Abstract
Free full text

Memory-enhancing effects of secreted forms of the β-amyloid precursor protein in normal and amnestic mice
Abstract
When administered intracerebroventricularly to mice performing various learning tasks involving either short-term or long-term memory, secreted forms of the β-amyloid precursor protein (APPs751 and APPs695) have potent memory-enhancing effects and block learning deficits induced by scopolamine. The memory-enhancing effects of APPs were observed over a wide range of extremely low doses (0.05-5,000 pg intracerebroventricularly), blocked by anti-APPs antisera, and observed when APPs was administered either after the first training session in a visual discrimination or a lever-press learning task or before the acquisition trial in an object recognition task. APPs had no effect on motor performance or exploratory activity. APPs695 and APPs751 were equally effective in the object recognition task, suggesting that the memory-enhancing effect of APPs does not require the Kunitz protease inhibitor domain. These data suggest an important role for APPss on memory processes.
Alzheimer’s disease (AD) is the most common cause of progressive cognitive decline and dementia in aged humans. The deposition of the β-amyloid peptide(s) (Aβ) in extracellular neuritic plaques of AD patients is an early and invariant feature of this neurodegenerative disorder (1). Aβ is derived from a large membrane-spanning β-amyloid precursor protein (APP), encoded by a single gene located on chromosome 21. Alternative splicing of this gene in humans leads to three major isoforms, either lacking (APP695) or containing (APP751 and APP770) a Kunitz protease inhibitor domain. APP695 is selectively expressed in the brain, whereas APP751 and APP770 also are abundantly expressed in peripheral tissues. Proteolytic processing of APPs at the N- and C- termini by β- and γ-secretases leads to the production of Aβ (2). An alternative cleavage by α-secretase(s) within the Aβ domain of APPs generates secreted N-terminal products, the secreted APPs (APPss) (2). The normal physiological functions of APPs and secreted derivatives are still poorly understood. However, neurotrophic as well as neuroprotective actions have been reported for both APPs751 and APPs695 (3–6). Recent behavioral studies have shown that intracerebroventricular (i.c.v.) administration of anti-APPs antisera results in memory impairment in rats performing a passive avoidance task (7, 8). Further, the induction of long-term potentiation in hippocampal slices is associated with increased APPs synthesis and secretion (9). These data suggest that APPss may be involved in learning and memory processes. In the present study, we investigated whether APPs751 and APPs695 have memory-enhancing actions when directly administered to mice performing various learning tasks and to mice rendered amnestic by administering the anticholinergic drug scopolamine.
MATERIALS AND METHODS
Animals and Surgical Procedures.
Male Swiss mice (10–12 weeks old) bred in our laboratory and maintained at 23–25°C under a 12–12 light-dark cycle (light on at 0700) with free access to food and water were used in all experiments. For i.c.v. injections, mice were stereotaxically implanted with a stainless steel guide cannulae in one of their lateral ventricles (coordinates: 2.3 anterior from lambda, 3.3 lateral from midline, −2.6 ventrally from dura) under sodium pentobarbital anesthesia (10). All experiments were carried out in accordance with the European Communities Directive of November 24, 1986.
Pharmacological Treatment.
Scopolamine hydrobromide (Sigma) was dissolved in 0.9% saline and injected s.c. The APPss used for this study were obtained from culture supernatants of human embryonic kidney 293 cells stably transfected with either human APP695 or APP751, as described elsewhere (11–13). Protein concentrations in the pooled fractions were estimated by the Pierce BCA protein assay. The protein concentrations of the purified APPs samples used as stock solutions in this study were 106 μg/ml for APPs751 and 640 μg/ml for APPs695. Western blotting was repeated after completion of the behavioral testing on the samples used (as well as on the stock solutions) and showed no significant degradation of either APPs751 or APPs695. For i.c.v. administration APPss were dissolved in 0.9% saline. Anti-5 antibody (Athena Neurosciences, San Francisco) raised in rabbits against the C-terminal portion of the ectodomain of secreted APP (residues 444–592 of human APP) and known to react only weakly with murine APP was used. APPs751 was preincubated with anti-5 antibody for at least 1 hr before administration. Rabbit Ig (IgG) was used as a control. Anti-5 antibody and APPs solutions (5 μl) were administered i.c.v. at a rate of 1 μl/12 s through a stainless steel injection cannulae.
