Abstract
Free full text

Differential Growth Characteristics and Streptomycin Susceptibility of Virulent and Avirulent Mycobacterium tuberculosis Strains in a Novel Fibroblast-Mycobacterium Microcolony Assay
Abstract
The ability to spread from cell to cell may be an important virulence determinant of Mycobacterium tuberculosis. An in vitro assay was developed to characterize this ability among four strains of M. tuberculosis: the attenuated strain H37Ra, the virulent strains H37Rv and Erdman, and a virulent clinical isolate (Stew). Confluent monolayers of human skin fibroblasts were infected with these strains and overlaid with agar-medium. M. tuberculosis infection developed over 21 days as microcolonies originating within the plane of the fibroblasts. Microcolonies of the virulent strains had an elongated appearance and exhibited extensive cording. The cords appeared to invade adjacent cells within the plane of the monolayer. Microcolony diameter of the Erdman strain was significantly larger than that of the other virulent strains, indicating that virulent strains can have distinguishing phenotypes in this assay. In contrast, avirulent H37Ra microcolonies were rounded and noncorded. H37Ra microcolonies were significantly smaller than those of the virulent strains. Microcolony diameter of the virulent strains was not reduced by the extracellularly acting antibiotic streptomycin at concentrations of up to 5.0 μg/ml. In contrast, H37Ra microcolony size was reduced at concentrations as low as 0.5 μg/ml. Growth of all strains was similarly inhibited by 1.0 μg of streptomycin per ml in fibroblast-conditioned tissue culture medium alone. When fibroblasts were infected with the M. tuberculosis strains without an agar overlay, with and without streptomycin, numbers of CFU mirrored the changes observed in the microcolony assay. There was a statistically significant decrease in H37Ra CFU compared to virulent strains after treatment with streptomycin. These differences between H37Ra and virulent strains in human fibroblasts suggest that H37Ra may be lacking a virulence determinant involved in cell-to-cell spread of M. tuberculosis.
Despite recent advances in the study of Mycobacterium tuberculosis, relatively little is known about the virulence determinants which allow M. tuberculosis to invade adjacent cells after establishment of primary infection and escape containment by the human immune system. This paucity of data is due, in part, to the limited number of models available for the study of M. tuberculosis pathogenesis.
Several M. tuberculosis strains have been studied in existing models. M. tuberculosis H37 was isolated in 1905 from the sputum of a 19-year-old patient suffering from chronic pulmonary tuberculosis. This strain was dissociated into two variants in 1934: H37Rv, the variant that retained virulence, and H37Ra, the attenuated variant (9). In the guinea pig model of tuberculosis, both strains can replicate 3 to 5 logs in the initial weeks following infection. After 4 weeks, while the H37Rv count remains high, H37Ra is cleared by the guinea pig (1). Thus, infection with the attenuated H37Ra strain can be established but not sustained in this model. One explanation for this observation might be that although H37Ra can establish infection, it is deficient in the ability to invade adjacent cells once the initially infected host cells have died. Although animal models such as the guinea pig model provide information about M. tuberculosis pathogenesis by allowing study of the bacterial population as a whole, examination of individual virulence characteristics of this pathogen is difficult in such models. To aid in characterization of M. tuberculosis virulence characteristics, a novel tissue culture model of M. tuberculosis infection has been developed. This model exploits the fact that in addition to mononuclear phagocytes, M. tuberculosis can infect a variety of nonprofessional phagocytes, including fibroblasts (6). Using this fibroblast-mycobacterium microcolony assay, we examined individual growth characteristics of the virulent strains Erdman and H37Rv, the avirulent strain H37Ra, and a patient isolate (Stew). In this report, we demonstrate that (i) virulent and attenuated strains of M. tuberculosis can be phenotypically characterized by using the fibroblast-mycobacterium microcolony assay; (ii) the virulent Erdman strain has the greatest capacity for directional growth within fibroblast monolayers and appears to preferentially grow in the intracellular environment; (iii) the avirulent H37Ra strain has the least capacity for directional cell-to-cell spread of the strains examined; (iv) growth rates in fibroblast monolayers as assessed by number of CFU are similar for all strains during the first 7 days of infection but thereafter diverge, with H37Ra showing the least growth over 21 days; and (v) in contrast to the virulent strains, the extracellularly acting antibiotic streptomycin is able to significantly inhibit growth of H37Ra in the fibroblast-mycobacterium microcolony assay.
MATERIALS AND METHODS
Tissue culture media.
Dulbecco’s modified Eagle’s medium, Iscove’s modified Dulbecco’s medium, and fetal calf serum (Sigma Chemical Co., St. Louis, Mo.) were used in experiments.
Fibroblasts.
