Abstract
Free full text

c-Myc Target Genes Involved in Cell Growth, Apoptosis, and Metabolism
The c-myc gene was discovered as the cellular homolog of the retroviral v-myc oncogene 20 years ago (23, 25, 167). The c-myc proto-oncogene was subsequently found to be activated in various animal and human tumors (37, 39, 42). It belongs to the family of myc genes that includes B-myc, L-myc, N-myc, and s-myc; however, only c-myc, L-myc, and N-myc have neoplastic potential (54, 82, 102, 118, 178). Targeted homozygous deletion of the murine c-myc gene results in embryonic lethality, suggesting that it is critical for development (43). Homozygous inactivation of c-myc in rat fibroblasts caused a marked prolongation of cell doubling time, further suggesting a central role for c-myc in regulating cell proliferation (121).
The frequency of genetic alterations of c-myc in human cancers (42) has allowed an estimation that approximately 70,000 U.S. cancer deaths per year are associated with changes in the c-myc gene or its expression. Given that c-myc may contribute to one-seventh of U.S. cancer deaths, recent efforts have been directed toward understanding the function of the c-Myc protein in cancer biology with the hope that therapeutic insights will emerge. Past efforts, which have contributed significantly to our current understanding of c-myc, are discussed in a number of excellent reviews (23, 29, 37, 40, 44, 52, 66, 82, 94, 102, 118, 125, 132, 145, 178, 182, 186).
ALTERATIONS OF THE C-MYC GENE IN HUMAN CANCERS
In human cancers, the c-myc gene is activated through several mechanisms. Unlike the normal c-myc gene, whose expression is under exquisitely fine control, translocations that juxtapose the c-myc proto-oncogene at chromosome 8q24 to one of three immunoglobulin genes on chromosome 2, 14, or 22 in B cells activate the c-myc gene and thereby promote the genesis of lymphoid malignancies (37, 118). Similarly, the murine c-myc proto-oncogene is activated through chromosomal translocations in pristane-induced murine plasmacytomas (140). Indeed, transgenic animals that overexpress c-myc in lymphoid cells or other tissues succumb to lymphomas or other tumors (1, 159, 174). The c-myc gene is amplified in various human cancers, including lung carcinoma (107), breast carcinoma (120, 128), and rare cases of colon carcinoma (9). In addition, elevated expression of the c-myc gene is found in almost one-third of breast and colon carcinomas (49, 50). Recent evidence suggests that activation of c-myc gene expression is central to signal transduction through the adenomatous polyposis coli (APC) tumor suppressor protein which negatively regulates β-catenin (Fig. (Fig.1)1) (80). β-Catenin is a coactivator for the transcription factor Tcf, which is able to directly activate c-myc expression, so that when APC is inactivated, activation of β-catenin results. The activities of human transforming proteins BCR-ABL (2, 158) and TEL-PDGFR (31) and proto-oncogenes c-src (13) and Wnt (33) have been shown to depend on the c-myc gene (Fig. (Fig.1).1). In retrospect, the emergence of c-myc as a central oncogenic switch in human cancers might have been predicted by the ability of the oncogenic retroviral v-myc gene to cause the rapid development of a variety of tumors in infected chickens (23, 25).

The c-myc gene is a central oncogenic switch for oncogenes and the tumor suppressor APC. The APC tumor suppressor protein mediates the degradation of β-catenin. The Wnt oncoprotein is shown activating its receptor, which results in the stabilization of free β-catenin. β-Catenin, which sustains activating mutations in human cancers, is a cofactor for the transcription factor Tcf. Tcf activates c-myc expression through specific DNA binding sites. The oncogenic fusion protein TEL-PDGFR hypothetically activates c-src, as does native PDGFR, resulting in the activation of c-myc. The BCR-ABL oncoprotein likewise requires c-myc for its activity.
In addition to activation of the c-myc gene through deregulated expression, point mutations in the coding sequence have been found in translocated alleles of c-myc in Burkitt’s lymphomas (21, 22, 36, 203). These point mutations, which perhaps arose from somatic hypermutation in B cells, cluster in the transactivation domain of c-Myc around two major phosphorylation sites, one of which is also subject to O-linked glycosylation (Fig. (Fig.2)2) (34, 35, 71, 85, 110–112). The consequence of these mutations is hypothesized to be abrogation of a negative regulation of c-Myc activity by phosphorylation of these sites, although hard evidence is still lacking (176). Alternatively, these mutations may prolong the half-lives of the mutant proteins, since the affected c-Myc regions have been implicated in the proteasome-mediated degradation of c-Myc (61).