Go-No Go Visual Discrimination Task.
The go-no go visual discrimination task was performed in two side-by-side Plexiglas runways (68 × 6.5 × 20 cm) that differed only in color (black or white). Only one runway color was reinforced (10). Before the training, mice were habituated to the apparatus (5 min per runway) and maintained at 80–85% of their ad lib body weight throughout the course of training. Learning was carried out over 3 consecutive days, with one session per day performed between 0800 and 1600. Each session consisted of 12 consecutive trials, randomly organized with six reinforced (go) trials and six unreinforced (no go) trials. Reinforcement consisted of food pellets (15 mg) in the goal box on reinforced trials. On each trial, a mouse was placed in the start box for 15 s after which a guillotine door was opened. The running time in the reinforced (R) or unreinforced (UR) runways, i.e., the time the mouse took to reach the goal box was recorded. Performance is quantified for each animal as a discrimination ratio D = R/(R + UR). A ratio of 0.5 corresponds to identical R and UR, i.e., no discrimination occurred. Learning is quantified by a decrease in this ratio. The treatment was administered 3 min after the first session of training and consisted of an i.c.v. injection of either saline or APPs751 (0.05–5,000 pg) immediately followed by a s.c. injection of 3 mg/kg of scopolamine or saline (n = 9–11 per group).
Lever-Press Task.
The apparatus was a Skinner box made of translucent Plexiglas (14 × 12 × 17.5 cm) with a grid floor. A metal lever (5.5 × 3.3 cm) and a food cup were separated by a 5-cm-long partition. After a lever press, the mouse had to go around the partition to reach the food cup. Food-deprived animals were submitted to two training sessions. On the first day, the mice were individually submitted to an acquisition session, terminating after completion of 15 reinforced responses. A reinforced response consisted of a lever press followed within 30 s by the consumption of a food pellet (5 mg). A 20-min retention session took place 24 hr later. Performance is expressed as the mean number of reinforced responses made during the first 5 min and the last 5 min of the acquisition session and the first 5 min of the retention session. Pharmacological treatments were administered 3 min after the acquisition session and consisted of an i.c.v. injection of either saline or 5 pg APPs751 immediately followed by a s.c. injection of 3 mg/kg of scopolamine or saline (n = 12–13 per group).
Traction Reflex Test.
The apparatus consisted of a wire stretched horizontally 40 cm above a table. Mice were submitted to two sessions separated by a 24-hr delay. Each session consisted of five consecutive trials separated by 5 min. On each trial (60 s maximum), the fore paws of the mouse were placed on the wire. The latency (s) the animal took to catch the wire by one of its hind paws was recorded. If the animal fell, it was credited with 60 s. Pharmacological treatments were administered 15 min before the first session and consisted of an i.c.v. injection of either saline or 5 pg APPs751 immediately followed by s.c. administration of 3 mg/kg of scopolamine or saline (n = 12–13 per group).
Object Recognition Task.
