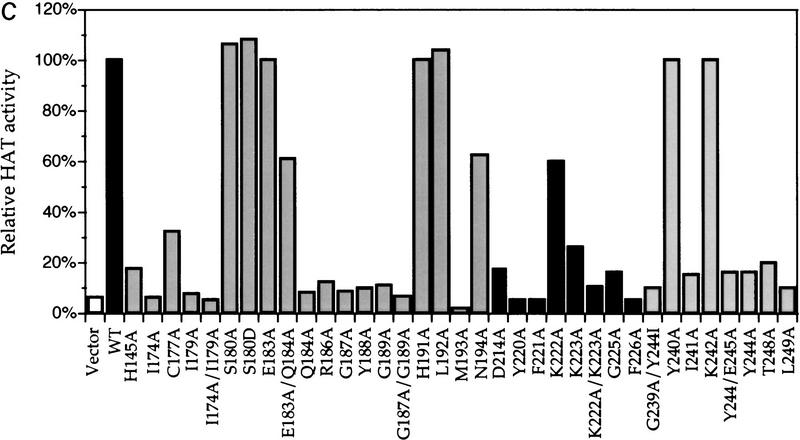
 Most conserved amino acid residues play an important role in histone acetylation in vitro. (A) Schematic diagram depicting the catalytic domain boundaries of yGcn5p (Brownell et al. 1996; Candau et al. 1997), amino acid sequences in HAT motifs I–IV, and sequence logos of each domain compiled from the sequences aligned in B. (B) Sequence alignments of Gcn5-type HATs, amino-acetyltransferases, and domain III homology regions from G10 proteins used in the analysis of the domain profiles shown in A. Color coding of the motifs corresponds to that in A. (C) Relative in vitro HAT activity of each mutant. Equivalent amounts of bacterially expressed Gcn5p derivatives were used for standard liquid HAT assays. Incorporation of the 3H-labeled acetate counts into histones was measured by scintillation counting and compared to wild-type Gcn5p. Shown are the relative HAT activity with the wild-type levels set to 100%. Vector only and wild-type controls were purified and assayed with every preparation of mutants analyzed to control for batch-to-batch variation; each mutant has been tested independently at least three times. Results are depicted from a single experiment; different shades of the bars represent each subdomain
Most conserved amino acid residues play an important role in histone acetylation in vitro. (A) Schematic diagram depicting the catalytic domain boundaries of yGcn5p (Brownell et al. 1996; Candau et al. 1997), amino acid sequences in HAT motifs I–IV, and sequence logos of each domain compiled from the sequences aligned in B. (B) Sequence alignments of Gcn5-type HATs, amino-acetyltransferases, and domain III homology regions from G10 proteins used in the analysis of the domain profiles shown in A. Color coding of the motifs corresponds to that in A. (C) Relative in vitro HAT activity of each mutant. Equivalent amounts of bacterially expressed Gcn5p derivatives were used for standard liquid HAT assays. Incorporation of the 3H-labeled acetate counts into histones was measured by scintillation counting and compared to wild-type Gcn5p. Shown are the relative HAT activity with the wild-type levels set to 100%. Vector only and wild-type controls were purified and assayed with every preparation of mutants analyzed to control for batch-to-batch variation; each mutant has been tested independently at least three times. Results are depicted from a single experiment; different shades of the bars represent each subdomain
To test whether similar residues are essential for the Gcn5p function in vivo, we first systematically substituted amino acid residues in the minimal catalytic domain of yGcn5p (amino acids 170–253) with alanine (Fig. (Fig.1A).1A). We chose to mutate residues that are well conserved among the known HATs that contain a clear acetyltransferase signature domain (domains II and III) and those that are shared only by histone acetyltransferases (domain IV). In vitro HAT activity of each mutant Gcn5p was tested after expressing and purifying these proteins from Escherichia coli. As seen in Figure Figure1C,1C, a large number, but not all, of these mutants showed significantly reduced HAT activity relative to wild-type Gcn5p. This result is consistent with the high degree of sequence conservation across these domains, and suggests that this region of the protein is critical for its ability to function as a HAT.
In vivo growth defects of gcn5 mutants
The above HAT assays were done with bacterially expressed Gcn5p in the absence of other yeast proteins. Because yGcn5p exists in several large, multisubunit complexes (Georgakopoulos et al. 1995; Candau and Berger 1996; Grant et al. 1997; Pollard and Peterson 1997; Saleh et al. 1997), we set out to determine whether any of the above Gcn5p mutants were also defective in vivo. To this end, each mutant was subcloned, along with GCN5 5′ and 3′ transcriptional regulatory sequences, into a low copy ARS/CEN plasmid and transformed into a gcn5Δ strain. Gcn5p is involved in maximal expression of certain amino acid biosynthesis genes (Georgakapolous and Thireos 1992); thus, loss of GCN5 leads to poor growth under amino acid starvation. The ability of each allele to complement growth of the gcn5Δ yeast strain in minimal medium was then investigated.
Shown in Figure Figure2A2A is a comparison of growth of several representative yeast transformants on synthetic minimal medium. As predicted, wild-type GCN5 rescued growth under amino acid starvation, whereas the vector alone control exhibited poor growth under these conditions. With each of the above point mutants as the sole source of Gcn5p, several mutants caused clear gcn5− phenotypes. Among these alleles, F221A caused the most profound defect followed by Y244A/E245A, D214A, G239A/Y244I, Y220A, I174A/I179A, and G187A/G189A. The last two double mutants caused subtle, but highly reproducible, growth phenotypes; such partial defects in the general control pathway can be augmented by applying amino acid analogs, such as ethionine or 3-aminotriazole to the medium (data not shown). The gcn− phenotypes associated with the above mutants were unlikely to be attributable to insufficient expression or protein instability as Western analyses showed roughly equal amounts of Gcn5p (Fig. (Fig.3A;3A; data not shown). It is also unlikely that F221A or other in vivo defective mutants possess gross conformational abnormality as coimmunoprecipitation, using an anti-Ada2p antibody, demonstrates that several of our in vivo defective mutants, including F221A, are still able to interact with Ada2p (data not shown).
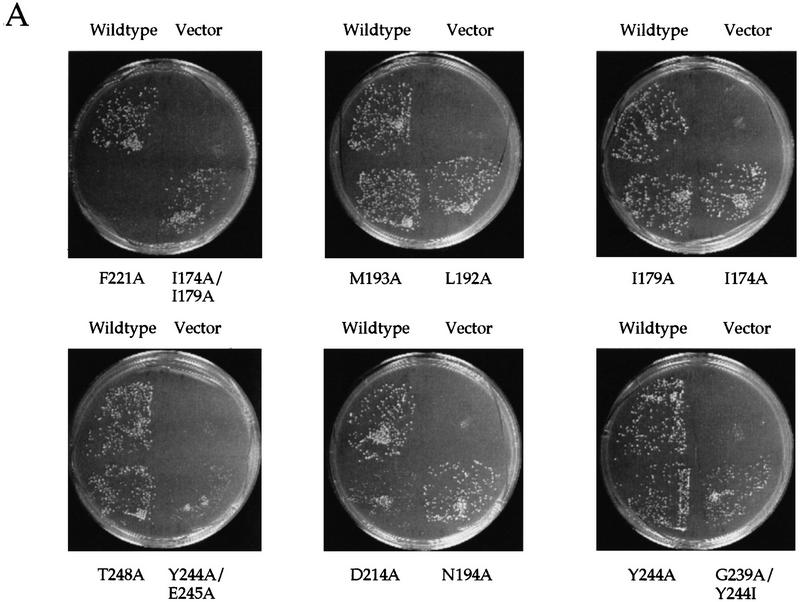
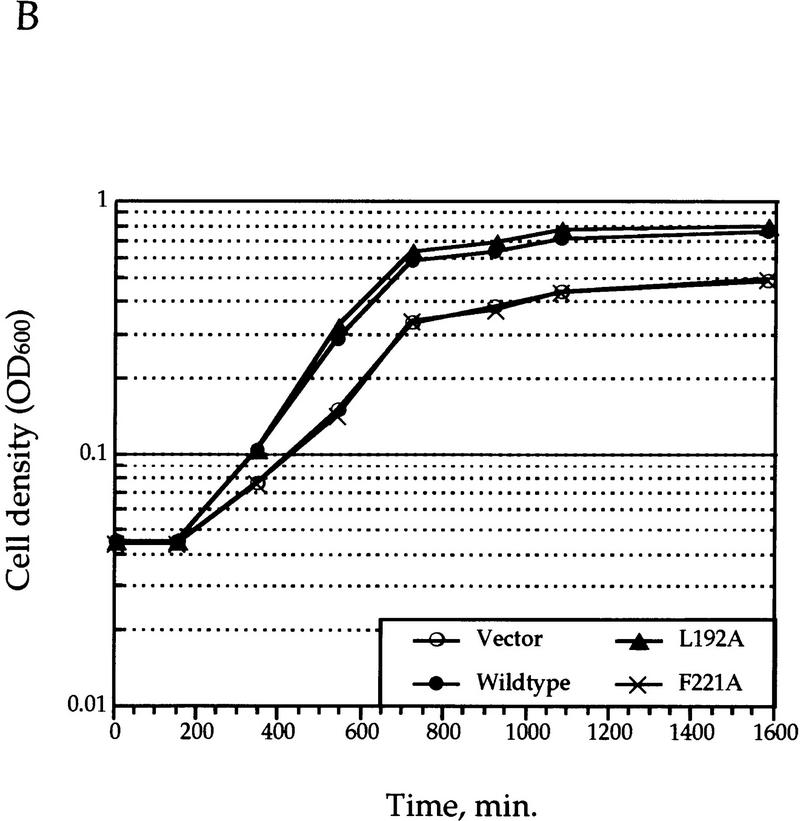
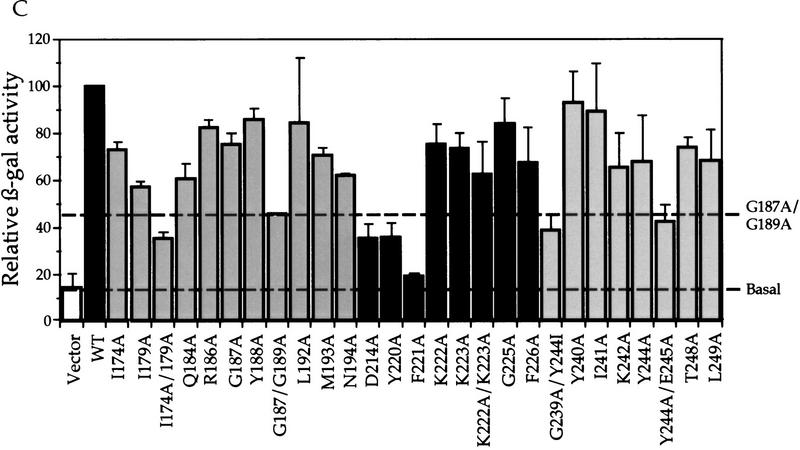
 In vivo tests of functions of mutant Gcn5p. (A) Growth complementation test. Shown are representative clones plated to synthetic minimal medium and grown at 30°C for 3 days before pictures were taken. All mutants have been tested at least three times for reproducibility. Note that certain mutations, such as Y220A, I174A/I179A, and G187A/G189A, consistently showed retarded growth. However, with long-termed storage at 4°C after 30°C incubation was completed, colonies of these strains reached the size close to that of wild-type strains. (B) Growth curves of four representative clones grown in minimal medium. (C) Transcriptional activation potency of Gcn5p derivatives. gcn5 null cells were first double-transformed with the GAL4–VP16 and β-gal expression constructs (both are 2 μ plasmids) followed by different GCN5 alleles (CEN/ARS minichromosomes). The β-gal expression was measured from exponentially growing cells and presented here as the percentage of that activated by wild-type Gcn5p; each mutant has been assayed independently three to ten times. The relatively wide range of standard errors of certain samples is at least partially attributable to the copy number variation of the GCN5 plasmid (data not shown).