Association of factors to functional domains of the c-Myc protein. O-GlcNAc marks a glycosylation site. GSK3 and CDK mark phosphorylation sites. Max is depicted in association with c-Myc through the HLH-LZ motif; b is the basic region. NTS is the nuclear target signal. TRD represents the transcriptional regulatory domain. The proteins Bin1, PAM, p107, and TBP are shown associated with the TRD of c-Myc. Miz1 and TFII-I are shown associated with the HLH-LZ region of c-Myc. YY1 may associate with the central domain of c-Myc.
C-MYC TRANSCRIPTION FACTOR, ITS BINDING PARTNER MAX, AND MAD PROTEINS
The c-myc gene, located on human chromosome 8, is comprised of three exons (15). Translation of the major 64-kDa polypeptide is initiated at the canonical AUG start codon (exon 2), and a longer polypeptide of 67 kDa results from translation initiated 15 codons upstream of the AUG at a CUG codon (exon 1) (76). An internal translationally initiated c-Myc 45-kDa polypeptide was recently recognized (179).
The primary sequence of the c-Myc protein suggests that it contains a potential transactivation domain within its N-terminal 140 amino acids and a dimerization interface consisting of a helix-loop-helix leucine zipper (HLH/LZ) domain at its C-terminal end (Fig. (Fig.2).2). Evidence from fusion proteins consisting of GAL4 and c-Myc suggested that the c-Myc transactivation domain is localized to its first 143 amino acids (93). Immediately N terminal to the dimerization domain is a domain rich in basic amino acids which directly contacts specific DNA sequences within the DNA major groove (41, 45, 56, 57, 60, 143, 144, 185). c-Myc DNA binding sites (both canonical [5′-CACGTG-3′] and noncanonical) have been identified by using a variety of in vitro protein-DNA binding assays (26, 27, 144). The search for a Myc binding partner protein resulted in the breakthrough discovery of an HLH/LZ human Max protein by Blackwood and Eisenman (28, 29) and the murine Max homolog, Myn, by Prendergast et al. (142). Max, in contrast to Myc, does not contain a transactivation domain (95). Initial models proposed that Myc/Max heterodimers bind to target sites to transactivate genes via the Myc transactivation domain (Fig. (Fig.3).3). Max homodimers were thought to counter the function of the Myc/Max heterodimers through competitive binding to target DNA sites (29, 95); however, functional Max homodimers are not readily detectable in vivo (20, 97, 177).
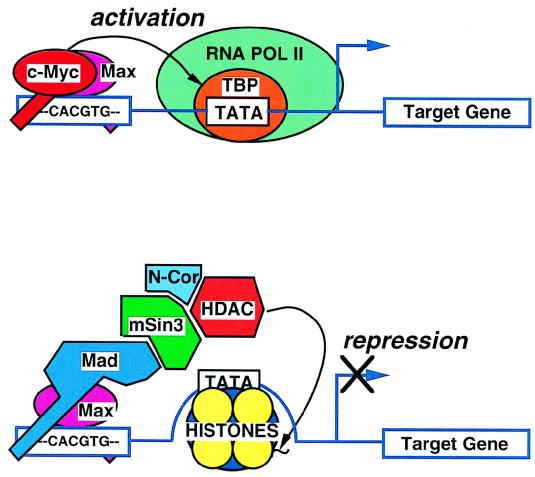
Models of c-Myc/Max and Mad/Max in transcriptional regulation. The c-Myc/Max heterodimer is shown at the top tethered to the E box 5′-CACGTG-3′. c-Myc contacts TBP, although the molecular mechanisms involved in c-Myc transactivation are not known. The bottom diagram depicts the association of the Mad/Max heterodimer with the E box, as well as with mSin3, N-Cor, and histone deacetylase (HDAC). HDAC deacetylates histones, causing the locking of nucleosomal DNA and, consequently, inhibition of transcription. POL, polymerase.
This simple model became more complex with the discovery of the Mad family of proteins, which were identified by their ability to bind Max (11, 87–89, 205). The Mad (Fig. (Fig.3)3) proteins contain the Sin3-interacting domain motif (12, 160), which recruits Sin3, the transcriptional corepressor N-Cor, and proteins that have histone deacetylase activity (4, 81, 129). Histone deacetylation is currently thought to be the major mode of transcriptional silencing by the Mad proteins. The Sin3-intacting domain motif, when tethered to an HLH/LZ transcriptional factor, TFEB, that binds Myc DNA sites, is able to inhibit c-Myc-mediated cellular transformation (78). This observation suggests that HLH/LZ proteins have overlapping binding sites within target genes, contributing to another level of gene regulation.
