Abstract
Free full text

Antibody Recognition of Plasmodium falciparum Erythrocyte Surface Antigens in Kenya: Evidence for Rare and Prevalent Variants
Abstract
Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is the name given to a family of parasite proteins that are inserted into the infected erythrocyte surface. Studies using agglutination assays have shown previously that PfEMP1 epitopes are extremely diverse. In a study in Kenya, 21 parasite isolates, including nine from children with severe malaria, were tested for agglutination by 33 pairs of plasma, 21 of which were from the corresponding children. Each plasma pair consisted of a sample taken at the time of disease (acute) and one taken 3 weeks later (convalescent). In agreement with previous studies, infection was generally followed by the induction of antibodies specific to the homologous parasite isolate. In addition however, the results show that (i) some isolates were agglutinated very frequently by heterologous plasma; (ii) unexpectedly, these frequently agglutinated isolates tended to be from individuals with severe malaria; (iii) an inverse relationship existed between the agglutination frequency of each parasite isolate in heterologous plasma and the agglutinating antibody repertoire of the homologous child at the time of disease; and (iv) A 3-month-old child apparently still carrying maternal antibodies was infected by a rarely agglutinated isolate. This child’s plasma agglutinated all isolates at the time of disease, apart from the homologous isolate. These results support the idea that preexisting anti-PfEMP1 antibodies can select the variants that are expressed during a new infection and may suggest the existence of a dominant subset of PfEMP1 variants.
The vast majority of childhood deaths from malaria follow infection by one parasite species, Plasmodium falciparum. The special pathology of this species compared to other species of Plasmodium that infect humans has been attributed to the ability of P. falciparum-infected erythrocytes to cytoadhere to receptors expressed on the surface of capillary endothelial cells (5, 18, 26, 34).
A family of parasite antigens collectively known as PfEMP1 (P. falciparum erythrocyte membrane protein 1) are thought to play an important role in cytoadherence through their ability to bind to various endothelial receptors (3, 12). They are therefore strongly implicated as virulence factors. These antigens are expressed on the erythrocyte surface from about 18 h into the asexual, erythrocytic phase of the parasite life cycle and undergo clonal antigenic variation. This mechanism of immune evasion was described over 30 years ago for analogous proteins expressed by the monkey malaria parasite Plasmodium knowlesi. In these early studies, antibodies raised in monkeys in response to infection were found to agglutinate infected erythrocytes in a variant-specific manner (6).
Agglutination has been similarly useful in describing antigenic variation in PfEMP1. Naturally induced antibodies agglutinate P. falciparum-infected erythrocytes in a predominantly variant-specific manner (24), and switches in agglutinating phenotype can be correlated with switches in cytoadherence characteristics (28). More recently, the var gene family encoding PfEMP1 has been cloned (4, 29, 31), and switches in agglutination phenotype have been directly correlated with switches in var gene expression (29).
Surveys of agglutination antibody responses to natural P. falciparum populations in Pakistan (16), The Gambia (20), and Papua New Guinea (11, 27) indicate that PfEMP1 antigens are very diverse since antibodies induced following an infection generally agglutinate only the homologous parasite isolate that caused that particular infection. This diversity together with their surface location and functional importance indicates that these molecules may be important targets for naturally acquired immunity, an idea supported by recent epidemiological data demonstrating that anti-PfEMP1 antibodies provide variant-specific protection against malarial disease (8).
Despite the apparent role of anti-PfEMP1 antibodies in the development of anti-disease immunity, their diversity may be thought to limit their potential as vaccine candidates. However, though the total pool of PfEMP1 epitopes is generally assumed to be large, antigen diversity does appear to be finite. Semi-immune serum has been found to agglutinate parasites isolated in different continents and those isolated from a similar location up to 19 years in the past (1). Limits to the diversity of PfEMP1, despite the huge genetic resources that are apparently invested in antigenic variation, might be imposed by the requirement of these molecules to mediate specific interactions with endothelial cells.
The present study was carried out as a preliminary exploration of the limits of PfEMP1 epitope diversity in an area of stable endemicity on the coast of Kenya. Though parasite isolates were very diverse in terms of the patterns of recognition by semi-immune plasma, some isolates were surprisingly frequently agglutinated by these samples. Frequently agglutinated isolates tended to be from children with severe disease.
MATERIALS AND METHODS
Study area.