In vivo tests of functions of mutant Gcn5p. (A) Growth complementation test. Shown are representative clones plated to synthetic minimal medium and grown at 30°C for 3 days before pictures were taken. All mutants have been tested at least three times for reproducibility. Note that certain mutations, such as Y220A, I174A/I179A, and G187A/G189A, consistently showed retarded growth. However, with long-termed storage at 4°C after 30°C incubation was completed, colonies of these strains reached the size close to that of wild-type strains. (B) Growth curves of four representative clones grown in minimal medium. (C) Transcriptional activation potency of Gcn5p derivatives. gcn5 null cells were first double-transformed with the GAL4–VP16 and β-gal expression constructs (both are 2 μ plasmids) followed by different GCN5 alleles (CEN/ARS minichromosomes). The β-gal expression was measured from exponentially growing cells and presented here as the percentage of that activated by wild-type Gcn5p; each mutant has been assayed independently three to ten times. The relatively wide range of standard errors of certain samples is at least partially attributable to the copy number variation of the GCN5 plasmid (data not shown).
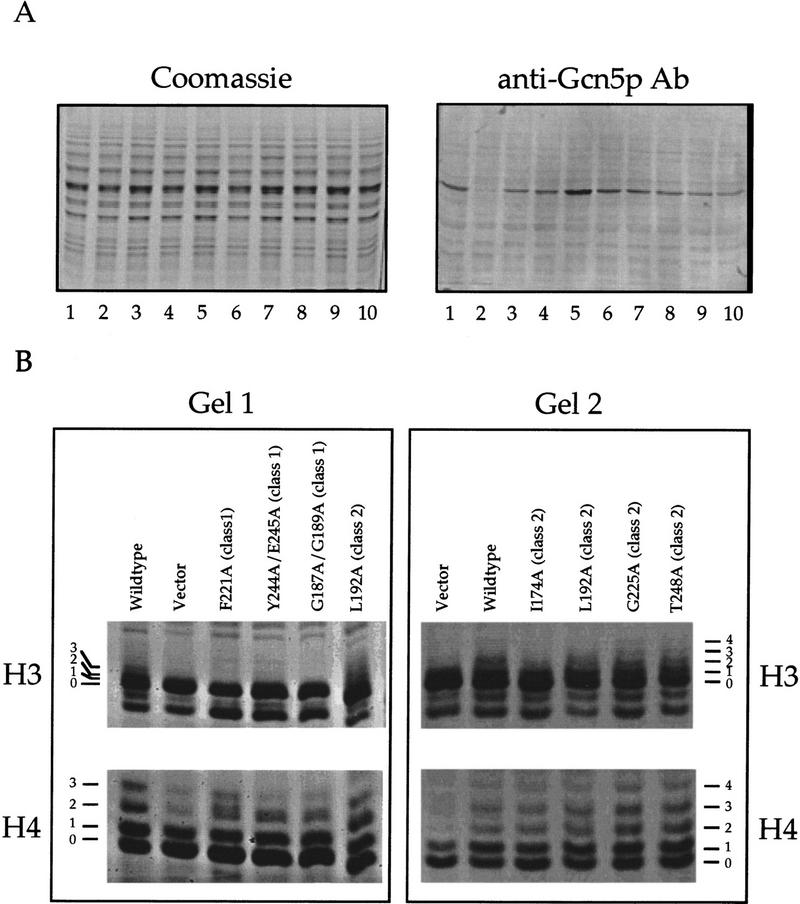
 Overproduction of functional Gcn5p leads to hyperacetylation of histones in vivo. (A) Western analysis of overproduced Gcn5p. Yeast whole cell extracts (20 μg) prepared from strains overproducing various Gcn5p derivatives were resolved by SDS-PAGE (left) and blotted for Western detection with Gcn5p antibodies (right). Note that the endogenous Gcn5p was in a low abundance under our standard Western conditions. The overproduced GCN5 alleles are as follows: (lane 1) Wild type; (lane 2) vector; (lane 3) F221A; (lane 4) Y244A/E245A; (lane 5) G187A/G189A; (lane 6) I174A; (lane 7) L192A; (lane 8) G225A; (lane 9) T248A; (lane 10) wild type. Although there is a certain degree of expression variation of different Gcn5p derivatives, this variation is not likely sufficient to account for the dramatic difference in in vivo activity displayed by each allele. (B) Histones H3 and H4 are hyperacetylated in the presence of overexpressed functional Gcn5p derivatives. Purified core histones were resolved by 15% Triton–acetic acid–urea gel electrophoresis and visualized by silver staining. Acetylation ladders are marked on the side. Samples shown (left and right) were electrophoresed on separate gels. The same preparation of wild-type and vector samples were included on both gels for reference. Note that all three class 1 mutants showed a lower degree of H3 acetylation and subtle, but readily visible, effects on H4 acetylation.
Overproduction of functional Gcn5p leads to hyperacetylation of histones in vivo. (A) Western analysis of overproduced Gcn5p. Yeast whole cell extracts (20 μg) prepared from strains overproducing various Gcn5p derivatives were resolved by SDS-PAGE (left) and blotted for Western detection with Gcn5p antibodies (right). Note that the endogenous Gcn5p was in a low abundance under our standard Western conditions. The overproduced GCN5 alleles are as follows: (lane 1) Wild type; (lane 2) vector; (lane 3) F221A; (lane 4) Y244A/E245A; (lane 5) G187A/G189A; (lane 6) I174A; (lane 7) L192A; (lane 8) G225A; (lane 9) T248A; (lane 10) wild type. Although there is a certain degree of expression variation of different Gcn5p derivatives, this variation is not likely sufficient to account for the dramatic difference in in vivo activity displayed by each allele. (B) Histones H3 and H4 are hyperacetylated in the presence of overexpressed functional Gcn5p derivatives. Purified core histones were resolved by 15% Triton–acetic acid–urea gel electrophoresis and visualized by silver staining. Acetylation ladders are marked on the side. Samples shown (left and right) were electrophoresed on separate gels. The same preparation of wild-type and vector samples were included on both gels for reference. Note that all three class 1 mutants showed a lower degree of H3 acetylation and subtle, but readily visible, effects on H4 acetylation.
To provide a more quantitative assessment of the growth defects of our gcn5 mutants, we compared the growth curves of four representative strains cultivated in the liquid minimal medium (see Fig. Fig.2B).2B). Consistent with the differential colony sizes observed on plates, the lack of functional GCN5 caused a significant lag of doubling time (cf. wild type, 132 ±
± 2 min vs. vector control, 179
2 min vs. vector control, 179 ±
± 5 min and L192A, 126
5 min and L192A, 126 ±
± 5 min vs. F221A, 165
5 min vs. F221A, 165 ±
± 2 min). In addition, the gcn5Δ and F221A mutants appear to enter stationary phase at lower cell densities. Apparently, the combination of these two phenotypic defects associated with gcn5 loss-of-function mutations results in the significantly smaller colonies of these mutants shown in Figure Figure22A.
2 min). In addition, the gcn5Δ and F221A mutants appear to enter stationary phase at lower cell densities. Apparently, the combination of these two phenotypic defects associated with gcn5 loss-of-function mutations results in the significantly smaller colonies of these mutants shown in Figure Figure22A.
Growth defects of gcn5 mutants correlate with defects in transcriptional activation
A second functional test for Gcn5p is to measure the expression level of lacZ driven by the UASgal–CYC1 cis-acting element in vivo. In this construct, lacZ reporter gene expression is activated by Gal4–VP16 chimeric activator and requires functional Gcn5p and other Ada proteins (Candau et al. 1997; Wang et al. 1997). Gal4–VP16 activator and lacZ reporter constructs were cotransformed into a gcn5Δ strain followed by a second transformation with low-copy plasmids bearing specific GCN5 alleles. β-Galactosidase activity was then measured, and the results are summarized in Figure Figure22C.