Increased expression of Mad proteins is associated with cellular differentiation and growth arrest, suggesting that certain Mad family members behave as tumor suppressors. The chromosomal localization of the Mxi-1 (Mad 2) protein to 10q24 initially suggested that it is the key tumor suppressor gene in human glioblastomas, which display frequent loss of heterozygosity (LOH) at this region (47, 166, 196–198). Although LOH of Mxi-1 at 10q24 is frequent, somatic mutations of Mxi-1 have not been found (3, 14, 46, 68, 96, 171). These findings are unable to confirm the observation that frequent mutations of the Mxi-1 gene occur in human prostate cancers (46). In contrast, the candidate 10q24 tumor suppressor PTEN gene was recently found to have LOH and somatic mutations in some cases of glioblastomas (105). To date, none of the Mad family members has been fully documented as a human tumor suppressor gene, although homozygous deletion of murine Mxi-1 potentiates skin tumor and lymphoma formation (161). Homozygous inactivation of Mad1 resulted in granulocyte differentiation abnormalities, supporting the role of Mad genes in cellular differentiation (62).
TRANSCRIPTIONAL PROPERTIES OF C-MYC
The c-Myc protein binds to and transactivates through consensus 5′-CACGTG-3′ sequences or E boxes in transient transfection experiments; however, the potency of transactivation by c-Myc pales when compared to those of other transcription factors, such as the HLH/LZ transcriptional factor USF, which also binds 5′-CACGTG-3′ (6, 7, 72, 98, 101). The variability of c-Myc transactivation has been questioned, and a study has provided evidence that endogenous levels of c-Myc may affect the outcome of transient-transfection experiments (101). Others suggest that the transactivation properties of c-Myc depend on whether the 64- or 67-kDa form is produced (75). The ability of c-Myc to interact with the TATA binding protein (TBP) and the transcriptional machinery (79, 85, 113, 123) may be modulated by its interaction with other factors, such as BIN1 (157), MIZ1 (138), PAM (73), p107 (17, 71, 74, 85), TFII-I (154), TRRAP (124), and YY1 (10, 172, 173) (Fig. (Fig.2).2). Understanding of how each of these proteins modulates the transcriptional activity of c-Myc requires further studies. Another as yet unresolved quagmire in the study of c-Myc is the inability to easily detect c-Myc gel shift activities in nuclear extracts of mammalian cells, although some progress has been achieved recently (108, 130, 177). Notwithstanding these unresolved concerns, evidence accumulated to date supports the model in which c-Myc is able to bind E boxes and transactivate genes.
In addition to its ability to activate transcription, c-Myc is able to repress transcription in in vitro transcription and transient-transfection assays (101, 106, 154). The in vitro data are compatible with the ability of c-Myc to inhibit transcription through the initiator or Inr element, which is a consensus transcriptional initiation motif found in certain gene promoters (175). Likewise, transfection studies using model promoter reporter constructs suggest that c-Myc is able to repress Inr-mediated transcription (100, 106, 138). c-Myc also represses genes that do not contain Inr sequences (202) and may modulate transcription through interactions with other transcription factors, such as C/EBP (127) or AP-2 (65). Since many genes bearing Inr sequences are differentiation marker genes, it is surmised that in addition to its ability to activate growth related genes through E boxes, c-Myc is also able to repress differentiation-related genes. The transcriptional repression function of c-Myc and its transactivation ability are both required for its transforming activity.
C-MYC TARGET GENES
The mechanisms by which c-Myc induces neoplastic transformation and apoptosis are beginning to emerge with the identification of authentic target genes, both direct and indirect (Table (Table11 and Fig. Fig.4).4). A direct target gene is one whose expression is altered by direct interaction of the c-Myc protein with the gene regulatory elements or with trans-acting factors that bind these cis elements. The time course of induction of a direct target gene should closely follow the expression of Myc. The Myc-estrogen receptor (Myc-ER) fusion protein system has become a standard for establishing the direct regulation of a candidate target gene by c-Myc (48). In this system, the Myc-ER fusion protein is retained in the cytoplasm via chaperone proteins. Upon exposure of cells expressing the Myc-ER protein to estrogenic ligands, the ligand-bound fusion protein is translocated into the nucleus. The Myc-ER protein then activates Myc target genes without requiring new intervening protein synthesis. Thus, exposure of cells simultaneously to estrogenic compounds and cycloheximide will result in the activation or repression of direct target genes. An indirect target gene of c-Myc is one whose expression is altered as a consequence of expression of the direct Myc target genes and whose expression is connected to c-Myc-dependent phenotypes such as cellular proliferation, transformation, or apoptosis. The search for target genes usually implies identification of the direct targets; however, it stands to reason that indirect targets may provide the missing links between deregulated c-Myc expression and neoplastic transformation or apoptosis.