The human skin fibroblast cell line ATCC 1635CRL was obtained from the American Type Culture Collection (ATCC). Fibroblasts were propagated in Dulbecco’s modified Eagle’s medium–1% fetal calf serum in tissue culture flasks (Costar, Cambridge, Mass.) maintained at 37°C in 5% CO2–95% air.
Bacterial culture media.
Middlebrook 7H9 broth (Difco, Detroit, Mich.) was used for dilution of culture supernatants prior to plating CFU. Middlebrook 7H11 agar (Difco) plates (100- by 15-mm bacteriologic petri dishes) were used for plating CFU from infected monolayers and supernatants.
Bacteria.
The Erdman (ATCC 35801), H37Ra (ATCC 25177), and H37Rv (ATCC 25618) strains were obtained from the ATCC as lyophilized cultures and were reconstituted as recommended. A clinical isolate, Stew, was obtained from a sputum culture from a patient with extensive cavitary pulmonary tuberculosis and was passaged once on 7H11 agar after initial isolation. Frozen bacterial stocks of the M. tuberculosis strains and the clinical isolate, preopsonized in normal human serum, were prepared, and inoculating suspensions of bacteria from these stocks were prepared for use in each experiment as previously described (2). For some experiments, animal-passaged M. tuberculosis was used. H37Rv and H37Ra strains (104 CFU) were inoculated intratracheally into BALB/c mice. After 21 days, lungs and spleens of infected mice were harvested. Organ homogenates were diluted into phosphate-buffered saline-Tween and streaked onto 7H11 agar plates. These bacteria were passaged once on 7H11 agar and used as described above. The Erdman strain from guinea pig lung was obtained from Marcus Horwitz (University of California, Los Angeles), passaged once on 7H11 agar, and used as described above.
Fibroblast-mycobacterium microcolony assay.
Fibroblasts were harvested from tissue culture flasks by using trypsin-EDTA (Sigma). After washing, fibroblasts were seeded into six-well, tissue culture-treated cluster plates (Costar) containing Dulbecco’s modified Eagle’s medium–1% fetal calf serum at a concentration of 1 × 105 to 2 × 105/well. After fibroblast monolayers had become confluent (3 to 6 days), the wells were washed three times with Iscove’s medium, and Iscove’s medium alone was readded. After a 24-h equilibration period, the wells were inoculated with various concentrations of M. tuberculosis. After a 1-h incubation at 37°C in 5% CO2–95% air, the monolayers were washed three times with 37°C Iscove’s medium, and 3.0 ml of 50 to 52°C Iscove’s medium-agar (Difco) overlay with or without streptomycin (Sigma) was added to each well. After hardening of the overlay at room temperature, the plates were incubated in a humidified incubator at 37°C in 5% CO2–95% air. Fibroblast monolayers were shown to be viable, as assessed by addition of a 0.5-ml agar overlay containing neutral red, which penetrated the agar and concentrated in the nuclei of viable cells. After 3 to 4 days, M. tuberculosis microcolonies were visible under the microscope; between 7 and 10 days, microcolonies were visible to the naked eye. At various time intervals, microcolonies were visually inspected for microcolony morphology and photographed with a tissue culture microscope (Nikon, Melville, N.Y.). At 21 days, microcolonies were fixed by adding 3.0 ml of 80% phosphate-buffered saline–20% formaldehyde to each well for 24 h, followed by removal of the agar-medium overlay. The bottoms of the wells were then stained for acid-fast bacilli by using carbol fushcin, followed by acid alcohol and several washes with double-distilled water. Precise quantitation of microcolony morphology was achieved by photographing the stained microcolonies at a magnification of ×20 with a tissue culture microscope (Nikon). The diameter of the individual microcolonies, measured in the longest dimension, was measured on 4- by 6-in. black-and-white prints, and corrected to actual size in millimeters. Mean microcolony diameters for different stains or for the same strain under different conditions were obtained.
Assessment of M. tuberculosis growth in human fibroblasts by plating CFU.
In addition to using the fibroblast-mycobacterium microcolony assay, we assessed M. tuberculosis growth in human fibroblasts by plating CFU. Fibroblasts were harvested as described above and seeded into 12-well, tissue culture-treated cluster plates with transwell inserts (Costar) containing Dulbecco’s modified Eagle’s medium–1% fetal calf serum at a concentration of 1 × 104 to 2 × 104/well. After reaching confluence, the monolayers were washed three times with Iscove’s medium, which was then used as the culture medium. The fibroblast monolayers were then inoculated with 105 bacteria, incubated at 37°C in 5% CO2–95% air for 1 h, washed three times with 37°C Iscove’s medium, and cultured in Iscove’s medium alone. Transwell inserts with 0.1-μm-pore-size membranes porous to solutes but not bacteria were then inserted into the wells containing M. tuberculosis-infected fibroblasts and inoculated with approximately 103 to 104 M. tuberculosis. This was done to control for any M. tuberculosis growth-enhancing effect of fibroblast-conditioned media and to assess the effect of streptomycin on extracellular M. tuberculosis growth in fibroblast-conditioned media. Wells were cultured in the presence or absence of streptomycin. At various time points, the culture supernatants, cell lysates, and transwell contents were serially diluted in 7H9 broth (Difco) and plated on 7H11 agar as previously described (2). The number of fibroblast nuclei per well was determined, as elsewhere described (2), in replicate infected wells. In each experiment, CFU counts were normalized to 105 fibroblast nuclei. Viability of the fibroblast monolayers was assessed by trypan blue (Sigma) exclusion to ensure that decreases in CFU were not due to toxic effects of streptomycin on the monolayers.