It is clear that wild-type Gcn5p activated lacZ expression efficiently. As expected, F221A, the most affected mutant (see Fig. Fig.2A)2A) was unable to activate lacZ much above that by the vector alone control. Moreover, in complete agreement with each mutant’s ability to rescue growth in minimal medium (Fig. (Fig.2A),2A), all mutants that showed gcn5− phenotypes activated lacZ less efficiently than those that supplied GCN+ function. Noteworthy is the G187A/G189A double mutant that exhibited the most subtle visible gcn5− growth defect in the growth complementation assay. In the lacZ expression assay, this allele appears to represent a “threshold” activity. Mutants expressing lacZ at a higher level all function well in rescuing growth under amino acid starvation, whereas others that exhibit lower transcriptional activation cannot fully complement growth in minimal medium. GCN5 mutants that we have assayed for in vivo functional integrity are listed in Table Table1.1. Alleles in the first class show reproducible defects in vivo, whereas class 2 includes all mutants that functionally complement gcn5Δ phenotypes in vivo. Because these two in vivo functional assays (growth under amino acid starvation and UASgal–CYC1–lacZ expression) reflect transcription activated by at least two separate transcription activators (Gcn4p and Gal4–VP16, respectively), we conclude that Gcn5p-dependent activation is likely to use a common mechanism (i.e., histone acetylation at promoters; see below).
Table 1
Amino acid residues in the HAT catalytic domain of Gcn5p that are critical for in vivo functions
functions
| Class
| Transcription and growth defects
| Mutations
|
|---|---|---|
| 1 | yes | I174A/I179A, G187A/G189A, D214A,Y220A, F221A, G239A/Y244I, Y244A/E245A |
| 2 | no | I174A, I179A, E183A, E183A/Q184A, R186A, G187A,Y188A, G189A, L192A, M193A, N194A, K222A, K223A, K222A/K223A, G225A, F226A, Y240A, K242A, I241A, Y244A, T248A, L249A |
Overproducing functional Gcn5p leads to histone hyperacetylation
To provide in vivo evidence that GCN5 encodes a histone acetyltransferase, we examined GCN5 dosage-dependent changes in histone acetylation. Consistent with the finding that genes under the control of Gcn5p are likely to occupy a small portion of the yeast genome (Pollard and Peterson 1997), we find that deleting GCN5 does not reveal any significant changes in steady-state histone acetylation patterns (data not shown). To circumvent this problem, we overexpressed wild-type and mutant Gcn5p (Fig. (Fig.3A),3A), and analyzed the acetylation status of individual histones by Triton–acetic acid–urea gel electrophoresis, a gel system capable of separating histones based on the number of acetyl moieties that each histone isoform contains.
As shown in Figure Figure3B,3B, characteristic “ladders” indicative of increasing levels of acetylation are seen in both H3 and H4, and to a lesser extent, H2A and H2B (not shown) when wild-type Gcn5p is overproduced. In the vector control sample, only a very small percentage of H3 is acetylated and most of the acetylated H4 exists in a monoacetylated isoform. However, when histones, isolated from strains overproducing various Gcn5p mutants, were analyzed under the same condition, the ability of each allele to acetylate histones correlates precisely with the ability to supply normal GCN5 functions in vivo. For example, F221A, Y244A/E245A, and G187A/G189A (as well as Y220A and G239A/Y244I; data not shown), mutants that exhibit clear in vivo defects (see Fig. Fig.2),2), all fail to produce high levels of H3 and H4 acetylation (gel 1; Fig. Fig.3B).3B). Compared with control histones from wild-type Gcn5p overproduction strain, underacetylated histones H3 and H4, as indicated by the lower intensity of acetylated isoforms, are associated with these three gcn5 mutants.
Histones from several mutants that function nearly normally in our in vivo assays were also examined; these histones all show comparable levels of hyperacetylation to those generated by wild-type Gcn5p (gel 2; Fig. Fig.3B).3B). The three alleles shown in gel 2 (i.e., I174A, G225A, and T248A) were selected because they function normally in vivo (see Fig. Fig.2)2) despite an apparent reduction in in vitro HAT activity. Thus, in vivo overproduction of such mutants provides a good indicator as to whether the HAT activity can be restored in vivo, and if so, whether the GCN+ phenotype is correspondingly maintained. Together with transcriptional assay and growth complementation tests, these results suggest strongly that Gcn5p is indeed a HAT in vivo and that histones are physiological substrates of this enzyme. Most important, our results are consistent with Gcn5p HAT activity being required for transcriptional activation of target genes (see below).
HIS3 activation mediated by Gcn5p is linked to histone hyperacetylation
The above data suggest that maintaining the HAT activity in vivo is a prerequisite to transcriptional activation. To link directly the HAT activity of Gcn5p to the activation of specific target genes, we performed chromatin immunoprecipitation (ChIP) experiments (Braunstein et al. 1993, 1996) with low-copy GCN5 alleles, to evaluate the status of nucleosomal histone acetylation. We first analyzed nucleosomal acetylation in the promoter region of HIS3 gene whose expression requires a functional Gcn5p. Yeast gcn5Δ strains bearing various GCN5 alleles (CEN/ARS, GCN5 5′ and 3′ native sequences) were starved for amino acids, including histidine, to induce HIS3 expression. After formaldehyde cross-linking and chromatin solubilization, sonication-sheared oligonucleosomes were incubated with acetylation-specific H3 antibody for coimmunoprecipitation of DNAs that are associated with hyperacetylated H3 (and likely other core histones). After reversal of cross-links, the DNA was analyzed by slot-blot hybridization to determine whether sequences of interest were enriched in the hyperacetylated H3 fraction under a given condition.
Derepression of HIS3 and some other amino acid biosynthesis genes requires functional Gcn5p. As shown in Figure Figure4B,4B, Gcn5p is required for both constitutive and activated expression of HIS3 gene, a phenomenon reported earlier by others (Georgakopoulos and Thireos 1992). For constitutive expression, the absence of functional Gcn5p leads to a twofold decrease in transcription. Under conditions of amino acid starvation, where HIS3 expression is fully derepressed, the lack of functional Gcn5p (i.e., with the null or F221A allele) causes a threefold decrease of the transcriptional output of this gene. Importantly, in excellent agreement with the above Northern data, histone H3 is hyperacetylated when HIS3 is activated by functional Gcn5p (Fig. (Fig.4C).4C). Association of the HIS3 promoter with hyperacetylated nucleosomes increases threefold in wild-type and L192A alleles of GCN5+ strains under repressive conditions. Furthermore, with amino acid starvation, hyperacetylation of the HIS3 promoter increases sixfold in the presence of wild-type or L192A Gcn5p. In contrast, in either a repressive or derepressive situation, the F221A mutant behaves similarly to the vector control (i.e., this allele cannot provide normal Gcn5p functions in either transcriptional activation or histone acetylation).