TABLE 1
Putative c-Myc target genesa
genesa
| Gene productb | Regulation | Technique | Relevance to c-Myc | Reference(s) |
|---|---|---|---|---|
| ARF or p19 | Up | Guess | Apoptosis | 206 |
| CAD | Up | Promoter | Growth and metabolism | 126 |
| Cdc2 | Up | Guess | Growth related | 30 |
| Cdc25A | Up | Guess | Growth related | 64 |
| Cyclin A | Up | Guess | Growth related | 38, 77, 84, 91 |
| Cyclin D1 | Up or down | Guess/diff | Growth related | 38, 139, 150 |
| Cyclin E | Up | Guess | Growth related | 103, 137 |
| DHFR | Up | Promoter | Growth and metabolism | 116 |
| ECA39 | Up | Diff | Amino acid transport | 19 |
| eIF-2α | Up | Guess | Growth-related metabolism | 151 |
| eIF4E | Up | Guess | Growth-related metabolism | 92, 151 |
| ISGF3γ | Up | Guess | Stress response | 199 |
| LDH-A | Up | Diff | Growth and metabolism | 170, 180 |
| MrDb | Up | Binding | Metabolism | 67 |
| ODC | Up | Diff/guess | Growth related | 18, 131, 134, 136, 190, 200 |
| PAI-1 | Up | Diff | ? | 141 |
| α-Prothymosin | Up | Diff | Growth related | 48 |
| p53 | Up | Diff/promoter | Growth related | 148, 180 |
| RCC1 | Up | Guess | Growth related | 183 |
| Rcl | Up | Diff | Growth related | 104 |
| Telomerase | Up | Guess | Immortality | 193 |
| TK | Up | Guess | DNA metabolism | 147 |
| Albumin | Down | Promoter | ? | 106 |
| Collagens α1(I), α2(I), α3(VI), α1(III) | Down | Diff | Adhesion | 180, 201, 202 |
| C/EBPα | Down | Promoter | Differentiation | 106 |
| Gadd 45 | Down | Diff | Growth | 119 |
| Ig lambda | Down | Promoter | ? | 117 |
| LFA-1 | Down | Guess | Adhesion | 90 |
| MHC class I | Down | Guess | Immune surveillance | 187 |
| Tdt | Down | Promoter | ? | 117 |
| Thrombospondin | Down | Diff | Metastasis | 181 |
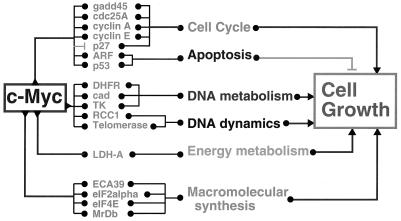
Links between c-Myc, selected putative target genes, cellular functions, and cell growth. This diagram illustrates the complexity of the connections between c-Myc and its putative target genes, which are shown clustered according to their functions. The various cellular functions cooperate to promote cell growth. It should be noted that this diagram does not reflect the controversies over the authentication of the various target genes.
The study of target genes is confounded, however, by the fact that target genes are likely to be necessary, but not sufficient, for Myc-mediated phenotypes. The use of antisense technology or dominant negative alleles of target genes is a logical approach to the establishment of the necessity of a target gene for a Myc-associated phenotype. This experimental approach is limited, however, to the study of target genes whose disruption leads to drastic fundamental cellular changes such as markedly slowed growth, which poses a problem for the interpretation of other cellular phenotypes that depend on growth rates. If a Myc target gene is sufficient for a specific c-Myc-induced phenotype, it would be expected that expression of this target gene will induce this specific Myc-mediated phenotype. It is more likely, however, that subsets of target genes collaborate to mediate specific Myc-related phenotypes.
There are three major approaches to the identification of target genes. The first, the candidate target gene approach, is based on the biology of c-Myc. The second identifies genes that are differentially expressed as a result of enforced Myc expression. The third implicates genes whose regulatory elements contain c-Myc/Max binding sites.
Candidate direct and indirect c-Myc target genes are listed in Table Table1.1. The gene for ornithine decarboxylase (ODC) is an example of the power of the candidate gene approach. It contains potential Myc/Max binding sites and has been independently verified to be c-Myc responsive by different investigators (18, 131, 134, 136, 190, 200). It is instructive to note that the gene for ODC is inducible by both cycloheximide and c-Myc (133). This characteristic accounts for the biphasic response of ODC expression to growth factor stimulation and demonstrates a weakness in the use of the Myc-ER chimeric system as a criterion for direct target genes. In this case, the high basal cycloheximide induction of ODC can mask the effect of the Myc-ER activator.
