Abstract
Free full text

Bone Morphogenetic Protein 10—A Novel Biomarker to Predict Adverse Outcomes in Patients With Atrial Fibrillation
Associated Data
Abstract
Background
Patients with atrial fibrillation (AF) face an increased risk of death and major adverse cardiovascular events (MACE). We aimed to assess the predictive value of the novel atrial‐specific biomarker BMP10 (bone morphogenetic protein 10) for death and MACE in patients with AF in comparison with NT‐proBNP (N‐terminal prohormone of B‐type natriuretic peptide).
Methods and Results
BMP10 and NT‐proBNP were measured in patients with AF enrolled in Swiss‐AF (Swiss Atrial Fibrillation Study), a prospective multicenter cohort study. A total of 2219 patients were included (median follow‐up 4.3 years [interquartile range 3.9, 5.1], mean age 73±9
years [interquartile range 3.9, 5.1], mean age 73±9 years, 73% male). In multivariable Cox proportional hazard models, the adjusted hazard ratio (aHR) associated with 1
years, 73% male). In multivariable Cox proportional hazard models, the adjusted hazard ratio (aHR) associated with 1 ng/mL increase of BMP10 was 1.60 (95% CI, 1.37–1.87) for all‐cause death, and 1.54 (95% CI, 1.35–1.76) for MACE. For all‐cause death, the concordance index was 0.783 (95% CI, 0.763–0.809) for BMP10, 0.784 (95% CI, 0.765–0.810) for NT‐proBNP, and 0.789 (95% CI, 0.771–0.815) for both biomarkers combined. For MACE, the concordance index was 0.732 (95% CI, 0.715–0.754) for BMP10, 0.747 (95% CI, 0.731–0.768) for NT‐proBNP, and 0.750 (95% CI, 0.734–0.771) for both biomarkers combined. When grouping patients according to NT‐proBNP categories (<300, 300–900, >900
ng/mL increase of BMP10 was 1.60 (95% CI, 1.37–1.87) for all‐cause death, and 1.54 (95% CI, 1.35–1.76) for MACE. For all‐cause death, the concordance index was 0.783 (95% CI, 0.763–0.809) for BMP10, 0.784 (95% CI, 0.765–0.810) for NT‐proBNP, and 0.789 (95% CI, 0.771–0.815) for both biomarkers combined. For MACE, the concordance index was 0.732 (95% CI, 0.715–0.754) for BMP10, 0.747 (95% CI, 0.731–0.768) for NT‐proBNP, and 0.750 (95% CI, 0.734–0.771) for both biomarkers combined. When grouping patients according to NT‐proBNP categories (<300, 300–900, >900 ng/L), higher aHRs were observed in patients with high BMP10 in the categories of low NT‐proBNP (all‐cause death aHR, 2.28 [95% CI, 1.15–4.52], MACE aHR, 1.88 [95% CI, 1.07–3.28]) and high NT‐proBNP (all‐cause death aHR, 1.61 [95% CI, 1.14–2.26], MACE aHR, 1.38 [95% CI, 1.07–1.80]).
ng/L), higher aHRs were observed in patients with high BMP10 in the categories of low NT‐proBNP (all‐cause death aHR, 2.28 [95% CI, 1.15–4.52], MACE aHR, 1.88 [95% CI, 1.07–3.28]) and high NT‐proBNP (all‐cause death aHR, 1.61 [95% CI, 1.14–2.26], MACE aHR, 1.38 [95% CI, 1.07–1.80]).
Conclusions
BMP10 strongly predicted all‐cause death and MACE in patients with AF. BMP10 provided additional prognostic information in low‐ and high‐risk patients according to NT‐proBNP stratification.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02105844.
Nonstandard Abbreviations and Acronyms
- AIC
- Akaike information criterion
- BMP10
- bone morphogenetic protein 10
- C‐index
- concordance index
- MACE
- major adverse cardiovascular events
- PITX2
- paired‐like homeodomain transcription factor 2
Despite improved treatment regimens, atrial fibrillation (AF) remains associated with a 3.5‐fold increased mortality risk compared with patients without AF. 1 , 2 In particular, patients with AF are at risk for heart failure, stroke, and hospitalizations. 3 , 4 , 5 , 6 , 7 Identifying patients at risk for adverse outcomes is crucial for the initiation of evidence‐based preventive therapies. However, risk assessment poses a major challenge and is currently mainly based on clinical parameters. 3 NT‐proBNP (N‐terminal prohormone of B‐type natriuretic peptide) is an established diagnostic and prognostic biomarker for heart failure. 8 It has marked advantages compared with clinical scores and also predicts adverse outcomes in patients with AF. 3 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Yet, its concentration is influenced by different variables including renal function, obesity, and AF in particular. 18
BMP10 (bone morphogenetic protein 10) was identified as an atrial‐specific biomarker. 19 , 20 , 21 , 22 , 23 Genome‐wide association studies found gene variants on chromosome 4q25 conferring an increased risk of AF. 24 , 25 The PITX2 (paired‐like homeodomain transcription factor 2) is located in this region and is one of the most differentially expressed atrium‐specific genes in patients. 20 , 26 Reducing PITX2 leads to a predisposition for AF. 26 , 27 , 28 , 29 BMP10 is a blood biomarker that is regulated by atrial PITX2 and that can be quantified in peripheral plasma samples. 19 , 20 , 26 , 30 , 31 , 32 , 33 So far, only limited information about influencing factors and the predictive value of BMP10 for adverse cardiovascular events in patients with AF is available. 19 , 34 , 35
In this study, we aimed to explore the association of BMP10 concentration with all‐cause death and major adverse cardiovascular events (MACE) in a large cohort of patients with AF. Our second aim was to determine whether BMP10 provides additional prognostic information compared with NT‐proBNP.