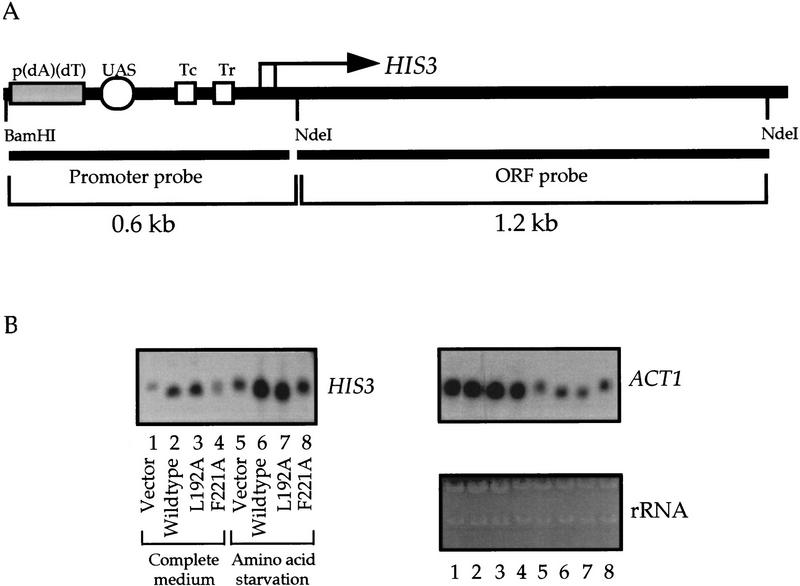
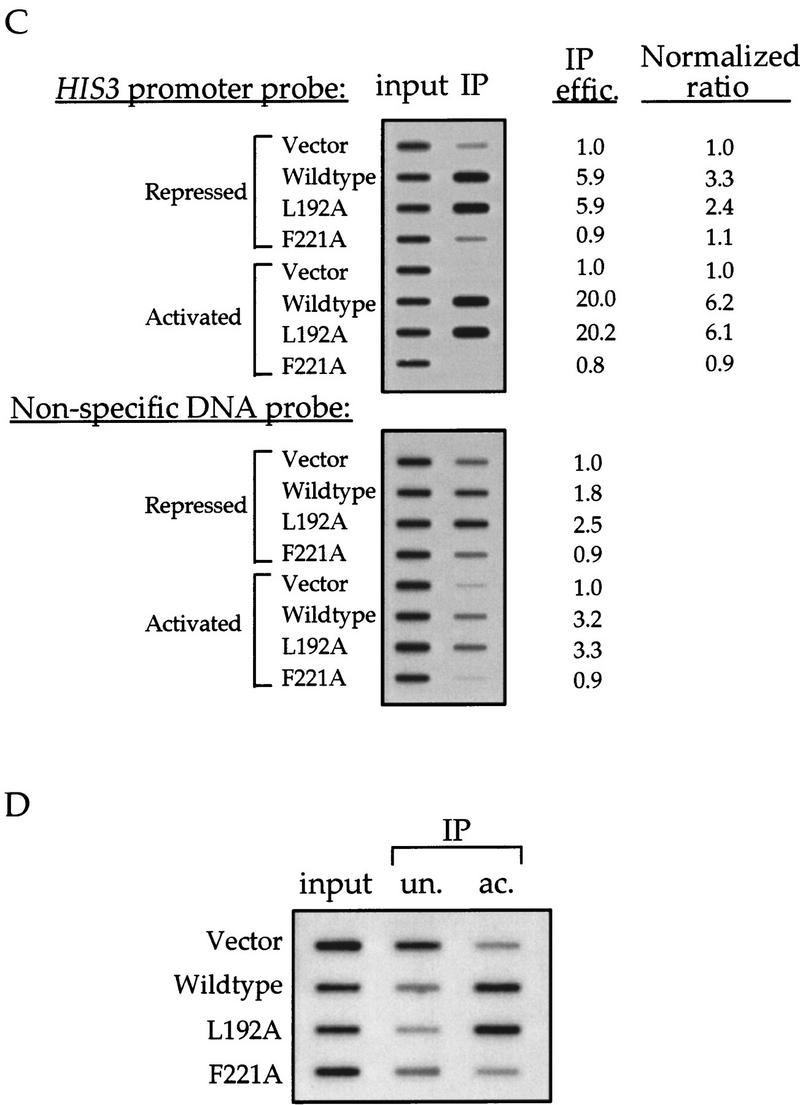
 Hyperacetylation of nucleosomal H3 is linked to the chromosomal copy of HIS3 activated by functional Gcn5p. (A) Source of the probes. The ORF probe was used only for Northern blot analyses. (B) Northern blot analysis of HIS3 transcription. The ratio of HIS3 expression, after normalization with ACT1 mRNA, was 1 : 1.8 : 2.5 : 0.9 : 6.0 : 19.4 : 19.3 : 8.2 (lanes 1–8). The generally lower expression of ACT1 under amino acid starvation is probably attributable to the complete lack of amino acids in the minimal medium during the 6-hr incubation before RNA extraction. (C) Chromatin IP results. Yeast cells were processed for immunoprecipitation of solubilized chromatin fragments using anti-H3.AcLys(9/14) antiserum. DNAs coprecipitated in these nucleosomal complexes were purified and analyzed by slot-blot hybridization. Relative IP efficiency (counts of the IP divided by those of the input) was obtained setting the value of the vector control slot as 1 in each hybridization result. The normalized ratio was derived by dividing the IP efficiency of each sample to that obtained when total genomic DNAs were used as a probe. (D) Chromatin IP using the antisera against unacetylated or hyperacetylated H3 shows comparable IP efficiency in different GCN5 backgrounds. Yeast extracts were subjected to ChIP using antisera against unacetylated (un.) or acetylated (ac.) H3 and the resultant slot-blots were first probed with HIS3 promoter sequence as above, followed by control probes, including ACT1 promoter and total genomic DNA, which showed essentially identical IP efficiency seen in Fig. Fig.4C4C (data not shown).
Hyperacetylation of nucleosomal H3 is linked to the chromosomal copy of HIS3 activated by functional Gcn5p. (A) Source of the probes. The ORF probe was used only for Northern blot analyses. (B) Northern blot analysis of HIS3 transcription. The ratio of HIS3 expression, after normalization with ACT1 mRNA, was 1 : 1.8 : 2.5 : 0.9 : 6.0 : 19.4 : 19.3 : 8.2 (lanes 1–8). The generally lower expression of ACT1 under amino acid starvation is probably attributable to the complete lack of amino acids in the minimal medium during the 6-hr incubation before RNA extraction. (C) Chromatin IP results. Yeast cells were processed for immunoprecipitation of solubilized chromatin fragments using anti-H3.AcLys(9/14) antiserum. DNAs coprecipitated in these nucleosomal complexes were purified and analyzed by slot-blot hybridization. Relative IP efficiency (counts of the IP divided by those of the input) was obtained setting the value of the vector control slot as 1 in each hybridization result. The normalized ratio was derived by dividing the IP efficiency of each sample to that obtained when total genomic DNAs were used as a probe. (D) Chromatin IP using the antisera against unacetylated or hyperacetylated H3 shows comparable IP efficiency in different GCN5 backgrounds. Yeast extracts were subjected to ChIP using antisera against unacetylated (un.) or acetylated (ac.) H3 and the resultant slot-blots were first probed with HIS3 promoter sequence as above, followed by control probes, including ACT1 promoter and total genomic DNA, which showed essentially identical IP efficiency seen in Fig. Fig.4C4C (data not shown).
To rule out the possibility that the observed difference in IP efficiency by hyperacetylated histone H3 antibody among different GCN5 alleles is attributable to variation of formaldehyde cross-linking efficiency or to a strain-specific accessibility of the HIS3 locus to the antibody, we conducted the ChIP experiments using an antibody against unacetylated histone H3 (Braunstein et al. 1993). As shown in Figure Figure4D,4D, consistent with the notion that transcriptional repression is associated with histone underacetylation, the HIS3 promoter sequence is enriched in the unacetylated histone fraction when GCN5 is either deleted (vector) or replaced with the F221A allele (cf. un. and ac. columns in Fig. Fig.4D).4D). The GCN5 allele-specific, differential enrichment of HIS3 promoter by a specific antiserum strongly suggests that there is a dynamic change in nucleosomal acetylation within Gcn5p-mediated transcriptional activation.
Promoter-specific histone hyperacetylation of UASgal–CYC1–lacZ
We also examined the nucleosomal histone acetylation status in the UASgal–CYC1–lacZ construct (Fig. (Fig.5B).5B). Again, using the ChIP assay, significant association of this promoter with hyperacetylated nucleosomes is observed in the presence of active transcription of lacZ driven by functional Gcn5p. Although this increase is lower than that seen in the HIS3 locus, we speculate that this is attributable to surplus copies of the reporter construct (a 2μ plasmid) in the cell where only a few copies of GCN5 allele (a CEN/ARS vector) are present.
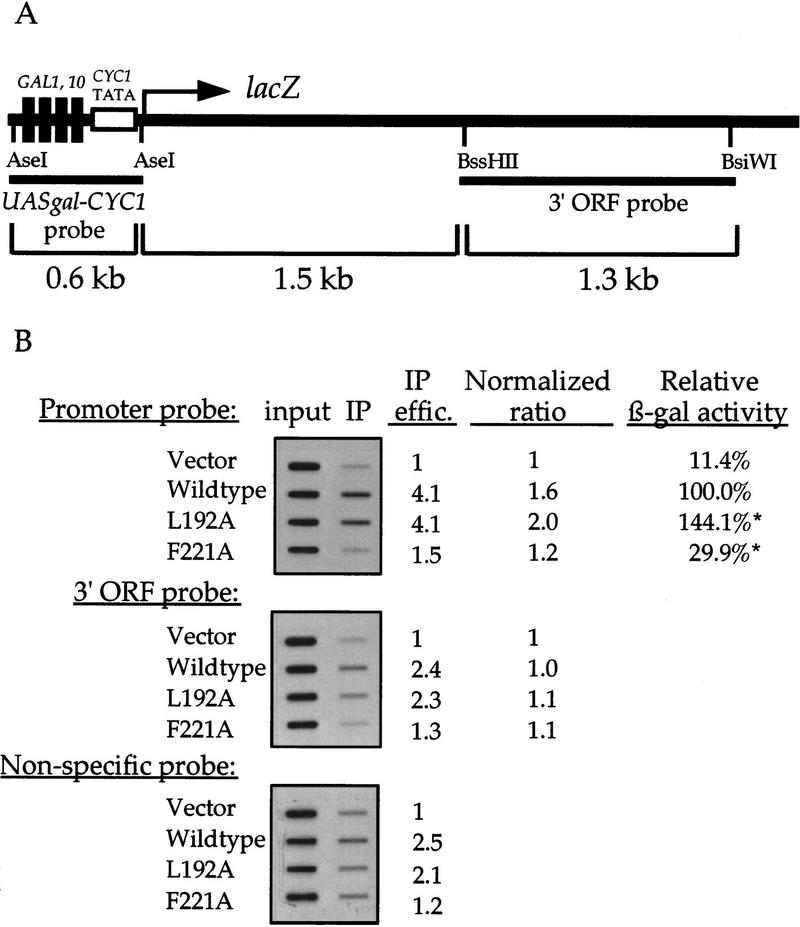
 UASgal–CYC1 promoter sequence is associated with hyperacetylated histones when lacZ is activated by functional Gcn5p. (A) A schematic diagram of the UASgal–CYC1–lacZ gene and the source of the probes used in the ChIP experiments. (B) Chromatin IP results. Assay conditions were essentially the same as HIS3 described in Fig. Fig.4.4. When quantified using GCN5 gene as the probe, two samples (*) contained a higher copy number of the GCN5 plasmid (not shown); this probably accounts for the roughly twofold increase in β-gal expression when compared with data presented in Fig. Fig.22C.
UASgal–CYC1 promoter sequence is associated with hyperacetylated histones when lacZ is activated by functional Gcn5p. (A) A schematic diagram of the UASgal–CYC1–lacZ gene and the source of the probes used in the ChIP experiments. (B) Chromatin IP results. Assay conditions were essentially the same as HIS3 described in Fig. Fig.4.4. When quantified using GCN5 gene as the probe, two samples (*) contained a higher copy number of the GCN5 plasmid (not shown); this probably accounts for the roughly twofold increase in β-gal expression when compared with data presented in Fig. Fig.22C.