Statistics.
Data were compared by Student’s t test. Data were considered significant at P < 0.05.
RESULTS
M. tuberculosis infection develops as microcolonies with distinctive morphology within the plane of the fibroblast monolayers.
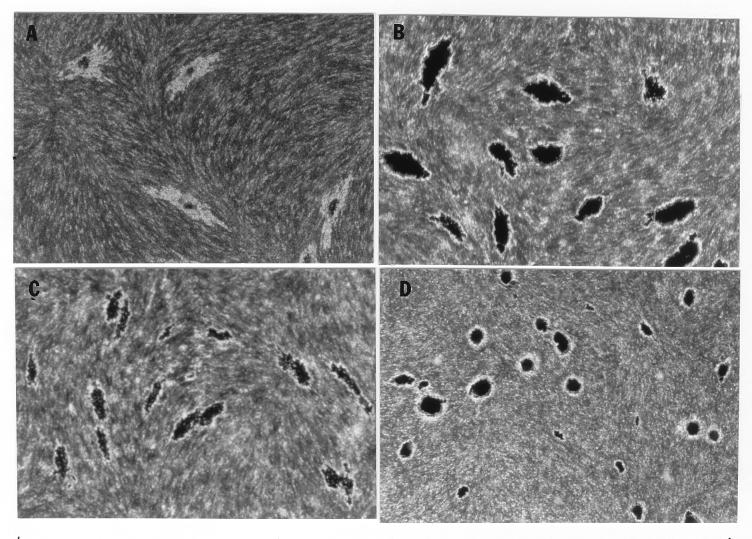
In the fibroblast-mycobacterium microcolony assay, M. tuberculosis formed discrete microcolonies within confluent monolayers when overlaid with agar (Fig. (Fig.1).1). The infection began in individual fibroblasts; mycobacteria became visible approximately 4 to 5 days after inoculation, after which the infected fibroblasts appeared to degenerate. From this point, there was a divergence in microcolony growth patterns. Erdman strain microcolonies grew in a linear fashion parallel to the long axes of the fibroblasts, remained within the plane of the fibroblasts, and had extensive cords which appeared to invade adjacent cells (Fig. (Fig.1A).1A). At the other extreme, H37Ra microcolonies were round and compact and did not cord. Initial growth was within fibroblasts, but at later stages of infection (14 to 21 days), the bacteria grew out of the plane of the fibroblasts into the agar (Fig. (Fig.1D).1D). Similar to Erdman, H37Rv (Fig. (Fig.1B)1B) and Stew (Fig. (Fig.1C)1C) grew in a linear fashion parallel to the long axes of the fibroblasts. However, unlike Erdman but similar to H37Ra, they also grew out of the plane of the fibroblasts into the agar. While individual cords could be clearly visualized in all Erdman microcolonies (Fig. (Fig.1A)1A) throughout the course of infection, the H37Rv and Stew microcolonies were extremely dense, with only a few individual cords visualized in H37Rv and Stew microcolonies on day 21 (Fig. (Fig.1B1B and C).
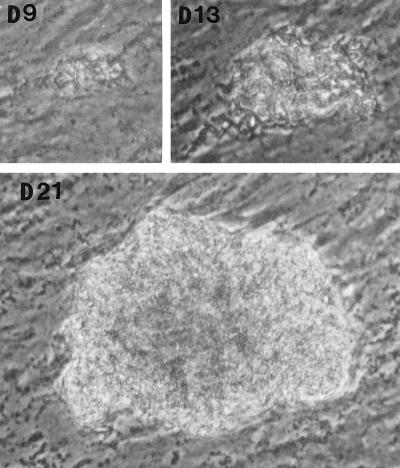
Microscopic appearance of unfixed, unstained M. tuberculosis at days 9, 13, and 21 in the fibroblast-mycobacterium microcolony assay. Over 21 days, M. tuberculosis infection developed as microcolonies within the plane of fibroblasts which had been overlaid with agar-tissue culture medium. (A) Erdman; (B) H37Rv; (C) Stew; (D) H37Ra. Magnifications, ×200 (A and B) and ×200 (C and D).