Many genes have been implicated as c-Myc targets, although their roles in c-Myc-mediated phenotypes have not been determined. An approach to the cloning of a subset of mid-G1 serum response genes that are regulated by c-Myc was undertaken via differential screening (180). Included in the putative Myc targets from this study were known genes encoding ODC, lactate dehydrogenase A (LDH-A), α-prothymosin, and one novel gene with limited homology to methylenetetrahydrofolate synthetase. Another approach, using representational difference analysis as a differential cloning strategy, was undertaken to identify c-Myc-responsive genes (104) that are expressed differentially between normal and Myc-transformed Rat1a fibroblasts grown in suspension. Under these conditions, genes whose expression is anchorage dependent are expected to be diminished in nontransformed fibroblasts but may be induced by c-Myc in Myc-transformed cells. Twenty genes were identified, i.e., 17 that are upregulated and 3 that are downregulated by c-Myc. Studies using the Myc-ER fusion protein system are consistent with the idea that one novel gene, rcl, is a direct target of c-Myc. In fact, rcl overexpression in Rat1a cells induces the cell transformation phenotype of anchorage-independent growth, albeit to a lesser extent than c-Myc overexpression.
Studies of gene promoters have led to the recognition of the 5′-CACGTG-3′ E box in a variety of genes. It should be noted that this E box could be bound by HLH/LZ protein USF, TFE-3, or TFE-B in addition to c-Myc. Thus, the existence of Myc-type E boxes in promoter regions should include the possibility that USF, TFE-3, or TFE-B can act as the transactivator (16, 59, 69). Several promoters have been proposed to be c-Myc targets based on this criterion; dihydrofolate reductase (DHFR) and carbamoyl-phosphate synthase (CAD) both contain E2F as well as the Myc E-box sequences (116, 126). However, there is no other established regulatory link between DHFR and Myc. The promoter of the p53 gene was noted to contain an E box resembling a Myc binding site (148, 155). Although many genes have been proposed to be targets of c-Myc (Table (Table1),1), the biology of these putative targets (CAD or p53) in c-Myc-mediated neoplastic transformation or apoptosis is only beginning to emerge and needs further study.
A physical approach to the identification of potential c-Myc target genes was recently undertaken (67). In this approach, immunoprecipitation of isolated chromatin with anti-Myc and anti-Max antibodies allowed the identification of potential target sites of Myc/Max complexes. One of the targets identified is a pseudogene whose authentic counterpart, the MrDb RNA helicase, appears to be regulated by c-Myc. Furthermore, pitchoune, the Drosophila homolog of MrDb, appears to be genetically linked to dMyc, the Drosophila homolog of myc (204). Indeed, the use of Drosophila genetics to study links to the diminutive phenotype caused by mutant dMyc may provide an additional approach to the identification of c-Myc target genes that may ultimately be relevant to mammalian biology.
C-MYC AND THE CELL CYCLE
The role of c-Myc in the cell cycle has been a confusing area due to the collection of data from different experimental models, although it is well established that c-myc is an early serum response gene. It should be noted that models of serum or growth factor stimulation of starved cells primarily address the G0/G1 and G1/S transitions. Therefore, early studies implicated c-Myc in the G0/G1 transition (63). In cycling cells, however, the participation of c-Myc in the cell cycle may be different (5). Furthermore, in anchorage-dependent cell growth, c-Myc may affect other components of the cell cycle.
The emergence of cyclins, cyclin-dependent kinases (CDKs), and CDK inhibitors as cell cycle regulators has provided some insights into c-Myc function (5). Regarding the regulation of G1, the connection between c-Myc and cyclin D1 is complex and may depend on specific stimuli and cell systems (152). Both c-myc and cyclin D1 are required for activation through the CSF1 receptor, and their relationship is nonlinear (153). With serum stimulation of fibroblasts, it is expected that c-Myc may activate the subsequent expression of cyclin D1; however, the role of c-Myc in regulating cyclin D1 expression is complex, since there are conflicting data in the literature (38, 139, 150).