It is reasonably well documented that hyperacetylated histones are associated with transcriptionally poised genes, sometimes marking an entire transcription unit (Hebbes et al. 1994, 1992). We wished to determine whether this was also the case for Gcn5p-activated genes in yeast. To this end, we used a probe that is 1.5 kb 3′ to the start codon of lacZ gene (see Fig. Fig.5A).5A). Unexpectedly, the amount of this DNA fragment associated with hyperacetylated H3 was not higher than the background levels, suggesting that H3 is hyperacetylated preferentially in the promoter region. Essentially identical results of this promoter-preferred nucleosomal hyperacetylation is seen in at least one other class 2 GCN5+ allele, G225A, but not in the I174A/I179A class 1 mutant (data not shown). Thus, we conclude that the Gcn5p-related histone hyperacetylation is a highly specific and local (promoter-specific) event.
Discussion
In this report, several lines of evidence are presented suggesting that yeast Gcn5p, a well-documented HAT in vitro, is likely a HAT in vivo, and that its HAT activity is critical for transcriptional activation of target genes. These data are summarized as follows: (1) All of the Gcn5p mutants that were able to activate transcription of the target genes maintained their ability to catalyze wild-type levels of histone acetylation in vivo and vice versa. (2) A single point mutation, F221A, in the minimal catalytic domain of Gcn5p not only significantly reduced HAT activity in vivo and in vitro, but also severely reduced the ability of Gcn5p to activate transcription. (3) Promoters of at least two different genes were associated with hyperacetylated nucleosomes only when the genes were activated by functional alleles of Gcn5p. In contrast, promoters of these genes were relatively underacetylated in mutants that failed to support Gcn5p functions both in vitro and in vivo. Together, these data provide strong evidence that Gcn5p is involved in a pathway of activating gene expression through promoter-specific histone acetylation and that the HAT activity of this protein is essential to bring about these events.
We acknowledge the formal possibility that there is a “master” HAT whose expression is critically dependent on a functional Gcn5p and that this HAT is responsible for most, if not all, transcription-related HAT activity that we have observed in this study. However, this scenario is unlikely for the following reasons. First, all class 1 mutants are catalytically impaired both in vivo and in vitro, making Gcn5p itself the most likely candidate mediating transcription-related histone acetylation in the promoters. Second, deleting GCN5 does not lead to detectable underacetylation of any histones in vivo, arguing against the presence of a predominant master HAT whose expression or function is under control of Gcn5p. Third, because the Gcn5/Ada complex is known to interact with transcriptional activators and TBP (Silverman et al. 1994; Barlev et al. 1995; Chiang et al. 1996), the physical presence of Gcn5p near the promoter makes it highly unlikely that another HAT is exclusively required to perform histone acetylation at the same genomic loci.
Conserved amino acids are critical for Gcn5p functions
Among the mutations we have created and analyzed, F221A is the most severely damaging allele. It is inactive as a HAT in vivo and in vitro, and it fails to support normal Gcn5p-mediated transcriptional activation. Because this mutant is able to interact with Ada2p normally (not shown), as reflected by in vivo coimmunoprecipitation assays, it is most likely that the in vivo defects are attributable to its inability to catalyze histone acetylation. Consistent with this hypothesis, we observed a strong dominant negative phenotype of this allele with overproduction (data not shown), which suggests that this HAT catalytic mutant, with otherwise unaffected functions in vivo, can titrate away functional interacting proteins and hence brings about a gcn5− phenotype. It is interesting that severe phenotypes are linked to this residue because phenylalanine is not likely to serve as an active site residue, although we point out that the catalytic mechanism used by Gcn5p is not known.
Some of our mutations in yGcn5p subdomains II and III, the regions likely to form an acetyl-CoA-binding motif, have been tested previously in human spermidine/spermine amino-acetyltransferase and yeast MAK3 (maintenance of killer) protein amino-terminal acetyltransferase (e.g., C177, I179, Q184, G187, Y188, G189, D214, F221, G225, and F226 of yGcn5p), and in general our in vitro HAT assay results are in excellent agreement with these studies (Lu et al. 1996). Domain IV, on the basis of our analyses, appears to be conserved only within the HAT family members. We speculate that this domain may be involved in histone substrate (amino-terminal tails) binding, although this hypothesis remains to be examined.
The in vitro HAT assays clearly show that Gcn5p, expressed and purified from bacteria, is sensitive to sequence alterations. There are two likely reasons for this observation. First, yeast Gcn5p are known to form complexes with several other subunits in vivo and in this form is better able to acetylate nucleosomal substrates (Grant et al. 1997; Pollard and Peterson 1997). None of these non-Gcn5p components were included in our in vitro HAT assays, and attempts to reconstitute nucleosomal HAT activity from individual components have so far been unsuccessful (Grant et al. 1997 and our unpublished data). It is likely that the association of accessory proteins with a Gcn5 mutant with, for example, an altered conformation could overcome or neutralize the mutation. Second, mutant Gcn5p may be less stable than the wild type, and therefore not able to fold during expression or purification. Others have observed mutant enzymes that maintain their activity in vivo, despite their altered stability in vitro, and in one case the mutations were made in the hydrophobic core of an enzyme (Serrano et al. 1992; Axe et al. 1996). One implication of our mutagenesis experiments is that many of the conserved residues in domains II and III form part of the conserved hydrophobic core of Gcn5p, and are not residues important specifically for catalytic function or interaction with Gcn5p-binding partners.
In a related study (see Wang et al. 1998), a number of alanine scan mutants in Gcn5p, many overlapping with our point mutants (including F221A), have been tested for both in vivo transcriptional activation and in vitro HAT activity. In general, our in vivo Gcn5p overproduction and histone hyperacetylation results agree well with their results assaying in vitro HAT activity using Gcn5p-containing complexes. These results are consistent with our notion that many mutants, when assayed in the recombinant and monomeric form, show in vitro enzymatic defects that can be “rescued” in vivo presumably by functioning in the context of large, multisubunit Gcn5/Ada or SAGA-type HAT complexes.
Gcn5p HAT activity is required for transcriptional activation
Our data suggest that recruitment of HATs to the promoter, possibly through interactions with activators, other coactivators, and components of the basal transcription machinery, leads to hyperacetylation of histones in the promoter region and transcriptional activation of target genes (Brownell et al. 1996; Wolffe and Pruss 1996; Wade and Wolffe 1997). In vivo phenotypes of Gcn5p mutants are tightly linked to the “potency” of each Gcn5p derivative to activate transcription of downstream genes and to acetylate histones. Importantly, the observed nucleosomal hyperacetylation at promoter region holds for two different transcription activators tested in this study. Collectively, these data provide compelling evidence that the HAT activity of Gcn5p is critically required for transcriptional activation of its target genes.
Our study also suggests that hyperacetylated histones may be confined to the promoter region of at least some genes in yeast (Fig. (Fig.5).5). This result differs from previous reports showing that the entire transcription units of some actively expressed loci are associated with hyperacetylated nucleosomes (Hebbes et al. 1994; O’Neill and Turner 1995). Such a discrepancy may be explained, at least in part, by the specific histone or site of acetylation that are under investigation. In our study, we used an antiserum that preferentially recognized a highly preferred site of Gcn5p-mediated acetylation in H3 (Lys-14; see Kuo et al. 1996). Depending on the substrate preference of the recruited HAT, histone hyperacetylation associated with transcriptional activation may escape detection from an antiserum with a different specificity.
Confinement of hyperacetylation to the promoter region is consistent with previous findings that tethering LexA–Gcn5p near a promoter can activate transcription (Georgakopoulos et al. 1995; Candau et al. 1997), suggesting that a “stationary” HAT is sufficient for facilitating transcription initiation by the basal transcription machinery on a nucleosomal template. It remains a formal possibility that another HAT, distinct from Gcn5p, conducts elongation-associated histone acetylation that spreads the hyperacetylation signal throughout the transcription unit. Recent reports of Gcn5p-independent HAT complexes in yeast (Grant et al. 1997) as well as the existence of an essential HAT in yeast (E.R. Smith, A. Eisen, J.C. Lucchesi, and C.D. Allis, in prep.) make this an intriguing possibility.
It is noteworthy that the three- to sixfold increase of association of HIS3 promoter with hyperacetylated histone H3 mediated by functional Gcn5p only reflects the minimal effects of Gcn5p in vivo. In the presence of functional Gcn5p [wild type, L192A, and G225A (not shown)], we observed consistently higher amounts of DNA associated with hyperacetylated histones even when ACT1 promoter (not shown) or bulk genomic DNA (Figs. (Figs.44 and and5)5) was used to probe the coprecipitated DNA sequences. It is tempting to speculate that Gcn5p is one of the major histone acetyltransferases by which histones are acetylated at specific lysine residues recognized by our antibody [anti-H3.AcLys(9/14)]. If this is the case, the relative IP efficiency we calculated (Figs. (Figs.44 and and5)5) would have been significantly underestimated.
Dynamics and universality of histone acetylation
In our ChIP experiments, we noticed that the efficiency of immunoprecipitation never approached 100% of the input materials. Outside of purely technical reasons (e.g., incomplete formaldehyde cross-linking), several biological explanations may account, at least in part, for the incomplete IP yield. First, cells that respond to the derepressing signals (e.g., amino acid starvation in this case) may comprise only part of the entire population (because of nonsynchronous cell growth or different physiological states of cells). A second interesting possibility is the potential interplay between histone acetyltransferases and deacetylases at the promoter of the genes that we have assayed (for review and references, see Hampsey 1997). In addition to the stable repression of genes conferred by histone deacetylases (Braunstein et al. 1993, 1996), there may be an obligatory equivalent requirement for rapid turnover of acetyl groups on histone tails in genes that need to be down-regulated quickly (see Pazin and Kadonaga 1997b; Wolffe 1997 and reference therein). Finally, it is also possible that nucleosomes are hyperacetylated before their “remodeling” near the promoters (e.g., by Swi/Snf complexes) rendering this region DNase I hypersensitive (Steger and Workman 1996). In this case, a smaller fraction of DNA sequences may remain associated with acetylated nucleosomes and be recovered by the ChIP technique.