The study was carried out at Kilifi District Hospital, situated 60 km north of Mombasa on the Kenyan coast. The hospital is equipped with a high-dependency ward to treat children with life-threatening illness. However, most children admitted to hospital are treated in the general pediatric ward. An area immediately surrounding the administrative town of Kilifi was defined in 1991 for surveillance (30). Over 10% of the children under the age of 5 years resident within the study area are admitted to the hospital each year. Following the long and short rains, the area has prolonged seasonal transmission of P. falciparum by Anopheles gambiae sensu lato complex (21).
Collection of parasites and plasma samples.
Parasite samples were collected between September 1994 and June 1995 from children attending or admitted to Kilifi District Hospital with a primary diagnosis of malaria. For this study, children were defined as severe cases and were admitted to the high-dependency ward if they were prostrated or had respiratory distress (19); cases were considered nonsevere if neither of these conditions was present. Blood samples were taken both at the time of disease (acute) and 3 weeks later (convalescent). The details for each patient were recorded in a FOXPRO database (Fox Holdings). After parental consent had been obtained, 5 ml of blood was taken into a tube containing heparin (Leo Laboratories). Following centrifugation, plasma was decanted and stored at −30°C.
Parasites were prepared from acute samples as follows. To remove mononuclear cells, the cell pellet was resuspended in 1 volume of RPMI 1640 (pH 7.2, containing 25 μg of gentamicin sulfate/ml, 1 mM glutamine, and 4 mg of d-glucose/ml [all from Gibco, BRL]) and layered onto a cushion of Lymphoprep (Nycomed). Following centrifugation at 3,000 × g for 10 min, the cell pellet was washed in RPMI 1640. To remove granulocytes, the cells were mixed with 4 volumes of 70% Plasmagel (Bellon) diluted in RPMI 1640, and the erythrocytes were allowed to settle for 15 min at 37°C. Following washing in RPMI 1640, erythrocytes were cryopreserved in liquid nitrogen (23).
Selection of samples for inclusion.
To avoid blood group incompatibility, only samples from children with group O cells were selected for inclusion in the study. Of these, only samples from children with parasitemias of >5% (i.e., >5 parasites per 100 erythrocytes) were selected, except for one sample (1134) from a child on the high-dependency ward with a parasitemia of <1%. This gave a total of 33 pairs of acute and convalescent plasma samples and 33 corresponding parasite isolates. Thirteen of the samples were from children with severe malaria: eight (1001, 1069, 1083, 1099, 1132, 1162, 1195, and 1134) from children with cerebral malaria and five (1016, 1047, 1054, 1064, and 1084) from children with noncerebral malaria. Of the severe cases, seven (1001, 1047, 1054, 1064, 1132, 1134, and 1195) had a blood transfusion after the acute sample was taken. The remaining 20 samples were from children with nonsevere malaria either from the pediatric ward (1019, 1026, 1028, 1029, 1031, 1032, 1041, 1042, 1057, 1068, 1076, 1079, 1086, 1089, 1094, 1097, 1145, 1235, and 1285) or from the outpatient department (1062). Plasma samples were thawed and recentrifuged to remove cryoprecipitates and cellular debris. Plasma was pipetted in 2.5-μl spots onto U-bottomed 96-well microtiter plates (Falcon), dried for 2 h at room temperature, and then stored frozen at −30°C in plastic bags so that one microtiter plate contained the complete panel of plasma samples to test against a single parasite isolate. In preliminary studies dried, frozen plasma samples were found to fully retain their potential for parasite agglutination (7). Plasma samples from children, together with plasma from a hyperimmune adult (positive control) and serum from a nonimmune European (negative control), were used as a panel against which each isolate was tested by agglutination.
Agglutination.