Deregulated c-Myc expression is linked to increased cyclin A and increased cyclin E expression (38, 77, 84, 91). Recent evidence has been provided that c-Myc is able to transactivate the expression of cyclin E directly, although the mechanism is unclear (103, 137). c-Myc increases CDK function through several mechanisms. In one study, c-Myc appeared to cooperate with RAS to induce the CDC2 (CDK1) promoter, which does not contain a consensus Myc E box (30). There are no other data, however, that support the elevation of CDC2 in response to Myc. More recently, evidence has been provided that the cdc25A gene is a direct target of c-Myc (64). The connection between c-Myc and cdc25A has not been confirmed in other studies (5), indicating that differences in experimental models might account for the discrepancy. This gene produces a protein phosphatase that activates both CDK2 and CDK4. Thus, a direct link between c-Myc and the cell cycle machinery may exist through its ability to activate the cdc25A and cyclin E genes directly. c-Myc expression also decreases the levels and interferes with the function of the p27 CDK inhibitor (103, 137, 156, 188). The mechanism by which c-Myc interferes with p27 activity is not known. These activities of c-Myc are all compatible with the ability of c-Myc to promote cell entry into S phase.
The ability of c-Myc to promote cell proliferation suggests that its deregulation contributes to deregulated DNA synthesis and genomic instability (114, 115). Several studies suggest that deregulated c-Myc expression triggers genomic instability as measured by gene amplification or the rate of development of aneuploidy. These studies are intriguing but preliminary, and therefore, additional, confirmatory studies are required for greater appreciation of the role and mechanism of action of c-Myc in genomic instability.
The role of c-Myc in the cell cycle is further highlighted by the marked prolongation of the doubling time of cells in which both alleles of c-myc were eliminated by homologous recombination (121). Cell cycle distribution analysis showed that myc-null cells, which express neither L-myc nor N-myc, have prolonged G1 and G2 phases, whereas the S phase is normal. Cell sizes were normal at each phase of the cell cycle, suggesting that c-Myc has a generalized effect on cell growth. Independent studies using elutriated cells overexpressing Myc-ER suggest that there is inappropriate expression of cyclin E in the G1 phase, but this ectopic cyclin E expression is insufficient to bypass the requirement for proper cell size before entry into S phase (146). These observations suggest that a subset of c-Myc target genes is involved in biosynthetic and metabolic pathways that regulate cell size.
C-MYC AND METABOLISM
Cells studied as monolayers under experimental conditions do not reflect the three-dimensional growth of an avascular tumor, which is mimicked in soft-agar anchorage-independent growth assays. When a tumor grows to a detectable size, the local environment of the tumor cells often becomes heterogeneous. Microregions of larger tumors, as well as small (<1 mm) tumor nodules, have microecological niches in which there are major gradients of critical metabolites, such as oxygen, glucose, and other nutrients or growth factors. Chronic hypoxia occurs in tumor tissue that is more than 150 μm away from a functional blood supply, and thus, survival of tumors depends on their ability to adapt to hypoxic conditions and to recruit new blood microvessels through angiogenic factors. Tumor cells must adapt to hypoxic conditions as a crucial step in tumor progression. The ability of cancer cells to consume glucose as an energy source without oxygen and overproduce lactic acid aerobically, termed the Warburg effect, was recognized over 7 decades ago, although its molecular basis has been elusive (194, 195).
The basis for the Warburg effect is likely to include activated oncogenes, inactivated tumor suppressors, and the hypoxia-inducible transcription factor 1 HIF-1. Increases in glucose transport and hexokinase activities in cancer cells appear to account for the increased flux of glucose through the cancer cells (122, 135, 149). The gene for LDH-A, which participates in normal anaerobic glycolysis and is frequently increased in human cancers, was recently identified as a c-Myc-responsive target (170). LDH-A has been used as a marker of neoplastic transformation and was previously shown to be induced by hypoxia through the activity of HIF-1 (58, 163, 164, 192). It is noteworthy that c-Myc and HIF-1 binding sites resemble the carbohydrate response element, 5′-CACGTG-3′ (109, 168). In fact, transgenic animals constructed to overexpress c-Myc in the liver have increased glycolytic liver enzymes and also overproduce lactic acid (184). Stably transfected rodent fibroblasts that overexpress LDH-A alone or those transformed by c-Myc overproduce lactate, suggesting that LDH-A is sufficient to induce the Warburg effect (170). LDH-A overexpression is required for c-Myc-mediated transformation, since lowering its expression reduces the soft-agar clonogenicity of c-Myc-transformed fibroblasts, as well as c-Myc-transformed human lymphoblastoid cells and Burkitt’s lymphoma cells.
In addition to the potential role of glycolysis in the transformed phenotype, glycolysis is elevated 20-fold after an initial increase in c-Myc expression during normal T-lymphocyte mitogenesis (70). The increased glycolytic rate results from elevated expression of numerous glycolytic enzymes (including LDH-A) and is thought to reduce oxygen radical damage from oxidative phosphorylation as cells enter S phase (32).