The object recognition task was performed in a Plexiglas open-field box (52 × 52 × 40 cm) with black vertical walls and a translucent floor divided into 25 equal squares. The open field was dimly illuminated by a 60-W lamp placed under the floor. The objects to be discriminated were a glass marble and a plastic dice. Mice first were habituated to the open field for 50 min. The next day, they were submitted to a 10-min acquisition trial (first trial) during which they were individually placed in the open field in the presence of object A (marble or dice). Locomotor activity (number of squares crossed), rearings, and the time the animal took to explore object A (when the animal’s snout was directed toward the object at a distance ≤1 cm) were recorded. A 10-min retention trial (second trial) occurred 3 hr or 24 hr later. During this trial, object A and another object B were placed in the open field, and locomotor activity, rearings, and the times (tA and tB) the animal took to explore the two objects were recorded. A recognition index (RI) was defined as (tB/(tA + tB)) × 100. Because deleterious effects on spontaneous recognition behavior can occur by using a posttraining administration schedule (A.U. and D. Eichenlaub, unpublished observations), we used a pretraining drug administration schedule for all experiments. Treatment was administered 20 min before the first trial and consisted of i.c.v. injection of APPs751 (0.005–50 pg), APPs695 (0.005–50 pg), or saline. When APPs751 and APPs695 were tested in combination with scopolamine, the i.c.v. injection of APPss was immediately followed by s.c. injection of 1 mg/kg of scopolamine or saline (n = 10–15). In experiments using antibody, treatment consisted of an i.c.v. injection of APPs751 (0.5 pg) alone, anti-5 antibody (2.5 ng/ml, alone), or APPs751 preincubated with 2.5 ng/ml of anti-5 antibody, 20 min before the first trial.
Statistical Analysis.
A global analysis of the data was made by using an ANOVA with repeated measures on one factor to compare performance in the go-no go visual discrimination task, the lever-press task, and the traction reflex test. The Dunnett’s two-tailed t test or the Student-Newman-Keuls t test were used to make comparisons of individual groups vs. control groups or between pairs of groups. A within-group analysis also was performed to compare the performance across training sessions. In the object recognition task, between-groups comparisons were made by using the Kruskall-Wallis test followed by the Kolmogorov-Smirnov test. A within-group analysis was made by using the Wilcoxon Signed Ranks test.
RESULTS
In the go-no go visual discrimination task, all groups significantly improved their performance across training sessions, as revealed by a significant decrease in the discrimination ratios (F ≥ 14.12, P < 0.0002) (Fig. (Fig.11a). The performance of the different groups did not differ during the first session, whereas comparison of discrimination ratios of the second and third sessions of training revealed a significant effect of treatment [F(5, 54) = 4.78, P < 0.002]. APPs751, when administered after the first training session, significantly improved the performance during the second and the third sessions at doses of 0.5 and 5 pg. The treatments had no effect on the absolute running times (reinforced + unreinforced) on the second [F(5, 54) = 2.3, not significant (NS); saline: 9.1 ± 0.8 s; APPs7510.5 pg: 8.3 ± .6 s; APPs7515 pg: 12.5 ± 1.2 s], or the third session [F(5, 54) = 1.93, NS] (data not shown).
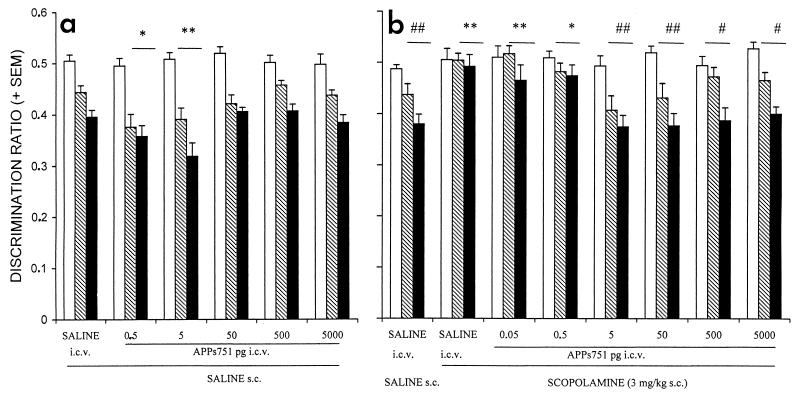
Administration of APPs751 results in a significant improvement of discrimination performance (a) and reduces learning deficits induced by scopolamine (b) in a go-no go visual discrimination task. APPs751 (0.05 to 5,000 pg) was administered i.c.v. to mice either alone or after s.c. treatment with scopolamine (3 mg/kg). See text for details. The discrimination ratios of the different groups evolved significantly [a: F(2, 54) = 119.01, P < 0.001; b: F(2, 70) = 50.72, P < 0.001] and differently [treatment x session interaction—a: F(10, 54) = 2.17, P < 0.03; b: F(10, 70) = 2.44, P < 0.01] across the three daily sessions. Empty, hatched, and filled bars represent the first, second, and third sessions, respectively.  , P < 0.05 and
, P < 0.05 and 
 , P < 0.01, vs. saline; #, P < 0.05 and ##, P < 0.01, vs. scopolamine, Dunnett’s two-tailed t test.