METHODS
Availability of Data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design, Setting, and Participants
We analyzed patients from the ongoing prospective multicenter observational cohort study Swiss‐AF (Swiss Atrial Fibrillation Study). 36 Detailed methodology was published previously (ClinicalTrials.gov identifier: NCT02105844). 37
The Swiss‐AF cohort study is being conducted at 14 centers in Switzerland involving 2415 patients with AF aged ≥65 years and a limited number of patients aged 45 to 64
years and a limited number of patients aged 45 to 64 years. Recruitment took place from 2014 to 2017, and patient follow‐up examinations are ongoing on a yearly basis. Clinically stable patients with AF were included. Exclusion criteria were reversible forms of AF (eg, after cardiac surgery), acute illness within the past 4
years. Recruitment took place from 2014 to 2017, and patient follow‐up examinations are ongoing on a yearly basis. Clinically stable patients with AF were included. Exclusion criteria were reversible forms of AF (eg, after cardiac surgery), acute illness within the past 4 weeks, or inability to sign informed consent. Eligible patients with AF were identified by screening in‐ and outpatients at participating sites and by contacting medical practices. Written informed consent was obtained from each participant. The study complied with the Helsinki declaration and was approved by local ethics committees in the participating centers (lead ethics committee: Ethikkommission Nordwest‐ und Zentralschweiz).
weeks, or inability to sign informed consent. Eligible patients with AF were identified by screening in‐ and outpatients at participating sites and by contacting medical practices. Written informed consent was obtained from each participant. The study complied with the Helsinki declaration and was approved by local ethics committees in the participating centers (lead ethics committee: Ethikkommission Nordwest‐ und Zentralschweiz).
For this analysis, we excluded 196 (8.1%) patients owing to missing baseline concentration of BMP10 (n=54) or NT‐proBNP (n=154) or dropout after the baseline visit only (n=28). We used all data available by May 31, 2021.
Blood Sampling
At the baseline visit, we obtained nonfasting venous blood samples from all study participants. The blood samples were centrifuged, aliquoted into cryotubes, and stored at −80 °C in a centralized biobank. BMP10 and NT‐proBNP concentrations of EDTA plasma were analyzed centrally at Roche Diagnostics, Penzberg, by laboratory personnel blinded to clinical information under constant quality control and calibration.
BMP10 was determined using a cobas e601 analyzer and a noncommercial robust prototype electro‐chemiluminescence immunoassay applying monoclonal antibodies specifically developed against BMP10 (lower detection limit=0.003 ng/mL, functional sensitivity lower limit of quantification=0.012
ng/mL, functional sensitivity lower limit of quantification=0.012 ng/mL). Run‐control measurements performed in the course of the study resulted in a coefficient of variation of 2.35% (mean 1.38
ng/mL). Run‐control measurements performed in the course of the study resulted in a coefficient of variation of 2.35% (mean 1.38 ng/mL). NT‐proBNP was determined using the Roche Elecsys proBNP II IVD on a cobas e601 (measuring range 10–35
ng/mL). NT‐proBNP was determined using the Roche Elecsys proBNP II IVD on a cobas e601 (measuring range 10–35 000
000 ng/L) with a coefficient of variation of 2.45% for the lower control measured (mean 133.1
ng/L) with a coefficient of variation of 2.45% for the lower control measured (mean 133.1 ng/L). The assays applied are based on the Elecsys electro‐chemiluminescence technology.
ng/L). The assays applied are based on the Elecsys electro‐chemiluminescence technology.