Following thawing (23), cells were cultured in RPMI 1640 in the presence of 10% European serum (33) until they matured into pigmented trophozoites. To synchronize growth, aphidicolin (1.5 μg/ml; Sigma) was included in the culture (15). In preliminary experiments, the presence of aphidicolin was found to have no effect on agglutination phenotype (7). Following culture, cells were washed once in RPMI 1640 and fresh uninfected erythrocytes were added, if necessary, to ensure that the parasitemia of pigmented trophozoites was between 1 and 5%. The agglutination method was modified from that described in reference 20. Cells were diluted to a 5% hematocrit in RPMI 1640 containing ethidium bromide (Sigma) at a final concentration of 10 μg/ml. A microtiter plate containing test plasma was removed from the freezer and allowed to warm to room temperature; 12.5 μl of diluted cells was added to each spot of dried plasma. The microtiter plate was rotated at 60 rpm on a vertical rotator for 60 min at room temperature. The entire reaction volume from each well was pipetted onto a slide and covered with an 18-mm-diameter coverslip, with the edges sealed with petroleum jelly. Following blinding, slides were scored for agglutination as follows. Fifteen fields (5% of the entire reaction volume) were read with a 20× objective, tracing the outline of a triangle along the top edge of the coverslip, down the left side, and diagonally across the coverslip to the starting point. A differential count was made within these fields, using a definition of size against a graticule. Only tightly formed agglutinates of infected cells were counted; agglutinates containing uninfected cells were not considered. Sizes were approximately as follows: size A, 3 to 5 cells; size B, 6 to 20 cells; size C, 20 to 100 cells; size D, >100 cells. Agglutination was scored on a scale of 0 to 4, based on both size and abundance of agglutinates, as follows: 1, (i) >10 size A or (ii) 5 to 10 size A and 1 size B; 2, (i) >10 size A and 1 size B or (ii) 2 to 5 size B; 3, (i) >5 size B or (ii) 2 to 5 size C; 4, (i) >5 size C or (ii) >1 size D.
This was used only as an initial scoring guide. Due to the frequent nonuniform distribution of agglutinates within a wet film, an additional complete scan of every slide was performed (this was not done for isolate 1069). Following this scan, 6% of the initial scores required adjustment by ±1 unit to account for the presence of previously unobserved large agglutinates or clusters of smaller agglutinates. This percentage ignores four of the parasite isolates (1029, 1054, 1064, and 1083) that exhibited autoagglutination (28) of infected cells (i.e., they agglutinated slightly in nonimmune serum and in all plasma samples). It was possible to assign only approximate scores for specific agglutination of these isolates by ignoring all agglutinates of the same size category as the largest autoagglutinate observed in nonimmune serum. An unblinded negative control slide was used as a reference while the assays were read.
Estimation of multiplicity of infections.
PCR was performed as described previously (17), using primers for MSP1 and MSP2, and the PCR products were fractionated by agarose gel electrophoresis. For both MSP1 and MSP2, the total number of bands amplified, whatever the intensity, was determined. The multiplicity of infection was estimated as the largest number of bands observed between the two sets of amplification reactions.
Data analysis.
Data were stored, formatted, and analyzed with Microsoft Excel (Microsoft Corp.); further statistical analysis was performed with STATA (Stata Corp.). Correlations were calculated by using Spearman’s rank correlation test (rho). The Mann-Whitney U test statistic (Z) was used to compare Δ scores (see Results for definition).
RESULTS
General observations.
Of the 33 cryopreserved parasite isolates tested, 22 grew successfully in culture. One of these isolates, 1028, was excluded because it exhibited strong agglutination in all plasma samples from children and in European serum samples. A total of 21 isolates were tested against 21 pairs of acute and convalescent plasma samples taken from the corresponding children and 12 pairs of acute and convalescent plasma taken from the children infected by isolates that could not be assayed. In all, the 21 isolates were tested against 66 plasma samples.
The agglutination patterns for the 21 isolates are shown in Fig. Fig.1.1. There was considerable variation in the frequency with which different parasite isolates were agglutinated by the panel of plasma samples and in the number of isolates recognized by each plasma sample.