, P < 0.01, vs. saline; #, P < 0.05 and ##, P < 0.01, vs. scopolamine, Dunnett’s two-tailed t test.
Effects of APPs751 also were analyzed on a model of scopolamine-induced amnesia in this task (10). Performance significantly improved over the three training sessions in the animals treated with saline [F(2, 9) = 17.71, P < 0.0001], and those treated with both scopolamine and APPs751 at doses of 5–5,000 pg after the first session (F ≥ 10.37, P < 0.002), whereas no evidence of learning was detected in animals treated with lower doses of APPs751 or scopolamine alone [F(2, 9) ≤ 1.43, NS] (Fig. (Fig.11b). The discrimination ratios differed significantly among groups on the second and third sessions [F(7, 70) = 5.99, P < 0.001], but not on the first session [F(7, 70) = 0.92, NS]. Administration of APPs751 after the first session dose-dependently blocked the deficit induced by scopolamine on the second and third sessions, with the most potent effect observed at doses of 5 and 50 pg. We confirmed these results on new sets of animals for saline, scopolamine, and APPs751 (0.05, 0.5, and 5 pg) groups. Again, APPs751 significantly blocked the effect of scopolamine at the dose of 5 pg (P < 0.01, vs. scopolamine), but not at the lower doses studied (data not shown).
The effects of APPs751 (5 pg i.c.v.) alone or against scopolamine-induced deficits were analyzed in a lever-press task using a continuous reinforcement schedule. This appetitively reinforced conditioning is characterized by spontaneous improvement in performance after partial acquisition of the task, which is thought to reflect the activation and maturation of memory traces allowing subsequent long-term storage (14, 15). There was a significant session x group interaction [F(3, 46) = 5.29, P < 0.004] and a significant session effect [F(1, 46) = 37.47, P < 0.0001] when considering the last 5 min of the acquisition session and the first 5 min of the retention session. Performance during the first 5 min of the retention session differed among groups [F(3, 46) = 3.35, P < 0.03] (Fig. (Fig.2).2). The deficit induced by posttraining administration of scopolamine was significantly blocked by APPs751. APPs751 had no significant effect by itself on retention performance. The spontaneous improvement of performance observed in control mice between the two sessions was suppressed by scopolamine, but reappeared in the animals treated with both scopolamine and 5 pg of APPs751 (Fig. (Fig.2).2).
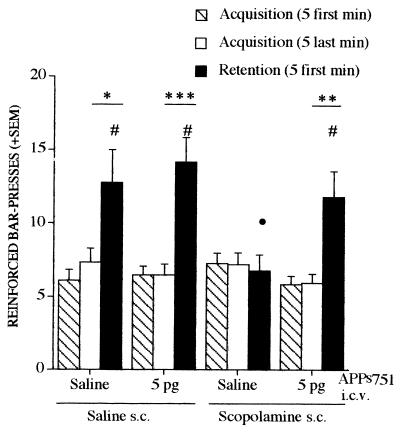
APPs751 significantly blocks retention deficits induced by scopolamine in the lever-press task. APPs751 (5 pg) was administered i.c.v. to mice either alone or after s.c. administration of scopolamine (3 mg/kg). See text for details. •, P < 0.05, vs. saline and #, P < 0.05, vs. scopolamine, Student-Newman-Keuls t test. (F ≥ 8.26,  , P < 0.02,
, P < 0.02, 
 , P < 0.002, and
, P < 0.002, and 

 , P < 0.0001), within group analysis between the last 5 min of the acquisition session and the first 5 min of the retention session.