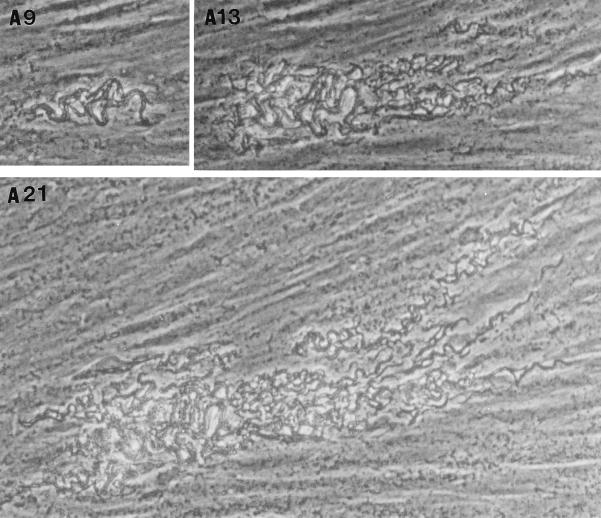
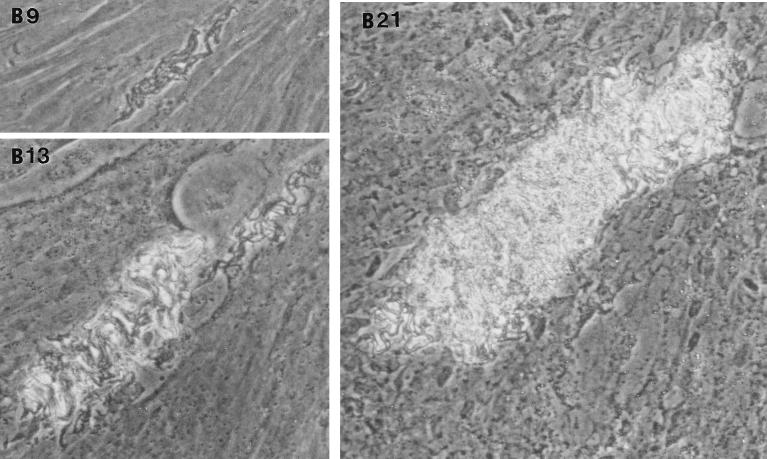
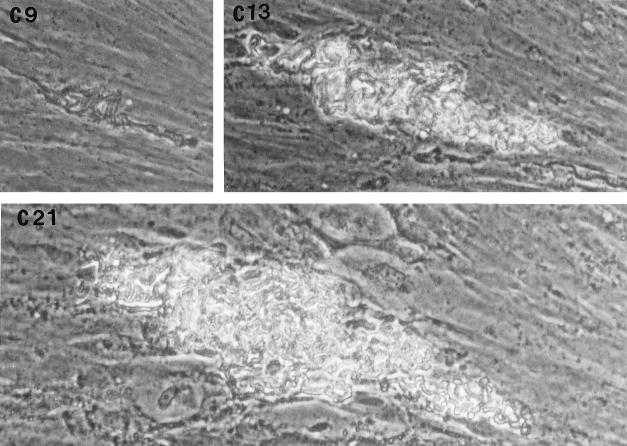
After removal of the agar overlay, 21-day-old microcolonies of M. tuberculosis were fixed and stained. Microscopically (Fig. (Fig.2),2), the Erdman strain microcolonies had a “fried egg” appearance, with central staining and a relative lack of peripheral staining (Fig. (Fig.2A),2A), in contrast to the other strains, which stained as dense, dark microcolonies (Fig. (Fig.2B2B to D). These results indicate that microcolonies from different strains of M. tuberculosis can be phenotypically characterized with this assay. When all M. tuberculosis strains were cultured on 7H11 agar, the resultant colonies were rounded rather than exhibiting the elongated shape of the virulent microcolonies in the fibroblast-mycobacterium microcolony assay. This finding indicates that this morphology is specific to growth in fibroblasts.
Various strains of M. tuberculosis are similar in efficiency of microcolony formation.
M. tuberculosis strains were examined for the ability to form microcolonies in relation to the number of infecting CFU. For all strains, the efficiency of microcolony formation after a 1-h incubation was approximately 2% (data not shown), which indicates that these strains are similar in the ability to associate with human fibroblasts. In addition, the phenotypic characteristics of the microcolonies from each of the four strains were unaffected by varying the number of microcolonies in the monolayer.
Microcolony diameter varies among M. tuberculosis strains.