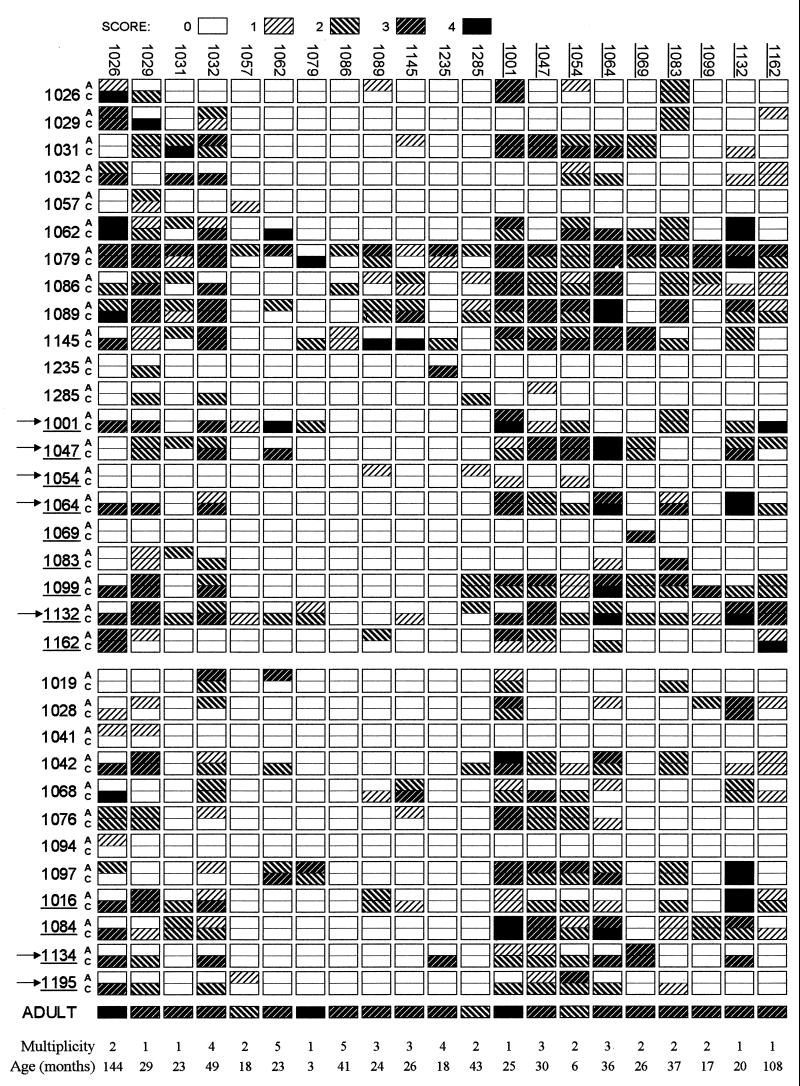
Parasite recognition profiles. Isolates are listed along the top; labels for nine isolates from patients with severe malaria are underlined. Each isolate was tested against 33 pairs of plasma samples listed at the left. Each row of boxes shows agglutination of 21 isolates by a single patient. Plasma samples from patients with severe disease are underlined. Each pair is labeled A (acute [sampled at the time of disease]) and C (convalescent [sampled 3 weeks later]). (a) Plasma pairs corresponding to the 21 isolates. Reactions against homologous isolates lie on a diagonal running from the top left to the bottom right. (b) Twelve additional pairs of plasma samples not corresponding to cultured isolates. Patients who received a blood transfusion are indicated with arrows. The bottom row is a profile of an immune adult. For each isolate, the estimated number of clones present (multiplicity) and the age of the corresponding child is indicated.
Reproducibility of the agglutination profiles.
Four of the isolates, 1054, 1029, 1145, and 1235, were grown twice from frozen aliquots and their agglutination profiles against the 66 plasma samples were compared to give an estimate of the variation introduced during culturing and in scoring. The percentage of 66 agglutination reactions that were scored within ±1 unit in duplicate experiments were 86, 91, 97, and 98, respectively.
Commonly agglutinated isolates.
A striking observation was the frequency with which some isolates were agglutinated by plasma samples from heterologous individuals.
We calculated the agglutination frequency of each parasite isolate by determining the percentage of 64 heterologous plasma samples, whether acute or convalescent, that gave agglutination scores of 1 or more. Since the isolates fell roughly into two groups, we defined the 10 isolates with the highest agglutination frequency (1001, 1026, 1029, 1032, 1047, 1054, 1064, 1083, 1132, and 1162) as common and the remaining isolates (1031, 1057, 1062, 1069, 1079, 1086, 1089, 1099, 1145, 1235, and 1285) as rare.
To compare the agglutination profiles of the common isolates with each other, we calculated correlation coefficients between the scores given to each parasite isolate by all 66 plasma. The four highest correlation coefficients were between (i) 1047 and 1064 (rho = 0.76, P < 0.0001), (ii) 1047 and 1054 (rho = 0.66, P < 0.0001), (iii) 1032 and 1064 (rho = 0.64, P < 0.0001), and (iv) 1054 and 1064 (rho = 0.62, P < 0.0001). The four lowest were between (i) 1026 and 1064 (rho = 0.22, P = 0.08), (ii) 1026 and 1047 (rho = 0.22, P = 0.08), (iii) 1162 and 1054 (rho = 0.22, P = 0.08), and (iv) 1162 and 1001 (rho = 0.27, P = 0.03). These correlations should be regarded only as a useful guide since (i) both acute and convalescent scores were included and (ii) resemblance between isolates is likely to be exaggerated by the fact that 11 plasma samples reacted with all 10 common isolates and may simply have been too reactive to distinguish between them. Figure Figure22 illustrates the diversity of the agglutination profiles of the common isolates against 49 plasma samples of intermediate reactivity (i.e., those that reacted with 1 to 9 of the 10 isolates).