Other putative target genes of c-Myc, such as those that encode CAD (126), ODC (18), DHFR (116), and thymidine kinase (TK) (147), are involved in DNA metabolism and, hence, affect the G1/S transition. CAD performs the first three rate-limiting steps of pyrimidine biosynthesis, whereas ODC participates in the synthesis of polyamines which are required for the activities of enzymes involved in nucleotide biosynthesis. It is noteworthy that a study of selected Myc target genes in myc-null fibroblasts revealed that only the expression of the gene for CAD was significantly decreased compared to its expression in wild-type fibroblasts (162a). While this observation confirms the contention that CAD is a downstream effector of Myc, it also raises a cautionary note on the use of these myc-null cells. Given that Myc is central to cell growth, its elimination by homologous recombination might only be tolerated by a minority of cells that have an inherent compensatory increase in critical c-Myc target genes. As such, expression of genes that are not essential for cell growth might not be increased in cells that tolerate homozygous elimination of myc. DHFR catalyzes the reduction of folate, a step that is necessary for the subsequent methylation of uridylate to produce thymidylate. TK catalyzes the conversion of thymidine to thymidylate.
The involvement of c-Myc in metabolism is further suggested by the roles of the target genes encoding translational regulatory factors eIF-2α (151) and eIF-4E (92, 151) and ECA39 (19), which has been implicated in amino acid transport (162). These connections between c-Myc and cellular metabolism suggest that it is the central integrator of cell proliferation and metabolism. From this vantage point, it is not surprising, in retrospect, that the role of c-Myc in cell growth has been elusive.
C-MYC, APOPTOSIS, AND IMMORTALITY
c-Myc-induced apoptosis was recognized initially in studies of the 32D.3 myeloid progenitor cell line (8). These cells are dependent on interleukin-3 for c-myc expression and growth, and enforced c-myc expression has no effect on 32D.3 under normal growth conditions. In the absence of IL-3, however, enforced c-myc expression continues to drive cells into S phase and accelerates the rate of cell death. Serum-deprived Rat1 fibroblasts overexpressing c-Myc or expressing activated MycER also undergo dramatic apoptosis (53). This apoptotic pathway appears to be dependent on the activity of wild-type p53 (83, 189) and might be related to an activated Fas/APO-1 (86) pathway. Glucose deprivation of c-Myc-overexpressing cells was recently found to induce extensive apoptosis that is p53 independent and may be linked to increased LDH-A expression (169). The Bcl-2 oncogene is able to protect Myc-overexpressing cells from either serum or glucose deprivation-induced apoptosis (24, 55, 169, 191).
Since the regions of c-Myc required for transcriptional regulation and cellular transformation are also those required for serum deprivation-induced apoptosis (53), it is surmised that c-Myc affects the transcription of genes which participate in apoptosis. ODC, the gene for which is probably the best characterized of the Myc targets, also induces apoptosis, albeit less effectively than Myc itself (134). The expression of cdc25A, however, appears to be necessary for c-Myc-induced apoptosis, since antisense cdc25A oligonucleotides block the serum deprivation-induced death of c-Myc-overexpressing cells (64). In contrast, overexpression of the rcl gene confers anchorage independence but does not predispose Rat1a cells to serum deprivation-induced apoptosis (104). These observations suggest that cellular transformation and apoptosis induced by c-Myc may occur through overlapping and nonoverlapping pathways.
Historically, c-Myc was touted to be an immortalizing gene, ectopic expression of which facilitates the immortalization of primary rodent fibroblasts. This simple view overlooked the initial events following ectopic c-Myc expression and the crisis period that cells must survive to achieve immortality. Since telomerase contributes to the immortality of tumor cells, the ability of increased expression of viral or cellular oncogenes to induce telomerase in normal human mammary epithelial cells and human fibroblasts (IMR-90) was studied (193). Among six candidates, c-Myc emerged as a key switch for induction of telomerase activity, as well as expression of the catalytic subunit of telomerase, termed TERT. Intriguingly, whereas TERT increases the life-span of human mammary epithelial cells, overexpression of TERT was unable to prolong the life-span of IMR-90 cells. It should be noted, however, that the construct used in that study produces a TERT with a C-terminal epitope tag that may have compromised its activity. In contrast to epitope-tagged TERT, c-Myc is able to immortalize IMR-90 cells, even though these cells do not show stabilization of telomeres. These observations suggest an alternative mechanism for c-Myc-mediated immortalization, in addition to the induction of telomerase.