M. tuberculosis microcolonies from different strains were characterized by measuring their diameter in the longest dimension to reflect their ability to grow in a directional manner. Microcolonies of the Erdman strain were the largest (1.7 ± 0.4 mm), with those of H37Rv (1.1 ± 0.2 mm) and Stew (1.1 ± 0.3 mm) having similar sizes and those of the avirulent strain H37Ra (0.6 ± 0.3 mm) being smallest (n = 50; Erdman versus all strains, P < 0.01; all strains versus H37Ra, P < 0.01; Stew versus H37Rv, nonsignificant; t test). Thus, the virulent Erdman strain has the greatest capacity for directional growth, followed by H37Rv and Stew (intermediate) and the attenuated strain H37Ra (deficient). The Erdman, H37Rv, and H37Ra strains have been passaged numerous times on bacterial culture media since their initial isolation from humans. To ensure that a loss of virulence in these ATCC laboratory-passaged strains was not a confounding variable in our assay system, animal-passaged Erdman, H37Rv, and H37Ra were examined. The same microcolony morphology and relative size results were obtained (n = 50; Erdman, 2.8 ± 0.5 mm; H37Rv, 2.0 ± 0.4 mm; H37Ra, 0.8 ± 0.2 mm; Erdman versus H37Rv and H37Ra, p < 0.01; Erdman and H37Rv versus H37Ra, p < 0.01; t test). These results indicate that animal passage is not a variable in our tissue culture system.
The extracellularly acting antibiotic streptomycin has different effects on microcolony formation by H37Ra and the virulent strains.
In contrast to the virulent strains, H37Ra formed the smallest microcolonies and had a nondirectional growth pattern, which raised the possibility that after initial infection, H37Ra spreads from cell to cell inefficiently relative to the virulent strains. To assess this possibility, we carried out the fibroblast-mycobacterium microcolony assay in the presence of the extracellularly acting antibiotic streptomycin, which was predicted to inhibit cell-to-cell spread of H37Ra if this strain requires an extracellular growth phase for microcolony formation (Fig. (Fig.3).3). There was a statistically significant decrease in the diameter of H37Ra microcolonies treated with streptomycin relative to nontreated microcolonies but no significant effect on microcolony diameter of the virulent strains. In addition, H37Ra microcolony number was reduced 98% at the 5.0-μg/ml drug concentration relative to no streptomycin. At 5.0 μg/ml, microcolony number of the other strains was reduced 30 to 60%. These results indicate that streptomycin has a selective effect on H37Ra growth in fibroblast monolayers relative to virulent strains.
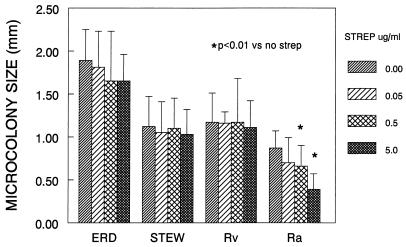
Streptomycin has a selective effect on H37Ra microcolony diameter. M. tuberculosis strains were incubated with various concentrations of streptomycin (strep), and microcolony diameter in the longest dimension was measured 21 days. Shown are the mean diameters and standard deviations for 20 consecutive colonies from each strain. ERD, Erdman; STEW, Stew; Rv, H37Rv; Ra, H37Ra.
With streptomycin treatment, microcolony density and acid fast staining were markedly diminished in the case of H37Rv, Stew, and H37Ra (Fig. (Fig.4),4), which exhibited intense acid-fast staining in the absence of streptomycin (Fig. (Fig.22 and and4).4). This finding indicates (i) that in the absence of streptomycin, the microcolony phenotype of these strains may be in part due to extracellular growth arising out of infected fibroblasts, and (ii) together with the data from Fig. Fig.3,3, that an extracellular growth phase is critical for H37Ra microcolony formation but not required for the virulent strains. In contrast, the relative lack of Erdman strain acid-fast staining, even in the absence of streptomycin, suggests that this strain preferentially exhibits intracellular growth (Fig. (Fig.4).4).
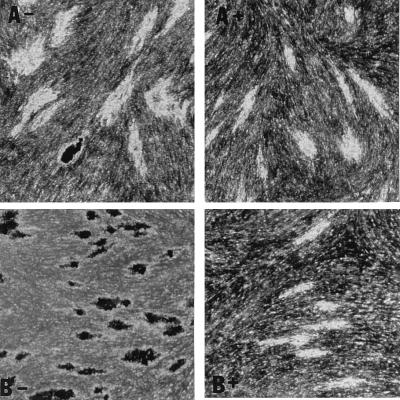
Acid-fast staining of M. tuberculosis H37Rv microcolonies is affected by streptomycin more than is acid-fast staining of Erdman strain microcolonies. M. tuberculosis Erdman (A) and H37Rv (B) were incubated in the absence (−) or presence (+) of streptomycin (0.5 μg/ml). At 21 days, microcolonies were fixed, stained with acid-fast stain, and photographed at a magnification of ×20. A rare, dark-staining Erdman microcolony is shown as a control for the staining procedure among the more typical Erdman microcolonies in panel A−.