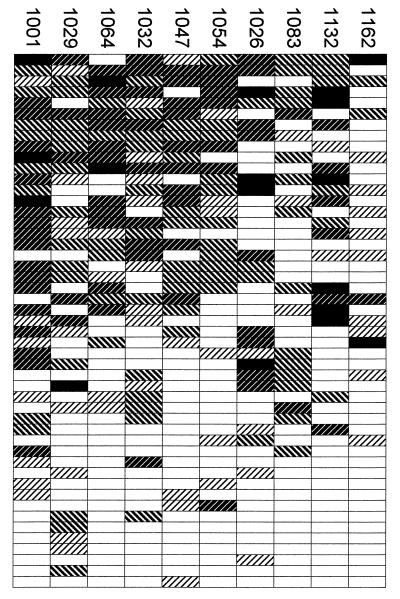
Diverse agglutination profiles of common isolates. The 49 plasma samples that agglutinated 1 to 9 of the 10 common isolates were treated as independent typing reagents and stacked vertically, with the most reactive at the top. Each row of rectangles represents agglutination of the 10 isolates by a single plasma sample. Scores are represented by shading as in Fig. Fig.11.
To establish that the agglutination frequency of each isolate reflected a true characteristic of the antigens on the surface of the infected erythrocytes rather than an artifact of the agglutination assay, four possible confounders were considered: a, the total parasitemia at which the agglutination assays were carried out, including cells that did not grow; b, the assay parasitemia counting only the cells containing pigment; c, the number of individual parasite genotypes detected in the infection; and d, the proportion of parasites that were pigmented (i.e., d = b/a), giving a measurement of the viability of the culture.
The correlations between these potential confounders and the agglutination frequency of each isolate were as follows: a, rho = 0.05, P = 0.82; b, rho = 0.19, P = 0.42; c, rho = −0.28, P = 0.21; d, rho = −0.09, P = 0.70. None of them correlated significantly with agglutination frequency. Thus the agglutination frequency of the isolates appeared to reflect a property of the infected erythrocyte surface at the time of assay.
Nonspecific agglutination.
Four of the common isolates (1029, 1083, 1054, and 1064) exhibited some nonspecific agglutination (autoagglutination) of infected erythrocytes, such that there was a general background level of agglutination in acute and convalescent plasma samples. This was taken into account by using an unblinded European serum negative control as a reference (see Materials and Methods). Isolates 1029, 1054, and 1064 also exhibited agglutination in RPMI 1640 at a level similar to that for European serum suggesting that autoagglutination was an intrinsic property of these isolates (not done for 1083). Six other isolates (common isolates 1026, 1032, and 1162; rare isolates 1079, 1145, and 1235) tested in RPMI 1640, exhibited no agglutination.
Parasite material remaining was sufficient to test two of the autoagglutinating isolates (1029 and 1054) and two other common isolates (1162 and 1032) against an additional panel of 13 plasma samples and corresponding serum samples taken from 13 nonimmune European adults. There was no reaction between these serum and plasma samples and isolates 1054, 1162, and 1032. However, for isolate 1029, although there was no apparent variation between the 13 individuals, the autoagglutinates observed in plasma samples (maximum size B) were larger than those observed in serum samples (maximum size A; data not shown), suggesting that factors present in plasma can sometimes enhance autoagglutination. Enhancement of autoagglutination by factors present in plasma may have contributed to the high agglutination frequency of isolate 1029 and possibly those of isolates 1083 and 1064. However, the fact that these three isolates still had distinct agglutination profiles (Fig. (Fig.2)2) suggests that the majority of the agglutination scored as positive was variant specific.
Agglutination frequency and disease severity.
Surprisingly, the common isolates included seven from children with severe malaria. In contrast, only two of the rare isolates were from children with severe malaria, suggesting that parasites causing severe disease may express variants that are prevalent in the community. Though there was no correlation between the agglutination frequency of each isolate and the parasitemia at the time of assay, there was a significant correlation with the parasitemia of the infection at the time of sampling (rho = 0.6, P = 0.005).