GCN5 is a nonessential gene in yeast (Georgakopoulos and Thireos 1992) whose product participates in the expression of only a small number of genes (Pollard and Peterson 1997) raising the issue of the universality of gene activation through histone acetylation. Recent reports indicate that at least two other Gcn5p-independent HAT complexes exist in yeast (Grant et al. 1997). Moreover, a poorly understood Gcn5p-independent function of the SAGA complex has been found functionally interacting with the Srb mediator complex (Roberts and Winston 1997). Therefore, it is important to determine whether expression of the majority of yeast genes is regulated through histone acetylation by different HATs or by other functionally redundant mechanisms.
In conclusion, multiple lines of evidence are presented demonstrating that Gcn5p functions as a histone acetyltransferase and that this activity is critical for transcriptional activation in vivo. In addition, we show that the transcription-related histone acetylation, mediated by functional Gcn5p, is concentrated in the promoter region of target genes. Collectively, our data lend strong support to the general idea that histone acetylation plays a causative role in relieving repressive nucleosomal effects on transcriptional activation (Allfrey et al. 1964). The phenomenon of localized (promoter-enriched) histone hyperacetylation provides new insights into mechanisms underlying transcriptional initiation from chromatin templates.
Materials and methods
Strains, plasmids, and manipulation of GCN5
Genotypes of the yeast strain EJ67 (Siliciano and Tatchell 1984) used in this study are MATα trp1 leu2-3,112 ura3-52 his4. The GCN5 knockout of this strain (yMK703) was created as described (Candau et al. 1997). The low-copy number plasmid of wild-type GCN5 gene (pRS414–GCN5) and GAL4–VP16FA constitutive expression vector, UASgal–CYC1–lacZ plasmid (pLGSD5), were provided by Shelley Berger (Wang et al. 1997).
Standard procedures for site-directed mutagenesis were used to introduce mutations into GCN5 in a pRSET B-based construct (Kuo et al. 1996). The great majority of mutants contain single mutations. Several double mutants were created to determine whether more severe phenotypes were to be obtained. The G239A/Y244I double mutant was generated fortuitously as a result of an error in the oligonucleotide sequence. Mutations were confirmed by restriction mapping and, in some cases, by sequencing. Sequences of the oligodeoxyribonucleotides are available upon request. For yeast vector construction, NcoI fragments of GCN5 containing each point mutation were used to replace the same region of pRS414–GCN5 (wild-type GCN5) before yeast transformation.
For Gcn5p overproduction in vivo, the plasmid pMK120 was created by substituting the BamHI–KpnI fragment of pYEULCB (a URA3 leu2-d plasmid with a CUP1 promoter controlling the inserted gene; Macreadie et al. 1991) with the BamHI–AseI fragment of pYES2 (both the KpnI and the AseI ends were flushed blunt by T4 DNA polymerase) (Invitrogen, Carlsbad, CA). The BamHI–SphI fragments of pRS414–GCN5 and its mutant derivatives were then subcloned into the same sites of pMK120 creating the pMK144 plasmid series that differed only at the amino acid residues being mutagenized for this study. pMK144 plasmids were then transformed into EJ66 strain (MATa, isogenic to EJ67).
Sequence analysis of histone acetyltransferases
Sequences of known histone acetyltransferases [GCN5 YEAST (Q03330), HATA1_TT (U47321), GCN5_HUMAN (U57316), PCAF_HUMAN (U57317), and HAT1_YEAST (S52530)] were screened for significant motifs using the programs ASSET (Neuwald and Green 1994) and GIBBS (Neuwald et al. 1995). The sequence of Drosophila GCN5 homolog is provided by E. Smith (E. Smith and C.D. Allis, unpubl.). Output from these programs was compared with that of the MEME program (Bailey and Elkan 1994) to confirm that these motifs were reproducible. Blocks generated using the above methods were also used to search the Blocks database (Henikoff and Henikoff 1991) using the LAMA block alignment software (Pietrokovski 1996). Hidden Markov models of the domains were built with HMMER (Eddy et al. 1995) using the blocks as guide alignments and used to search GenPept, the protein section of GenBank. Sequence logos (Schneider and Stephens 1990) were constructed using the Blocks server. Fold recognition using H3P2 (Rice and Eisenberg 1997) and TOPITS (Rost 1995) detected no significant similarity between HATs and known structures. A sequence match was observed between the carboxy-terminal end of HAT subdomain III and a region of the G10 proteins, nuclear factors that are well conserved in eukaryotes from yeast to human; edg2, a human G10 protein, appears to be expressed in response to induction by phorbol esters (Hla et al. 1995).
YEAST (Q03330), HATA1_TT (U47321), GCN5_HUMAN (U57316), PCAF_HUMAN (U57317), and HAT1_YEAST (S52530)] were screened for significant motifs using the programs ASSET (Neuwald and Green 1994) and GIBBS (Neuwald et al. 1995). The sequence of Drosophila GCN5 homolog is provided by E. Smith (E. Smith and C.D. Allis, unpubl.). Output from these programs was compared with that of the MEME program (Bailey and Elkan 1994) to confirm that these motifs were reproducible. Blocks generated using the above methods were also used to search the Blocks database (Henikoff and Henikoff 1991) using the LAMA block alignment software (Pietrokovski 1996). Hidden Markov models of the domains were built with HMMER (Eddy et al. 1995) using the blocks as guide alignments and used to search GenPept, the protein section of GenBank. Sequence logos (Schneider and Stephens 1990) were constructed using the Blocks server. Fold recognition using H3P2 (Rice and Eisenberg 1997) and TOPITS (Rost 1995) detected no significant similarity between HATs and known structures. A sequence match was observed between the carboxy-terminal end of HAT subdomain III and a region of the G10 proteins, nuclear factors that are well conserved in eukaryotes from yeast to human; edg2, a human G10 protein, appears to be expressed in response to induction by phorbol esters (Hla et al. 1995).
Enzyme assays
Recombinant Gcn5p was expressed and purified from E. coli as described before (Kuo et al. 1996) with the following modifications: 20 mm imidazole was included in the Ni–NTA agarose binding reaction. Bound Gcn5p was washed three times for 10 min each [50 mm NaPi (pH 6.0), 300 mm NaCl, 10% glycerol] followed by a 5-min wash with the same buffer at pH 5.5. Gcn5p was then eluted with a 5-min incubation with pH 4.0 elution buffer; this elution step was repeated once before eluates were pooled. One-twentieth of the volume of 1 m Na2HPO4 was then added immediately to neutralize the pH. Gcn5p remains active in this buffer for at least several months when stored at −80°C.
HAT assays were conducted in 20-μl reactions containing 10-μg of free chicken histones, 0.1 μCi of 3H-acetyl CoA (4–6 Ci/mmole), 50 mm Tris HCl (pH 8.0), 10% glycerol, 1 mm DTT, and 10 mm n-butyrate. The reaction was incubated at 30°C for exactly 20 min. Approximately 15 ng of wild-type Gcn5p saturates the reaction; typically, 10 and 20 ng of each Gcn5p derivative was used to obtain the relative HAT activities. Each mutant has been purified and assayed independently for HAT activity at least three times.
Yeast histone preparation and analyses
Gcn5p overproduction was done by growing yeast strains bearing pMK144 series plasmids in synthetic complete −Ura, −Leu medium (to increase the copy number of pMK144) containing 200 μm of Cu2SO4 (to fully induce Gcn5p expression) until mid-log phase. Yeast histones were purified according to Edmondson et al. (1996).
The 15% Triton X-100–acetic acid–urea polyacrylamide gels (typically, 1.5 mm thick, 13 ×
× 14 cm2) were used to separate yeast histones according to Mullen et al. (1989) except that gels were run at 250 V for 16 hr to obtain good resolution of isoforms of H2A, H2B, and H4. For better resolution of H3, the gel was run for 20 hr. Proteins were visualized by silver staining.
14 cm2) were used to separate yeast histones according to Mullen et al. (1989) except that gels were run at 250 V for 16 hr to obtain good resolution of isoforms of H2A, H2B, and H4. For better resolution of H3, the gel was run for 20 hr. Proteins were visualized by silver staining.
Transcriptional and growth complementation assays
Expression of lacZ and growth complementation assays were as described by Candau et al. (1997). gcn5Δ cells (yMK703) were transformed with each mutant derivative of Gcn5p on pRS414-GCN5. For colony size comparison, log-phase transformants in complete drop-out medium were plated to synthetic minimal medium and grown at 30°C for 3 days. In addition, late log-phase cells were inoculated into liquid minimal medium for growth curve analyses. For β-gal expression and measurement, yMK703 was transformed with GAL4–VP16FA expression vector, UASgal–CYC1–lacZ plasmid, and different alleles of Gcn5p mutants. Log-phase transformants were harvested for β-gal measurement. Three to ten of each mutant transformants were analyzed.
Immunochemical procedures and chromatin immunoprecipitation
For immunoprecipitation of formaldehyde cross-linked chromatin, 100 ml of log-phase culture grown in synthetic medium at 0.4–0.6 OD600/ml was made into 1 ml of final whole cell extracts according to Strahl-Bolsinger et al. (1997). Ten microliters of the antiserum specific for histone H3 acetylated at Lys-9 and Lys-14 (Braunstein et al. 1996) (or 40 μl of unacetylated H3 antiserum) was used for every 200 μl of whole cell extract. The Ag–Ab reaction was done at 4°C for 4 hr to overnight followed by protein A–Sephadex (six volumes of antisera in 1:1 slurry) incubation for an additional hour at 4°C. The remaining procedures were essentially the same as those described previously (Strahl-Bolsinger et al. 1997).