, P < 0.0001), within group analysis between the last 5 min of the acquisition session and the first 5 min of the retention session.
Scopolamine causes transient motor disturbances revealed by an increase in latencies in a traction reflex test, when administered 15 min before testing (saline: 9.06 ± 1.20 s; scopolamine: 33.22 ± 2.93 s; P < 0.01, Student-Newman-Keuls test). However, the motor effects of scopolamine could not explain the memory deficits observed in the learning tasks because they disappeared 24 hr later (saline: 7.89 ± 0.82 s; scopolamine: 7.07 ± 0.88 s; NS). Furthermore, APPs751 (5 pg i.c.v.) coadministered with scopolamine did not block scopolamine-induced motor deficits (29.77 ± 2.74 s; P < 0.01 vs. saline) and had any effect by itself. These data suggest that the effects of APPs751 observed in the various memory tasks studied were not caused by alterations of motor behavior.
The effects of APPs751 also were analyzed by using an object recognition task in an open field. This two-trial task is based on the spontaneous tendency of rodents to explore a novel object (16). Recognition performance is expressed by a RI representing the relative time spent exploring a novel object and a familiar one during the second trial and is highly dependent on the delay between the two trials. Indeed, the RI is high at a 3-hr delay and decreases to a chance level at a 24-hr delay (17). At the 24-hr delay, injection of APPs751 (0.005–50 pg) 20 min before the first trial dose-dependently improved recognition performance, as revealed by significantly higher RIs at APPs751 doses of 0.05 to 5 pg (Fig. (Fig.33a). There was no group effect on locomotor activity [first trial: F(5, 63) = 0.37, NS; second trial: F(5, 63) = 0.81, NS] and rearings [F(5, 63) = 1.46, NS], again suggesting that APPs751 did not affect general motor activity.

APPs751 and APPs695 improve retention performance and block retention deficits induced by scopolamine in an object recognition task. When using a 24-hr delay, both APPs751 and APPs695 (a and b, respectively) dose-dependently (0.05–5 pg) improve retention performance. When using a 3-hr delay, both APPs751 and APPs695 (c and d, respectively) block retention deficits induced by scopolamine (1 mg/kg). In each experiment, the recognition index significantly differed among groups [Kruskall-Wallis test: (a) H = 15.19, P < 0.01; (b) H = 29.96, P < 0.0001; (c) H = 34.39, P < 0.0001; (d) H = 37.52, P < 0.001].  , P < 0.05,
, P < 0.05, 
 , P < 0.01, and
, P < 0.01, and 

 , P < 0.001 vs. saline, and #, P < 0.05, ##, P < 0.01, and ###, P < 0.001 vs. scopolamine, Kolmogorov-Smirnov test.
, P < 0.001 vs. saline, and #, P < 0.05, ##, P < 0.01, and ###, P < 0.001 vs. scopolamine, Kolmogorov-Smirnov test.
The effects of APPs751 were evaluated on scopolamine-induced deficits in the object recognition task by using an intertrial delay of 3 hr. At this delay, control mice exhibit high levels of recognition performance, which are strongly impaired by administration of 0.1 mg/kg of scopolamine 20 min before the first trial (17). APPs751 (0.5–50 pg, i.c.v.) dose-dependently blocked the impairment induced by scopolamine on recognition performance (Fig. (Fig.33c). Indeed, mice treated with 5 and 50 pg of APPs751 in combination with scopolamine explored the novel object significantly more than the familiar one, as observed in the saline group (Wilcoxon Signed Ranks test: P < 0.01), whereas mice treated with 1 mg/kg of scopolamine (alone or in combination with 0.5 pg of APPs751) similarly explored the two objects (P > 0.05 in each case). No treatment significantly affected exploration of object A during the first trial (Kruskal-Wallis test: H = 4.13, NS). Locomotor activity of groups treated with scopolamine, with or without APPs751, remained unchanged during the 10-min exploration period, whereas that of the saline control group decreased gradually (data not shown), suggesting that habituation in the open field was affected by scopolamine, but not APPs751. Scopolamine also induced a significant decrease in rearings during the first trial (P < 0.01 vs. saline, Student-Newman-Keuls test), which was not affected by coadministration of APPs751 (P < 0.01 vs. saline, P > 0.05 vs. scopolamine). These findings confirm that APPs751 has no effect on motor activity and suggest that APPs acts selectively to reduce recognition memory deficits induced by scopolamine.