In collaboration with activated RAS, c-Myc was able to transform primary fibroblasts in the classic experiments of Weinberg and coworkers (99). In this role, c-Myc appears to inactivate cellular responses that are normally required for RAS-mediated growth inhibition, thereby switching the gene for RAS into a growth-promoting gene (165). Reciprocally, RAS is able to inhibit Myc-mediated apoptosis (51). Given that p19 ARF-null murine embryonic fibroblasts (MEFs) are immortal and can be transformed by oncogenic RAS independently of c-Myc, it was hypothesized that c-Myc might regulate ARF (206). Indeed, it has been demonstrated that ARF and p53 are induced by ectopic c-Myc expression in wild-type MEFs, triggering a replicative crisis and apoptosis. MEFs that survive myc overexpression and the crisis period sustain ARF loss or p53 mutations. MEFs that lack ARF or p53 showed a decreased apoptotic response to c-Myc overexpression. These observations indicate that ARF participates in a p53-dependent checkpoint that safeguards cells against oncogenic signals, such as overexpression of c-Myc. These new observations indicate that immortalization of primary cells by oncogenes is a complex phenomenon in which normal safeguard apoptotic mechanisms are inactivated, thereby allowing immortalized cells to emerge from a crisis period of massive cell death.
CONCLUSION
In conclusion, the c-Myc molecule has continued to emerge as a centerpiece and key to the many secrets of cancer biology. Recent studies suggest that c-Myc is able to activate the cell cycle machinery and its safeguards. Intriguingly, its ability to activate glycolysis suggests that in addition to triggering the cell cycle, c-Myc also sustains the fuel necessary to run the cell cycle machinery. Indeed, its ability to enhance the activities of specific enzymes involved in DNA metabolism and other metabolic pathways further suggests that it is a key molecular integrator of cell cycle machinery and cellular metabolism. The future of the study of c-Myc target genes lies in the use of arrayed gene expression analysis to determine the common and divergent patterns of c-Myc target gene expression in a variety of physiological and neoplastic conditions. The benefits from such advances in technology, however, will require the expertise of biologists who are able to tease out the roles of the target genes in producing the multitude of c-Myc-mediated phenotypes. The greatest challenge, however, is the development of a discipline that is capable of dynamically and comprehensively linking transcription factor activities to their target genes and, in turn, to cellular phenotypes.
ACKNOWLEDGMENTS
I have relied on review articles and apologize for the omission of original references. Comments by L. Lee and D. Wechsler are greatly appreciated.
My original work was supported by NIH grants.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.19.1.1
Read article for free, from open access legal sources, via Unpaywall:
https://mcb.asm.org/content/mcb/19/1/1.full.pdf
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/content/full/19/1/1
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/19/1/1
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/19/1/1
Citations & impact
Impact metrics
Article citations
Transcriptional regulation by MYC: an emerging new model.
Oncogene, 28 Oct 2024
Cited by: 0 articles | PMID: 39468222
Review
The 24-hour molecular landscape after exercise in humans reveals MYC is sufficient for muscle growth.
EMBO Rep, 31 Oct 2024
Cited by: 0 articles | PMID: 39482487
Endothelial PHD2 deficiency induces apoptosis resistance and inflammation via AKT activation and AIP1 loss independent of HIF2α.
Am J Physiol Lung Cell Mol Physiol, 327(4):L503-L519, 19 Aug 2024
Cited by: 0 articles | PMID: 39159362 | PMCID: PMC11482463
AFP-HSP90 mediated MYC/MET activation promotes tumor progression in hepatocellular carcinoma and gastric cancers.
Cancer Cell Int, 24(1):283, 12 Aug 2024
Cited by: 0 articles | PMID: 39135041 | PMCID: PMC11321088
Missense Mutations in Myc Box I Influence Nucleocytoplasmic Transport to Promote Leukemogenesis.
Clin Cancer Res, 30(16):3622-3639, 01 Aug 2024
Cited by: 0 articles | PMID: 38848040
Go to all (1,030) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transcriptional regulation. Flipping the Myc switch.
Curr Biol, 5(8):859-861, 01 Aug 1995
Cited by: 19 articles | PMID: 7583141
Review
The Myc:Max protein complex and cell growth regulation.
Cold Spring Harb Symp Quant Biol, 56:109-117, 01 Jan 1991
Cited by: 22 articles | PMID: 1819481
Review
Myc/Max/Mad regulate the frequency but not the duration of productive cell cycles.
EMBO Rep, 2(12):1125-1132, 21 Nov 2001
Cited by: 32 articles | PMID: 11743027 | PMCID: PMC1084169
Cytokine-induced inhibition of Myc activity in monocytic cells.
Curr Top Microbiol Immunol, 224:191-200, 01 Jan 1997
Cited by: 0 articles | PMID: 9308242