In the case of UASgal–CYC1–lacZ chromatin IP, loading of DNA recovered from immunoprecipitates was first normalized on a test blot using lacZ ORF DNA as the probe. Copy number of the lacZ reporter construct apparently varied, as reflected later by using total yeast genomic DNA as the probe. Input material for the blot hybridization comprised 10% of that subjected to immunoprecipitation. The same blot was first probed with promoter probe, followed by 3′ ORF, rDNA (not shown), and total genomic DNA probes with stripping of the previous probe before each new round of hybridization. The radioactivities were quantitated by ImageQuant software using the PhosphorImager (Molecular Dynamics) and normalized by comparing with the same samples probed by the total genomic DNA probes.
For HIS3 expression, cells were grown in complete medium to log phase. Twenty-five percent of the culture was harvested for RNA extraction, and another 25% for formaldehyde fixation of ChIP; these samples represent repressive condition for transcriptional activation. The rest of the cultures was transferred to synthetic minimal medium (without amino acids) containing 10 mm 3-AT (3-aminotriazole) for 6 hr before RNA extraction or fixation for ChIP. Twenty micrograms of total RNAs were used for Northern blot hybridization. rRNA, as reflected by acridine orange staining, and ACT1 mRNA, detected by hybridization, were used as the control for loading and blotting.
The procedure for preparing yeast total extracts for Western blot analyses was the same as that used for ChIP (Strahl-Bosinger et al. 1997) except that 0.1% of SDS and 1 mm PMSF was included in the FA–lysis buffer. Gcn5p was detected by standard Western blot analysis procedure using rabbit anti-Gcn5p antibodies (a gift of Shelley Berger, Wistar Institute, Philadelphia, PA).
Acknowledgments
We thank Shelley Berger for communicating data before publication and for providing us with strains, plasmids, and antisera. We acknowledge Ian Macreadie for sharing plasmids for overproducing Gcn5p. We are grateful to Martin Gorovsky, Beth Grayhack, and Sharon Roth for critical comments on this manuscript. M.H.K. thanks Beth Grayhack for constant encouragement and suggestions throughout this work and Martin Gorovsky for introducing him to the field of chromatin studies. This work is supported by the National Institutes of Health (NIH) (GM53512) to C.D.A. M.E.A.C. acknowledges support from The American Heart Association, NIH (Shannon Award GM52100), and a Cellular and Molecular Biology NIH Pre-doctoral Training Grant (P.J.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
References
- Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in regulation of RNA synthesis. Proc Natl Acad Sci. 1964;51:786–794. [Europe PMC free article] [Abstract] [Google Scholar]
- Axe DD, Foster NW, Fersht AR. Active barnase variants with completely random hydrophobic cores. Proc Natl Acad Sci. 1996;93:5590–5594. [Europe PMC free article] [Abstract] [Google Scholar]
- Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In: Altman R, Brutlag D, Karp P, Lathrop R, Searls D, editors. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. Menlo Park, CA: AAAI Press; 1994. pp. 28–36. [Abstract] [Google Scholar]
- Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. [Abstract] [Google Scholar]
- Barlev NA, Candau R, Wang L, Darpino P, Silverman N, Berger SL. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. [Abstract] [Google Scholar]
- Bone JR, Lavender J, Richman R, Palmer MJ, Turner BM, Kuroda MI. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes & Dev. 1994;8:96–104. [Abstract] [Google Scholar]
- Bradbury EM. Reversible histone modifications and the chromosome cell cycle. BioEssays. 1992;14:9–16. [Abstract] [Google Scholar]
- Brandl CJ, Martens JA, Margaliot A, Stenning D, Furlanetto AM, Saleh A, Hamilton KS, Genereaux J. Structure/functional properties of the yeast dual regulator protein NGG1 that are required for glucose repression. J Biol Chem. 1996;271:9298–9306. [Abstract] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes & Dev. 1993;7:592–604. [Abstract] [Google Scholar]
- Braunstein M, Sobel RE, Allis CD, Turner BM, Broach JR. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356. [Europe PMC free article] [Abstract] [Google Scholar]
- Brownell JE, Allis CD. Special HATs for special occasions: Linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184. [Abstract] [Google Scholar]
- Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: A homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. [Abstract] [Google Scholar]
- Candau R, Berger SL. Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo. J Biol Chem. 1996;271:5237–5245. [Abstract] [Google Scholar]
- Candau R, Zhou JX, Allis CD, Berger SL. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 1997;16:555–565. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. [Abstract] [Google Scholar]
- Chiang YC, Komarnitsky P, Chase D, Denis CL. ADR1 activation domains contact the histone acetyltransferase GCN5 and the core transcriptional factor TFIIB. J Biol Chem. 1996;271:32359–32365. [Abstract] [Google Scholar]
- Coleman CS, Huang H, Pegg AE. Structure and critical residues at the active site of spermidine/spermine-N1-acetyltransferase. Biochem J. 1996;316:697–701. [Europe PMC free article] [Abstract] [Google Scholar]
- Coon SL, Roseboom PH, Baler R, Weller JL, Namboodiri MA, Koonin EV, Klein DC. Pineal serotonin N-acetyltransferase: Expression cloning and molecular analysis. Science. 1995;270:1681–1683. [Abstract] [Google Scholar]
- Eddy SR, Mitchison G, Durbin R. Maximum discrimination hidden Markov models of sequence consensus. Comp Biol. 1995;2:9–23. [Abstract] [Google Scholar]
- Edmonson DG, Smith MM, Roth SY. Repression domain of the yeast global repressor Tup1 directly interacts with histones H3 and H4. Genes & Dev. 1996;10:1247–1259. [Abstract] [Google Scholar]
- Felsenfeld G. Chromatin unfolds. Cell. 1996;86:13–19. [Abstract] [Google Scholar]
- Georgakopoulos T, Thireos G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 1992;11:4145–4152. [Europe PMC free article] [Abstract] [Google Scholar]
- Georgakopoulos T, Gounalaki N, Thireos G. Genetic evidence for the interaction of the yeast transcriptional co-activator proteins GCN5 and ADA2. Mol & Gen Genet. 1995;246:723–728. [Abstract] [Google Scholar]
- Grant PA, Duggan L, Cote J, Roberts SM, Brownell JE, Candau R, Ohba R, Owen-Hughes T, Allis CD, Winston F, Berger SL, Workman JL. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: Characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes & Dev. 1997;11:1640–1650. [Abstract] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 carboxy-terminal domain. Cell. 1997;90:595–606. [Abstract] [Google Scholar]
- Hampsey M. A SAGA of histone acetylation and gene expression. Trends Genet. 1997;13:427–429. [Abstract] [Google Scholar]
- Hebbes TR, Thorne AW, Clayton AL, Crane-Robinson C. Histone acetylation and globin gene switching. Nucleic Acids Res. 1992;20:1017–1022. [Europe PMC free article] [Abstract] [Google Scholar]
- Hebbes TR, Clayton AL, Thorne AW, Crane-Robinson C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 1994;13:1823–1830. [Europe PMC free article] [Abstract] [Google Scholar]
- Henikoff S, Henikoff JG. Automated assembly of protein blocks for database searching. Nucleic Acids Res. 1991;19:6565–6572. [Europe PMC free article] [Abstract] [Google Scholar]
- Hla T, Jackson AQ, Appleby SB, Maciag T. Characterization of edg-2, a human homologue of the Xenopus maternal transcript G10 from endothelial cells. Biochim Biophys Acta. 1995;1260:227–229. [Abstract] [Google Scholar]
- Horiuchi J, Silverman N, Pina B, Marcus GA, Guarente L. ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3. Mol Cell Biol. 1997;17:3220–3228. [Europe PMC free article] [Abstract] [Google Scholar]
- Imhof A, Yang X-J, Ogryzko VV, Nakatani Y, Wolffe AP. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. [Abstract] [Google Scholar]
- Jeppesen P, Turner BM. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell. 1993;74:281–289. [Abstract] [Google Scholar]
- Kingston RE, Bunker CA, Imbalzano AN. Repression and activation by multiprotein complexes that alter chromatin structure. Genes & Dev. 1996;10:905–920. [Abstract] [Google Scholar]
- Kuo M-H, Brownell JE, Ranalli TA, Cook RG, Edmonson DG, Roth SY, Allis CD. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383:269–272. [Abstract] [Google Scholar]
- Lin R, Allis CD, Elledge SJ. PAT1, an evolutionarily conserved acetyltransferase homologue, is required for multiple steps in the cell cycle. Genes Cells. 1996;1:923–942. [Abstract] [Google Scholar]
- Lu L, Berkey KA, Casero Jr RA. RGFGIGS is an amino acid sequence required for acetyl coenzyme A binding and activity of human spermidine/spermine N1-acetyltransferase. J Biol Chem. 1996;271:18920–18924. [Abstract] [Google Scholar]
- Macreadie IG, Horaitis O, Verkuylen AJ, Savin KW. Improved shuttle vectors for cloning and high-level Cu(2+)-mediated expression of foreign genes in yeast. Gene. 1991;104:107–111. [Abstract] [Google Scholar]
- Marcus GA, Silverman N, Berger SL, Horiuchi J, Guarente L. Functional similarity and physical association between GCN5 and ADA2: Putative transcriptional adaptors. EMBO J. 1994;13:4807–4815. [Europe PMC free article] [Abstract] [Google Scholar]