M. tuberculosis growth in human fibroblasts as assessed by CFU count parallels the findings observed in the microcolony assay.
The changes in microcolony phenotype induced by streptomycin were likely due to antibiotic inhibition of extracellular growth. To provide further evidence for this hypothesis, we examined the growth characteristics, as assessed by CFU count, of the M. tuberculosis strains by using a transwell system. The advantage of this system is that transwell inserts are porous to solutes but not bacteria. By placing bacteria in the upper chamber and M. tuberculosis-infected fibroblasts in the lower chamber, the influence of streptomycin on the growth of M. tuberculosis in the presence and absence of fibroblasts under identical culture conditions can be observed (Fig. (Fig.5).5). CFU in the fibroblast monolayer and transwell insert were counted at 2, 7, and 21 days after infection. CFU in the fibroblast monolayers showed similar increases for all strains from days 2 to day 7 of infection (Fig. (Fig.5A),5A), which indicates that during the early phase of M. tuberculosis infection of human fibroblasts, the growth rates of H37Rv and H37Ra are similar. During this 5-day period, M. tuberculosis CFU in accompanying transwells decreased 0.5 log for H37Ra and 0.1 log for H37Rv. Since M. tuberculosis growth was not supported by the infected fibroblast-conditioned medium, the results indicate that these strains entered and multiplied within the fibroblasts. Along with data indicating a similar efficiency of microcolony formation, this result indicates that H37Ra is initially taken up by fibroblasts to a similar extent as the virulent strains and subsequently multiplies within these cells. After 7 days, M. tuberculosis growth showed an increase in the transwells of all strains, indicating that after prolonged conditioning, the tissue culture medium of infected fibroblasts supports M. tuberculosis growth (data not shown). This may in part provide an explanation for the intense acid-fast staining of H37Rv, H37Ra, and Stew observed in the microcolony assay: the extracellular growth of these strains produces increased numbers of mycobacteria to take up the acid-fast stain, resulting in the dense appearance and the observation that at late time points these strains appear to be growing out of the plane of the fibroblast monolayers.
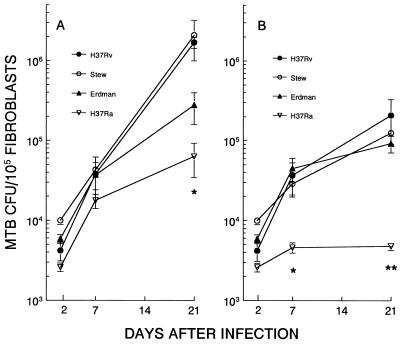
Differential M. tuberculosis (MTB) growth and streptomycin susceptibility in fibroblast monolayers as assessed by CFU count. Fibroblast monolayers were infected with H37Rv, Stew, Erdman, or H37Ra and cultured in the absence (A) or presence (B) of streptomycin (1.0 μg/ml) (with no agar overlay). At 2, 7, and 21 days, CFU from the fibroblast monolayers were counted. Data are means ± standard deviations for duplicate cultures. (A)  , H37Rv and Stew versus H37Ra, P < 0.05; (B)
, H37Rv and Stew versus H37Ra, P < 0.05; (B)  and
and 
 , virulent strains versus H37Ra, days 7 and 21, P < 0.05. CFU of all strains seeded into transwell inserts over infected fibroblast monolayers were uniformly inhibited by this concentration of streptomycin.
, virulent strains versus H37Ra, days 7 and 21, P < 0.05. CFU of all strains seeded into transwell inserts over infected fibroblast monolayers were uniformly inhibited by this concentration of streptomycin.
In contrast to early infection, after 7 days the growth rates of these strains diverged markedly (Fig. (Fig.5A).5A). The virulent strains H37Rv and Stew grew at similar rates and to the same extent from days 7 to 21, paralleling their behavior in the microcolony assay, in which they were similar with respect microcolony size and intensity of acid-fast staining (Fig. (Fig.2).2). In contrast, the Erdman strain grew to a lesser extent than H37Rv and Stew. Although the Erdman strain had the largest microcolony diameter of all strains in the fibroblast-mycobacterium microcolony assay, it had markedly diminished microcolony density and acid-fast staining relative to the other strains (Fig. (Fig.2),2), which likely explains its lower CFU count during this time frame. H37Ra showed minimal growth from days 7 to day 21, consistent with its markedly decreased microcolony size in the microcolony assay. Thus, growth in the microcolony assay is a reflection of both (i) microcolony density and intensity of acid-fast staining and (ii) microcolony diameter.