Induction of antibodies to homologous and heterologous isolates.
By comparing the profiles of agglutinating antibodies in each child’s plasma samples at two time points (acute and convalescent), we could identify antibody specificities that were newly acquired or lost following each infection.
To compare the induction of antibodies to heterologous (another child’s) and homologous (the corresponding child’s) parasites, we defined the Δ score as the difference between the acute and convalescent agglutination scores given by a single child’s plasma samples from children against a single parasite isolate. For simplicity, we treated the checkerboard of agglutination reactions shown in Fig. Fig.11 as a set of 693 plasma pair tests (i.e., 33 plasma pairs tested against 21 parasite isolates). For each plasma pair test, the Δ score was calculated. Figure Figure33 compares frequency distributions of various sets of plasma pair tests as a function of their Δ scores.
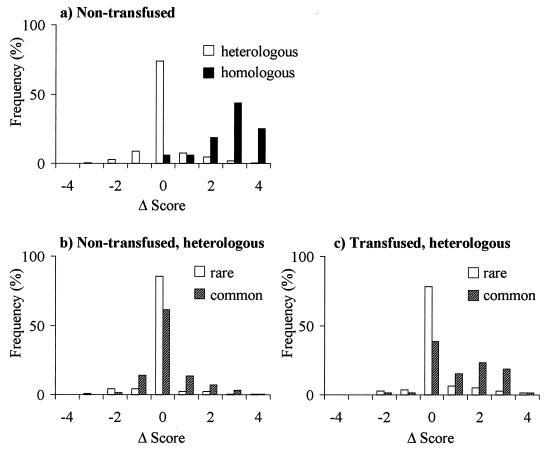
Induction of agglutinating antibodies. Histograms of Δ scores (the change in agglutination score between disease and convalescence) are compared between various sets of plasma pair tests (plasma-parasite combinations [see text]). (a) Comparison between plasma pair tests against homologous isolates (n = 16) and against heterologous isolates (n = 530), excluding plasma pairs from transfused children. (b) Comparison between plasma pair tests against common heterologous isolates (n = 255) and against rare heterologous isolates (n = 275), excluding plasma samples from transfused children. (c) Evidence for passive antibody transfer during blood transfusion: comparison between plasma pair tests against common heterologous isolates (n = 65) and against rare heterologous isolates (n = 77), considering only plasma pairs from transfused children.
A characteristic feature of previous studies of this kind was the tendency for children to respond strongly to homologous isolates, creating a diagonal from the top left to the bottom right of the agglutination checkerboard. This diagonal is clearly visible in Fig. Fig.1.1. As expected, therefore, the mean Δ score given by plasma pair tests against homologous isolates (excluding children who had a blood transfusion [see Materials and Methods]) was higher than the mean Δ score given by tests against heterologous isolates (means = 2.8 and 0.1, respectively; Z = 7.7, P < 0.0001 [Fig. 3a]). When plasma pair tests against heterologous isolates (again excluding children who had a blood transfusion) were subdivided into those against rare isolates and those against common isolates, the mean Δ scores were both close to zero (−0.1 and 0.2, respectively; Z = −3.1, P = 0.001 [Fig. 3b]). The higher proportion of cross-reactive responses made against common isolates can be partly explained by the apparent induction of antibodies by many individuals to a single isolate, 1026.
Evidence for antibodies passively transferred during blood transfusion.
Since most blood donors in Kilifi are immune adults, it is likely that agglutinating antibodies are frequently passively transferred during blood transfusions. When only plasma pairs from transfused children were considered, there was a marked difference between the mean Δ scores of plasma pair tests against rare isolates compared to those against common isolates, though both were positive (means = 0.2 and 1.2 respectively; Z = −5.1, P < 0.0001 [Fig. 3c]). It appears that passively transferred antibodies were stable enough to be detectable after 3 weeks although, curiously, this was only strikingly evident for antibodies against the common isolates.
Evidence for immune selection of rare variants.
As shown in Fig. Fig.4,4, there was evidence for a negative relationship between each child’s acute immune response and the agglutination frequency of the child’s own parasites. Thus, individuals with a larger repertoire of anti-PfEMP1 responses appear to have been infected by parasites carrying rarer or less immunogenic PfEMP1 variants.