To test whether the Kunitz protease inhibitor domain is involved in the memory-enhancing effects of APPs751, APPs695 was tested alone or against scopolamine-induced deficits in the object recognition task. After a 24-hr delay, the RIs were significantly increased by APPs695 at doses of 0.05 to 5 pg (Fig. (Fig.33b). The deficit in recognition performance induced by scopolamine also was significantly blocked by APPs695 at doses of 0.05 to 50 pg (Fig. (Fig.33d). As shown for APPs751, APPs695 did not affect the animal’s general motor activity (locomotor activity and rearings). Moreover, the locomotor activity of the group treated with both scopolamine and APPs695 remained unchanged during the 10-min exploration period on the first trial, which suggests that habituation was not affected by APPs695.
To confirm that the memory-enhancing effects observed in our experiments are caused by APPss contained in our diluted stock solutions, we attempted to block these effects by using an antibody directed against the C-terminal portion of the secreted APP ectodomain (anti-5 antibody) in the object recognition task by using a 24-hr delay. I.c.v. administration of APPs751 (0.5 pg) 20 min before the first trial significantly improved recognition performance (control RI: 58.5 ± 3.4%; APPs751 RI: 70.2 ± 2.1%, P < 0.05), as observed in previous experiments. The latter, however, was blocked when APPs751 was preincubated with anti-5 antibody (2.5 ng/ml) (anti-5 + APPs751 RI = 50.5 ± 3.4%, P < 0.001 vs. APPs751). Although memory deficits have been reported after i.c.v. administration of APP antisera (7), the required concentrations were 4 × 106 times higher than that of the anti-5 antibody used in our experiments. In addition, it has been verified that anti-5 antibody alone did not impair recognition performance at the 3-hr delay (control RI: 69.6 ± 3.6%; anti-5 RI: 68.1 ± 2.1%, NS). Thus, the poor performance in the group treated with APPs751 (preincubated with anti-5 antibody) at the 24-hr delay is caused by blockade of exogenous APPs751 and is not an effect of anti-5 antibody on endogenous APPs.
DISCUSSION
Our study clearly demonstrates that APPs751 and APPs695 enhance memory performance and block the amnestic effects of scopolamine in a variety of learning tasks involving either short-term or long-term memory. The enhancement of memory performance occurs at doses as low as 0.05 pg (about 10−19 mol) with both APPss in the object recognition task, and 5 pg of APPs751 blocked scopolamine-induced memory deficits in all of the learning tasks studied. The lack of effect of APPss on exploratory activity, locomotor activity (including habituation), and motor abilities shortly after administration or 24 hr later suggests a selective effect of this protein on memory-related processes. The memory-enhancing effects of APPs751 are already manifest within 3 hr posttraining, as it blocks scopolamine-induced recognition deficits at this delay. This finding is in agreement with the results of Doyle et al. (7), supporting a role for endogenous APPs in early memory processes, as antibodies to the extracellular domain of APP impair passive avoidance retention performance when administered within 2.5 hr, but not 4 hr and 6 hr posttraining. In addition, posttraining administration of APPs751 enhanced visual discrimination memory when performance is evaluated 24 hr and 48 hr later, presumably under drug-free conditions, which is consistent with an action of the protein on the mechanisms underlying consolidation and/or maturation of memory traces. This hypothesis also is supported by the ability of APPs751 to block the scopolamine-induced suppression of the spontaneous improvement of retention performance in the lever-press task, which is thought to reflect the activation of mechanisms involved in posttraining reorganization of long-term memory traces (14, 15). The fact that lever-press retention performance was not further improved by APPs751 alone might be related to a “ceiling effect” (i.e., performance already maximally improved), as suggested in the same testing conditions with other promnestic drugs (14). APPs751 and APPs695 also improve short-term memory in the object recognition task when administered before the first trial. Although APPss may have improved the mechanisms underlying recognition memory, we cannot exclude an action on acquisition of new information in this working memory task.