As part of this experiment, we compared M. tuberculosis growth in the presence of the extracellularly acting antibiotic streptomycin with that seen in the fibroblast-mycobacterium microcolony assay (Fig. (Fig.5B).5B). In transwells for which the initial CFU count was similar to that for the fibroblast monolayers, CFU counts of all strains at 21 days decreased by over 99.9% in the transwells receiving streptomycin relative to companion transwells not receiving streptomycin. Thus, growth of these strains is equally affected by streptomycin in the culture medium of M. tuberculosis-infected fibroblasts. In the accompanying fibroblast monolayers infected with the virulent strains of M. tuberculosis, there was minimal to no inhibition of growth at this concentration of streptomycin over the initial 7 days of infection. However, after 7 days, in the presence of streptomycin, growth rates of the virulent strains overlapped. This result indicates that the increased H37Rv and Stew CFU relative to Erdman in the absence of streptomycin are due to extracellular as well as intracellular growth of these strains. In contrast to the virulent strains, streptomycin had a much greater effect on H37Ra. While streptomycin had no effect on the virulent strains over the initial 7 days, H37Ra growth was significantly inhibited (Fig. (Fig.5B)5B) and subsequently decreased from days 7 through 21. These results support the fibroblast-mycobacterium microcolony assay findings which indicate that streptomycin has a selective effect on H37Ra microcolony size relative to the virulent strains.
DISCUSSION
Previously, we found that the ability of M. tuberculosis to spread from cell to cell is facilitated by tumor necrosis factor alpha and that this ability may be an important virulence determinant of M. tuberculosis (2, 3). To examine the phenomenon of M. tuberculosis cell-to-cell spread, we developed a fibroblast-mycobacterium microcolony assay which allows for long-term observation of M. tuberculosis growth. Results obtained with this assay indicate that cell-to-cell spread of virulent M. tuberculosis occurs in fibroblast monolayers. Furthermore, different M. tuberculosis strains can be quantitatively and qualitatively characterized. Results obtained with this assay and an assay directly assessing CFU counts in fibroblast monolayers reveal significant differences between M. tuberculosis strains. Among the strains studied, Erdman appears to have the greatest ability for directional growth, as assessed by microcolony diameter. However, as assessed by numbers of CFU, Erdman growth is less than that of the virulent H37Rv strain and the Stew isolate. An explanation for this finding relates to the acid-fast staining of each of these strains. In the absence of streptomycin, Erdman microcolonies remain within the plane of the fibroblast monolayer and consist of a loose network of clearly visible cords which take up acid-fast stain poorly. This lack of acid fastness of the mycobacterial cords at the periphery of the Erdman strain microcolonies at 21 days suggests that these cords may be in an intracellular location. Intracellular M. tuberculosis has been reported to take up acid-fast stain poorly (5). Alternatively, the decreased acid-fast staining of the Erdman microcolonies may reflect a less compact, more diffuse microcolony. In contrast, H37Rv and Stew form dense, heavily stained, compact microcolonies which, in addition to showing linear growth in the plane of the fibroblast monolayers, grow out of the fibroblasts into the agar-tissue culture medium. When the extracellularly acting antibiotic streptomycin is added to the fibroblast-mycobacterium microcolony assay, there is relatively little change in Erdman acid-fast microcolony staining or appearance but markedly diminished staining of H37Rv and Stew microcolonies, accompanied by an absence of growth into the agar-tissue culture medium. This correlates with a large decrease in the growth rate of H37Rv and Stew as assessed by number of CFU but relatively little effect on Erdman CFU in the CFU assay, with the result that the growth rates of the virulent strains overlap. Thus, when growth of H37Rv and Stew out of the fibroblasts into the tissue culture medium is eliminated by streptomycin, the three virulent strains have similar growth rates. One interpretation of these results is that the Erdman strain preferentially invades adjacent fibroblasts after establishment of primary infection compared to H37Rv and Stew, which invade adjacent fibroblasts but also grow out into the extracellular environment.
In contrast to the virulent strains, microcolony size and CFU counts of the avirulent strain H37Ra are markedly diminished. Interestingly, when CFU counts are assessed over the initial 5 days of infection, the growth rate of H37Ra is similar to that of the virulent strains, consistent with the observation that H37Ra and H37Rv have similar growth rates in human monocytes over a 6-day period (7). Beyond 7 days, however, the growth rate of H37Ra diverges from the growth rates of the virulent strains, similar to what we (unpublished observations) and others (8, 10) have noted in M. tuberculosis-monocyte infection assays of longer duration. One explanation for this observation is that the early growth phase represents only intracellular growth. After infected fibroblasts are lysed by the original inoculum, the ability to spread from cell to cell influences the growth rate in the fibroblast monolayers. If lysis of initially infected fibroblasts occurs at approximately 7 days, and if H37Ra has an inefficient mechanism for entering adjacent fibroblasts, allowing exposure to the toxic effects of streptomycin, microcolony diameter and CFU count would be decreased.