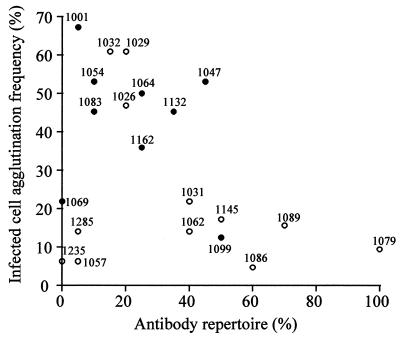
Evidence for immune selection of rare surface variants: relationship between each child’s repertoire of agglutinating antibodies at the time of disease and the agglutination frequency of that child’s parasites. Antibody repertoire = percentage of heterologous parasite isolates agglutinated by each patient’s acute plasma samples (i.e., 21 plasma samples tested against 20 isolates). Each point is labeled by the identification number of the plasma-parasite sample. Samples from patients with severe disease are shown with filled circles.
Child 1079 is particularly interesting since this individual recognized all parasite isolates at the time of disease except for the child’s own parasites (Fig. (Fig.1).1). Three weeks after the infection, however, the child had developed a strong response to these parasites. The agglutination frequency of this child’s parasites was very low. Child 1079, the youngest patient in the study, was only 3 months old at the time of disease. The broad reactivity of the acute serum samples together with the young age of the patient suggests the presence of maternal antibodies at the time of acute infection. A simple explanation is that this individual was infected with parasites expressing rare surface antigens corresponding to a gap in the repertoire of antibodies passed on from the child’s mother.
DISCUSSION
Most serological surveys of wild parasite isolates have emphasized the specific induction of antibodies to homologous isolates during the 3 weeks following infection. Though this is clearly demonstrated by the present study, an unexpected feature was the high frequency with which some isolates were agglutinated by heterologous plasma samples.
Variation in agglutination frequency between different isolates is evident from previous studies but has not previously been described in detail. Most notably, a recent study from Sudan shows that there was an inverse relationship between the agglutination frequency of four parasite isolates in semi-immune plasma samples and the age of the parasite donor (13).
In the present study, the agglutination profiles of the common isolates were all different, showing that agglutination was variant specific. Though there was considerable variation in the ability of different children to agglutinate the various parasite isolates, the ability of each child to agglutinate any particular heterologous common isolate was generally stable over time. Individual acute and convalescent agglutination scores against these isolates were highly correlated with each other (rho = 0.71, P < 0.0001, excluding transfused children), and the mean Δ score was close to zero (Fig. (Fig.33b).
Taken together, the data provide evidence for the existence erythrocyte surface antigens recognized by relatively prevalent antibodies. Though it is possible that other molecules are involved, the diverse agglutination profiles exhibited by the common isolates make PfEMP1 the most likely target. Both common and rare isolates were agglutinated at similar intermediate levels by plasma samples from an immune adult (Fig. (Fig.1),1), suggesting that the isolates expressed similar levels of PfEMP1. Common and rare isolates were apparently equally immunogenic, since both groups of isolate generally elicited strong responses in the homologous children. Furthermore, there was no evidence that common isolates formed smaller agglutinates (data not shown), an observation that might be expected if they merely expressed a more diverse population of variants. We therefore propose that the common isolates expressed members of a subset of PfEMP1 epitopes which had been frequently encountered and responded to by the children in the study.
It is likely that the prevalence of different PfEMP1 epitopes varies both between different geographical regions and from year to year, as previously described for other parasite antigens (2, 10) and discussed theoretically (14). The existence of rare and common isolates could reflect fluctuations in the prevalence of different genotypes over space and time. An alternative possibility, consistent with the observed association between high infected cell agglutination frequency and severe disease, is that the common isolates express members of a subset of dominant PfEMP1 variants that have optimal binding ability. Such variants could be highly adapted to competitive growth in immunologically naive hosts, but if the cost of optimized binding ability was low epitope diversity, they would be rapidly countered by variant-specific antibodies in semi-immune individuals, leading to the selection of variants carrying novel, rare epitopes. This idea is supported by the fact that the common isolates in the present study infected children with a small antibody repertoire and that a child apparently carrying maternal antibodies was infected by a rare isolate. The idea of a restricted subset of dominant variants would be consistent with the fact that some of the var genes are located in stable central regions of the chromosomes (9, 32). Such variants would be potentially analogous to the parental variants described in Plasmodium chabaudi (22).