- Marcus GA, Horiuchi J, Silverman N, Guarente L. ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription. Mol Cell Biol. 1996;16:3197–3205. [Europe PMC free article] [Abstract] [Google Scholar]
- Martens JA, Genereaux J, Saleh A, Brandl CJ. Transcriptional activation by yeast PDR1p is inhibited by its association with NGG1p/ADA3p. J Biol Chem. 1996;271:15884–15890. [Abstract] [Google Scholar]
- Mizzen CA, Yang X-J, Kokubo T, Brownell JE, Bannister AJ, Owen-Hughes T, Workman J, Berger SL, Kouzarides T, Nakatani Y, Allis CD. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. [Abstract] [Google Scholar]
- Mullen JR, Kayne PS, Moerschell RP, Tsunasawa S, Gribskov M, Colavito-Shepanski M, Grunstein M, Sherman F, Sternglanz R. Identification and characterization of genes and mutants for an N- terminal acetyltransferase from yeast. EMBO J. 1989;8:2067–2075. [Europe PMC free article] [Abstract] [Google Scholar]
- Neuwald AF, Green P. Detecting patterns in protein sequences. J Mol Biol. 1994;239:698–712. [Abstract] [Google Scholar]
- Neuwald AF, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. [Abstract] [Google Scholar]
- Neuwald AF, Liu JS, Neuwald CE. Gibbs motif sampling: Detection of bacterial outer membrane protein repeats. Protein Sci. 1995;4:1618–1632. [Europe PMC free article] [Abstract] [Google Scholar]
- O’Neill LP, Turner BM. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J. 1995;14:3946–3957. [Europe PMC free article] [Abstract] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. [Abstract] [Google Scholar]
- Owen-Hughes T, Workman JL. Experimental analysis of chromatin function in transcription control. Crit Rev Euk Gene Expression. 1994;4:403–441. [Abstract] [Google Scholar]
- Pazin MJ, Kadonaga JT. SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions? Cell. 1997a;88:737–740. [Abstract] [Google Scholar]
- ————— What’s up and down with histone deacetylation and transcription? Cell. 1997b;89:325–328. [Abstract] [Google Scholar]
- Pietrokovski S. Searching databases of conserved sequence regions by aligning protein multiple-alignments. Nucleic Acids Res. 1996;24:3836–3845. [Europe PMC free article] [Abstract] [Google Scholar]
- Pollard KJ, Peterson CL. Role of ADA/GCN5 products in antagonizing chromatin-mediated transcriptional repression. Mol Cell Biol. 1997;17:6212–6222. [Europe PMC free article] [Abstract] [Google Scholar]
- Reifsnyder C, Lowell J, Clarke A, Pillus L. Yeast SAS silencing genes and human genes associated with AML and HIV-1 Tat interactions are homologous with acetyltransferases. Nature Genet. 1996;14:42–49. [Abstract] [Google Scholar]
- Rice DW, Eisenberg D. A 3D-1D substitution matrix for protein fold prediction that includes predicted secondary structure of the sequence. J Mol Biol. 1997;267:1026–1038. [Abstract] [Google Scholar]
- Roberts SM, Winston F. SPT20/ADA5 encodes a novel protein functionally related to the TATA-binding protein and important for transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3206–3213. [Europe PMC free article] [Abstract] [Google Scholar]
- ————— Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics. 1997;147:451–465. [Europe PMC free article] [Abstract] [Google Scholar]
- Rost B. TOPITS: Threading one-dimensional predictions into three-dimensional structures. In: Rawlings C, Clark D, Altman R, Hunter L, Langauer T, Wodak S, editors. Proceedings of the third international conference on intelligent systems for molecular biology. Menlo Park, CA: AAAI Press; 1995. pp. 314–321. [Abstract] [Google Scholar]
- Saleh A, Lang V, Cook R, Brandl CJ. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J Biol Chem. 1997;272:5571–5578. [Abstract] [Google Scholar]
- Schneider TD, Stephens RM. Sequence logos: A new way to display consensus information. Nucleic Acids Res. 1990;18:6097–6100. [Europe PMC free article] [Abstract] [Google Scholar]
- Serrano L, Kellis Jr JT, Cann P, Matouschek A, Fersht AR. The folding of an enzyme. II. Substructure of barnase and the contribution of different interactions to protein stability. J Mol Biol. 1992;224:783–804. [Abstract] [Google Scholar]
- Siliciano PG, Tatchell K. Transcription and regulatory signals at the mating type locus in yeast. Cell. 1984;37:969–978. [Abstract] [Google Scholar]
- Silverman N, Agapite J, Guarente L. Yeast ADA2 protein binds to the VP16 protein activation domain and activates transcription. Proc Natl Acad Sci. 1994;91:11665–11668. [Europe PMC free article] [Abstract] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai M-J, O’Malley BW. Steroid receptor coactivator one is a histone acetyltransferase. Nature. 1997;389:194–198. [Abstract] [Google Scholar]
- Steger DJ, Workman JL. Remodeling chromatin structures for transcription: What happens to the histones? BioEssays. 1996;18:875–884. [Abstract] [Google Scholar]
- Strahl-Bolsinger S, Hecht A, Luo K, Grunstein M. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes & Dev. 1997;11:83–93. [Abstract] [Google Scholar]
- Tercero JC, Wickner RB. MAK3 encodes an N-acetyltransferase whose modification of the L-A gag NH2 terminus is necessary for virus particle assembly. J Biol Chem. 1992;267:20277–20281. [Abstract] [Google Scholar]
- Tsukiyama T, Wu C. Chromatin remodeling and transcription. Curr Opin Genet Dev. 1997;7:182–191. [Abstract] [Google Scholar]
- Turner BM, O’Neill LP. Histone acetylation in chromatin and chromosomes. Semin Cell Biol. 1995;6:229–236. [Abstract] [Google Scholar]
- Ura K, Kurumizaka H, Dimitrov S, Almouzni G, Wolffe AP. Histone acetylation: Influence on transcription, nucleosome mobility and positioning, and linker histone-dependent transcriptional repression. EMBO J. 1997;16:2096–2107. [Europe PMC free article] [Abstract] [Google Scholar]
- Wade PA, Wolffe AP. Histone acetyltransferases in control. Curr Biol. 1997;7:R82–R84. [Abstract] [Google Scholar]
- Wang L, Mizzen C, Ying C, Candau R, Barlev N, Brownell J, Allis CD, Berger SL. Histone acetyltransferase activity is conserved between yeast and human GCN5 and is required for complementation of growth and transcriptional activation. Mol Cell Biol. 1997;17:519–527. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang, L., L. Liu, and S.L. Berger. 1998. Critical residues for histone acetylation by Gen5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes & Dev. (this issue). [Europe PMC free article] [Abstract]
- Welihinda AA, Tirasophon W, Green SR, Kaufman RJ. Gene induction in response to unfolded protein in the endoplasmic reticulum is mediated through Ire1p kinase interaction with a transcriptional coactivator complex containing Ada5p. Proc Natl Acad Sci. 1997;94:4289–4294. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolffe AP. The transcription of chromatin templates. Curr Opin Genet Dev. 1994;4:245–254. [Abstract] [Google Scholar]
- ————— Transcriptional control. Sinful repression. Nature. 1997;387:16–17. [Abstract] [Google Scholar]
- Wolffe AP, Pruss D. Targeting chromatin disruption: Transcription regulators that acetylate histones. Cell. 1996;84:817–819. [Abstract] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. [Abstract] [Google Scholar]
Articles from Genes & Development are provided here courtesy of Cold Spring Harbor Laboratory Press
Full text links
Read article at publisher's site: https://doi.org/10.1101/gad.12.5.627
Read article for free, from open access legal sources, via Unpaywall:
http://genesdev.cshlp.org/content/12/5/627.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1101/gad.12.5.627
Article citations
PARylation of GCN5 by PARP1 mediates its recruitment to DSBs and facilitates both HR and NHEJ Repair.
Cell Mol Life Sci, 81(1):446, 07 Nov 2024
Cited by: 0 articles | PMID: 39508866 | PMCID: PMC11544116
Acetylation of histones and non-histone proteins is not a mere consequence of ongoing transcription.
Nat Commun, 15(1):4962, 11 Jun 2024
Cited by: 1 article | PMID: 38862536
RebL1 is required for macronuclear structure stability and gametogenesis in Tetrahymena thermophila.
Mar Life Sci Technol, 6(2):183-197, 26 Mar 2024
Cited by: 0 articles | PMID: 38827131 | PMCID: PMC11136921
Diverse and dynamic forms of gene regulation by the S. cerevisiae histone methyltransferase Set1.
Curr Genet, 69(2-3):91-114, 31 Mar 2023
Cited by: 0 articles | PMID: 37000206
Review
The SAGA HAT module is tethered by its SWIRM domain and modulates activity of the SAGA DUB module.
Biochim Biophys Acta Gene Regul Mech, 1866(2):194929, 24 Mar 2023
Cited by: 0 articles | PMID: 36965704 | PMCID: PMC10226619
Go to all (296) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Genes & Proteins
- (1 citation) UniProt - Q03330
Nucleotide Sequences (3)
- (1 citation) ENA - U57316
- (1 citation) ENA - U57317
- (1 citation) ENA - U47321
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase.
EMBO J, 17(11):3155-3167, 01 Jun 1998
Cited by: 224 articles | PMID: 9606197 | PMCID: PMC1170654
GCN5, a yeast transcriptional coactivator, induces chromatin reconfiguration of HIS3 promoter in vivo.
Biochem Biophys Res Commun, 242(1):84-87, 01 Jan 1998
Cited by: 18 articles | PMID: 9439614
Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines.
Nature, 383(6597):269-272, 01 Sep 1996
Cited by: 378 articles | PMID: 8805705
Transcription: gene control by targeted histone acetylation.
Curr Biol, 8(12):R422-4, 01 Jun 1998
Cited by: 82 articles | PMID: 9637914
Review
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: P41 RR012408
Grant ID: P41 RR012408-080045
NIGMS NIH HHS (3)
Grant ID: R37 GM053512
Grant ID: GM52100
Grant ID: GM53512