The data presented in this study suggest important differences in the ability of these strains to spread from cell to cell, particularly at late time points after infection. One striking aspect of these results is the finding that growth of the virulent M. tuberculosis strains occurs almost exclusively parallel to the long axes of the fibroblasts. As a mechanism of virulence, focused bacterial growth in one direction may facilitate rapid spread of M. tuberculosis. Such directional growth would allow greater distances to be traversed by the growing mass of bacteria than could be achieved by bacteria growing in a nondirectional manner. Small distances between cells could be rapidly traversed, and the growing directional mass of bacteria might evade the ability of lymphocytes, macrophages, etc., to effectively focus an immune response.
While fibroblasts are not the natural host cells for most intracellular pathogens, including M. tuberculosis, they have been extremely useful as infection models for the study of bacterial virulence determinants. For example, Listeria monocytogenes has been analyzed in fibroblast models, and virulence correlates with its ability to spread within the fibroblast monolayer (4). However, in contrast to the pattern of spread by M. tuberculosis, L. monocytogenes spreads in a radial fashion from the initial site of infection (4). The mechanism(s) for the linear spread of virulent M. tuberculosis through fibroblast monolayers is currently under investigation in this laboratory.
In summary, this is the first study to show phenotypic differences among different strains of M. tuberculosis in an in vitro tissue culture assay using human cells. The data indicate that the ability to spread directionally from cell to cell differs for different strains of M. tuberculosis and is reduced in the avirulent strain H37Ra. The enhanced susceptibility of H37Ra to streptomycin may relate to an inefficient mechanism of cell-to-cell spread. Future efforts using this model will be directed at correlating intracellular events with the microcolony phenotype of virulent and attenuated strains of M. tuberculosis. In addition, correlating the microcolony phenotype of clinical isolates with clinical presentations (e.g., miliary versus cavitary tuberculosis) may provide further insights into M. tuberculosis pathogenesis. The results of this study suggest that a greater emphasis on the assessment of late events in M. tuberculosis pathogenesis may be warranted in future studies. In this context, the fibroblast-mycobacterium microcolony assay should prove useful for studying mycobacterial pathogenesis.
ACKNOWLEDGMENTS
This work was supported by the VA Medical Center, Albuquerque, N.Mex., and by National Institutes of Health grants AI35249 to Thomas F. Byrd and HL55776 to C. Rick Lyons. C. R. Lyons is a Culpeper Medical Scholar, and this work was supported in part by the Culpeper Medical Scholar Foundation.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.66.11.5132-5139.1998
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/66/11/5132.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/content/full/66/11/5132
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/66/11/5132
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/66/11/5132
Citations & impact
Impact metrics
Citations of article over time
Article citations
Updated Review on the Mechanisms of Pathogenicity in Mycobacterium abscessus, a Rapidly Growing Emerging Pathogen.
Microorganisms, 11(1):90, 29 Dec 2022
Cited by: 8 articles | PMID: 36677382 | PMCID: PMC9866562
Review Free full text in Europe PMC
Bacterial Strain-Dependent Dissociation of Cell Recruitment and Cell-to-Cell Spread in Early M. tuberculosis Infection.
mBio, 13(3):e0133222, 13 Jun 2022
Cited by: 9 articles | PMID: 35695454 | PMCID: PMC9239178
Mycobacterium abscessus: Shapeshifter of the Mycobacterial World.
Front Microbiol, 9:2642, 01 Nov 2018
Cited by: 44 articles | PMID: 30443245 | PMCID: PMC6221961
Review Free full text in Europe PMC
Evolution and role of corded cell aggregation in Mycobacterium tuberculosis cultures.
Tuberculosis (Edinb), 93(6):690-698, 13 Aug 2013
Cited by: 14 articles | PMID: 24011631
Prison break: pathogens' strategies to egress from host cells.
Microbiol Mol Biol Rev, 76(4):707-720, 01 Dec 2012
Cited by: 52 articles | PMID: 23204363 | PMCID: PMC3510522
Review Free full text in Europe PMC
Go to all (21) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors.
J Immunol, 150(7):2920-2930, 01 Apr 1993
Cited by: 309 articles | PMID: 8454864
Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages.
Infect Immun, 66(2):794-799, 01 Feb 1998
Cited by: 72 articles | PMID: 9453643 | PMCID: PMC107972
Expression of virulence of Mycobacterium tuberculosis within human monocytes: virulence correlates with intracellular growth and induction of tumor necrosis factor alpha but not with evasion of lymphocyte-dependent monocyte effector functions.
Infect Immun, 66(3):1190-1199, 01 Mar 1998
Cited by: 81 articles | PMID: 9488413 | PMCID: PMC108033
Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis.
Infect Immun, 10(4):742-746, 01 Oct 1974
Cited by: 40 articles | PMID: 4214780 | PMCID: PMC423015
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: HL55776
NIAID NIH HHS (1)
Grant ID: AI35249