The immune selection of rare PfEMP1 variants proposed here should be distinguished from the previously demonstrated reduction in number of parasite genotypes infecting individuals as they developed immunity in Dielmo, Senegal (25), where an abrupt fall in number was observed after the age of 15 years. The immune selection proposed in the present study occurs within a group of 21 children of under 12 years of age, all but two of whom are actually under 5 years of age. Consistent with the Dielmo study, we found no evidence of a drop in the number of genotypes within this age group, either with age or with increasing repertoire of anti-PfEMP1 antibodies in acute plasma samples. An extensive survey of MSP1, MSP2, and S-antigen genotypes in Kilifi supports this finding (17). Thus, a decrease in number of infecting genotypes appears over a relatively long time scale but selection of rare parasite variants may occur within a time scale similar to that of the development of anti-disease immunity (8).
The assessment of variant-specific agglutination is complicated by the occasional occurrence of autoagglutination in field samples. Though all four of the autoagglutinating isolates in the present study were also defined as common, preliminary data indicate that autoagglutination and agglutination frequency are independent. Parasite clone C10, in which autoagglutination was originally identified (28), apparently expresses a rare variant. This clone reacted relatively infrequently with a panel of child serum samples from Kilifi (8) and did not react with any of a panel of 15 acute plasma 2- to 3-year-old children in Kilifi (7).
In summary, we have found that some parasite isolates are frequently agglutinated in plasma samples from children. This may suggest the existence of a dominant subset of PfEMP1 variants. To determine whether commonness is a stable feature of some variants, further studies are needed to examine how the prevalence of antibodies to common isolates fluctuates over space and time.
ACKNOWLEDGMENTS
This work was supported by KEMRI and the Wellcome Trust. K.M. is a Wellcome Trust Senior Research Fellow (grant 631342).
We thank the head of the KEMRI unit at Kilifi, N. Peshu, and the staff, especially Sam Kinyanjui, Garama Baya, Asha Juma, and B. M. Chome. We thank our colleagues in Oxford, especially Sue Kyes and Dave Roberts, and we are particularly grateful to Chris Newbold for his sustained critical input and collaboration. We thank an anonymous reviewer for some helpful criticism of the manuscript.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.67.2.733-739.1999
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/67/2/733.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/67/2/733
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/content/full/67/2/733
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/67/2/733
Citations & impact
Impact metrics
Citations of article over time
Article citations
Antigenic strain diversity predicts different biogeographic patterns of maintenance and decline of antimalarial drug resistance.
Elife, 12:RP90888, 16 Feb 2024
Cited by: 0 articles | PMID: 38363295 | PMCID: PMC10942604
Antibody to Plasmodium falciparum Variant Surface Antigens, var Gene Transcription, and ABO Blood Group in Children With Severe or Uncomplicated Malaria.
J Infect Dis, 228(8):1099-1107, 01 Oct 2023
Cited by: 0 articles | PMID: 37341543 | PMCID: PMC10582907
Breadth of Antibodies to Plasmodium falciparum Variant Surface Antigens Is Associated With Immunity in a Controlled Human Malaria Infection Study.
Front Immunol, 13:894770, 30 May 2022
Cited by: 4 articles | PMID: 35711446 | PMCID: PMC9195513
Longitudinal analysis of naturally acquired PfEMP1 CIDR domain variant antibodies identifies associations with malaria protection.
JCI Insight, 5(12):137262, 18 Jun 2020
Cited by: 17 articles | PMID: 32427581 | PMCID: PMC7406271
Cerebral Plasmodium falciparum malaria: The role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it.
Immunol Rev, 293(1):230-252, 27 Sep 2019
Cited by: 67 articles | PMID: 31562653 | PMCID: PMC6972667
Review Free full text in Europe PMC
Go to all (123) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria.
Nat Med, 4(3):358-360, 01 Mar 1998
Cited by: 413 articles | PMID: 9500614 | PMCID: PMC3836255
Induction of strain-transcending antibodies against Group A PfEMP1 surface antigens from virulent malaria parasites.
PLoS Pathog, 8(4):e1002665, 19 Apr 2012
Cited by: 55 articles | PMID: 22532802 | PMCID: PMC3330128
Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults.
Am J Trop Med Hyg, 76(5):860-864, 01 May 2007
Cited by: 23 articles | PMID: 17488905
The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria.
Trends Microbiol, 10(2):55-58, 01 Feb 2002
Cited by: 92 articles | PMID: 11827798
Review