As APPs are widely expressed throughout the brain of rodents (18), their localization does not give much information on the brain structures that could mediate their memory-enhancing effects. However, the go-no go visual discrimination task and the lever-press task both involve the hippocampus shortly after the initial acquisition session (19, 20), whereas a learning-specific activation of several cortical areas, such as frontal, entorhinal, parietal, and cingulate cortices, was shown at a 3-hr posttraining delay in the lever-press task (20). The memory-enhancing effects of APPss might be mediated through an action on the hippocampus, as APPss have been shown to modulate hippocampal synaptic plasticity (21), thought to be involved in learning and memory processes (22, 23). However, nonspatial/item recognition memory depends on cortical structures (e.g., visual association areas, perirhinal, entorhinal, and the frontal cortices) rather than the hippocampus per se (24). A peptide derived from APP was shown to increase the number of presynaptic terminals in the fronto-parietal cortex of rats at doses improving memory retention in a water maze (25).
We have demonstrated that APPss have potent memory-enhancing properties in rodents by using direct in vivo administration of the protein. An involvement of APP in early memory processing previously has been suggested by studies using i.c.v. administration of antibodies directed against APP (7, 8). Mice expressing very low levels of APP, after targeted disruption of the APP gene, display spatial memory deficits in the water maze (26). However, transgenic mice overexpressing human APP also show memory deficits in the water maze and the spontaneous alternation spatial learning task (27, 28). The latter could be caused, however, by the overproduction of neurotoxic Aβ peptide(s), previously shown to produce memory deficits (29), and/or developmental abnormalities often observed in these mice. We postulate that physiological levels of APP are necessary for normal learning and memory functions.
Several possible cellular mechanisms could underlie the very potent memory-enhancing effects of APPss observed in our experiments. It has been shown that APPss can be released by different cell types after either cholinergic (30–33) or glutamatergic (33–35) stimulation. This stimulated release of APPs reflects increased α-secretase activity (involved in nonamyloidogenic APP processing) and could be mediated by activation of the protein kinase C (PKC) (36, 37). Moreover, in vivo studies in the rat support a role for the cholinergic system in the regulation of APP secretion (38, 39). Recent studies suggest that a common feature among these two neurotransmitters, long implicated in memory processes, is that both activate PKC. Thus, stimulated release of APPss by activated PKC may, in part, underlie the role of PKC in learning and memory processes (40). The secretion of APPss after the induction of long-term potentiation (LTP) in hippocampal slices (9) provides another potential link between APP and the cellular mechanisms underlying learning and memory (22, 23). LTP induction is accompanied by a rapid activation of PKC (23), which in turn triggers various intracellular events involved in the maintenance of enhanced synaptic function (e.g., possibly APPs secretion). However, most studies suggesting a role for PKC on the secretion of APPs have been done on cells in culture, and other PKC-independent mechanisms have been shown to induce APPs secretion (41, 42). Thus, a direct link between PKC and APPs needs to be confirmed in vivo.
What are the physiological consequences of APPs release and how could these be related to memory processes? It has been shown that APP can function as a cell adhesion molecule (43–45) because the membrane-bound form has structural similarities to other known adhesion molecules (46). Further, APPs also has been reported to have trophic and neuroprotective properties both in vitro (47–49) and in vivo (6). These findings may be relevant to a possible function of this protein in memory consolidation by stabilizing contacts between cells, and particularly by consolidating or strengthening certain synapses involved in the memory trace. Indeed, studies on neuronal or non-neuronal cultures have demonstrated that APPs can promote cell growth. In this regard, Roch et al. (25) have shown that a peptide containing the neurotrophic domain of APP increases the number of synapses in the cortex of rats during learning in the water maze. Moreover, Huber et al. (50) recently have reported that the number of synapses and the level of endogenous APP at synapses in the cerebral cortex are increased in rats bred in an enriched environment, compared with rats bred in standard conditions, again suggesting a role for APP in the formation of new synapses during learning. Finally, Ishida et al. (21) have shown that APPss enhance long-term potentiation and modulate the induction of long-term depression (LTD) in hippocampal slices, presumably through their stimulating action on cGMP production in hippocampal neurons (51), suggesting another way by which APPss could act on synaptic events underlying memory processes. The effects of APPss on LTD induction are in agreement with their cGMP-mediated ability to potently stimulate potassium currents, and hence decrease intracellular calcium levels (51). However, the entire cascade of cellular mechanisms underlying the neuromodulatory properties of APPss are still to be elucidated, and no specific receptor(s) for APPss has been identified.
The present study clearly suggests that APPss play an important role in the formation and/or the consolidation of the memory trace. As memory function is one of the earliest and most affected functions in Alzheimer’s disease (AD), it is tempting to speculate that altered levels of APPss in AD patients could explain, at least in part, some of the memory deficits observed in this disease.
Acknowledgments
We are grateful to Drs. Jean De Barry and Jérôme Mouton for their help in testing the purity of the solutions of APPss in their laboratory, to Athena Neurosciences for providing the APPs695, and to J. Hale and E. Johnstone at Lilly for their work on APPs751. Many thanks to M. Dubois, M. Elyacoubi, and A. Klepper for their technical help. This work was supported by Eli Lilly, Indianapolis.
ABBREVIATIONS
| APP | β-amyloid precursor protein |
| APPs | secreted form of APP |
| i.c.v. | intracerebroventricular |
| RI | recognition index |
| NS | not significant |
| PKC | protein kinase C |
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.95.21.12683
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc22891?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.95.21.12683
Article citations
Alpha-lipoic acid alleviates cognitive deficits in transgenic APP23/PS45 mice through a mitophagy-mediated increase in ADAM10 α-secretase cleavage of APP.
Alzheimers Res Ther, 16(1):160, 19 Jul 2024
Cited by: 0 articles | PMID: 39030577 | PMCID: PMC11264788
Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis.
Glia, 72(1):167-183, 05 Sep 2023
Cited by: 4 articles | PMID: 37667994
Recent Mechanisms of Neurodegeneration and Photobiomodulation in the Context of Alzheimer's Disease.
Int J Mol Sci, 24(11):9272, 25 May 2023
Cited by: 8 articles | PMID: 37298224 | PMCID: PMC10253105
Review Free full text in Europe PMC
Secreted Amyloid Precursor Protein Alpha (sAPPα) Regulates the Cellular Proteome and Secretome of Mouse Primary Astrocytes.
Int J Mol Sci, 24(8):7165, 12 Apr 2023
Cited by: 2 articles | PMID: 37108327 | PMCID: PMC10138557
How Can Insulin Resistance Cause Alzheimer's Disease?
Int J Mol Sci, 24(4):3506, 09 Feb 2023
Cited by: 12 articles | PMID: 36834911 | PMCID: PMC9966425
Review Free full text in Europe PMC
Go to all (231) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A secreted form of the beta-amyloid precursor protein (sAPP695) improves spatial recognition memory in OF1 mice.
Neurobiol Learn Mem, 81(1):27-38, 01 Jan 2004
Cited by: 43 articles | PMID: 14670356
Secreted forms of beta-amyloid precursor protein protect against ischemic brain injury.
J Neurochem, 63(2):781-784, 01 Aug 1994
Cited by: 124 articles | PMID: 8035204
The neurosteroid pregnenolone sulfate reduces learning deficits induced by scopolamine and has promnestic effects in mice performing an appetitive learning task.
Psychopharmacology (Berl), 126(4):323-330, 01 Aug 1996
Cited by: 44 articles | PMID: 8878348
Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein.
Neuron, 10(2):243-254, 01 Feb 1993
Cited by: 483 articles | PMID: 8094963