For BMP10, we tested the stability of the measurements in biological plasma samples with a routine of stress testing conditions, as assumed variances of monitored storage conditions are expected to be less critical on sample stability. The test covered incubation of plasma samples stored after thawing from −80 °C at room temperature for 1 as well as 2 days of 3 independent samples, resulting in recovery of 104% each. Application of freeze/thaw cycles to 10 independent plasma samples revealed high stability for a single cycle (−80 °C/room temperature/−80 °C) of 101% as well as for 3 cycles resulting in 103%. The mean time (±SD) from collection of the blood samples to measurement of the biomarker concentrations was 720±332
days of 3 independent samples, resulting in recovery of 104% each. Application of freeze/thaw cycles to 10 independent plasma samples revealed high stability for a single cycle (−80 °C/room temperature/−80 °C) of 101% as well as for 3 cycles resulting in 103%. The mean time (±SD) from collection of the blood samples to measurement of the biomarker concentrations was 720±332 days for NT‐proBNP and 1144±332
days for NT‐proBNP and 1144±332 days for BMP10 in our study population. All blood samples drawn were stored at −80 °C throughout the entire duration from blood draw until measurement.
days for BMP10 in our study population. All blood samples drawn were stored at −80 °C throughout the entire duration from blood draw until measurement.
The estimated glomerular filtration rate of patients was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation. 38
Other Study Variables
At the baseline visit, standardized questionnaires were used to collect data on the patients' lifestyle, medication, and medical history. Smoking status was categorized into current smokers and nonsmokers. The CHA2DS2‐VASc score was calculated (congestive heart failure [1 point], hypertension [1 point], age ≥75 years [2 points], diabetes [1 point], prior stroke/transient ischemic attack/thromboembolism [2 points], vascular disease [1 point], age 65 to 74
years [2 points], diabetes [1 point], prior stroke/transient ischemic attack/thromboembolism [2 points], vascular disease [1 point], age 65 to 74 years [1 point], and female sex [1 point]). AF‐related symptoms, such as palpitations, dyspnoea, dizziness, fatigue, chest pain, syncope, exercise intolerance, or other, were elicited. AF was classified in compliance with the 2010 AF guidelines of the European Society of Cardiology and then categorized into paroxysmal and nonparoxysmal (persistent and permanent) AF.
39
Clinical measures including body weight and height were obtained with calibrated devices. The body mass index was calculated (weight in kilograms divided by height in meters squared). Blood pressure was measured 3 times in a supine position after 5
years [1 point], and female sex [1 point]). AF‐related symptoms, such as palpitations, dyspnoea, dizziness, fatigue, chest pain, syncope, exercise intolerance, or other, were elicited. AF was classified in compliance with the 2010 AF guidelines of the European Society of Cardiology and then categorized into paroxysmal and nonparoxysmal (persistent and permanent) AF.
39
Clinical measures including body weight and height were obtained with calibrated devices. The body mass index was calculated (weight in kilograms divided by height in meters squared). Blood pressure was measured 3 times in a supine position after 5 minutes of rest. We used the mean of these 3 measurements for the analysis. A resting 16‐lead ECG of 5
minutes of rest. We used the mean of these 3 measurements for the analysis. A resting 16‐lead ECG of 5 minutes duration was obtained to determine the rhythm at enrolment. We categorized the rhythm into sinus rhythm, AF, or other.
minutes duration was obtained to determine the rhythm at enrolment. We categorized the rhythm into sinus rhythm, AF, or other.
Clinical Outcome Events
At the ongoing annual follow‐up examinations performed by in‐person visits or telephone interviews, we assessed clinical adverse outcomes. After collecting all available information from the treating physician/hospital, the outcome events were independently adjudicated by 2 physicians. In case of disagreement, a third specialist was involved to make the final decision.
For the present analysis, the primary outcomes were all‐cause death and MACE. MACE was defined as a composite of hospitalization for heart failure, cardiovascular death, stroke, systemic embolism, and myocardial infarction. Secondary outcomes included the individual components of MACE. Definitions of the adverse outcome events are provided in Table S1.
Statistical Analysis
Baseline characteristics were stratified by observed BMP10 quartiles (QI–QIV). Categorical variables are presented as numbers (percentages) and compared using chi‐square tests. Continuous variables are presented as mean±SD and compared using ANOVA, or as median (interquartile range) and compared using Kruskal–Wallis tests if strongly skewed.
We calculated the incidence rates for BMP10 quartiles and 3 clinically relevant NT‐proBNP categories (<300, 300–900, >900 ng/L),
40
,
41
expressed as numbers of events per 100 patient‐years of follow‐up. Follow‐up patient‐years were calculated from time of enrolment until the occurrence of the respective outcome event. Patients were censored at their last observation or at the focal outcome events. Kaplan–Meier curves were constructed for the main outcomes, and log‐rank tests were performed.
ng/L),
40
,
41
expressed as numbers of events per 100 patient‐years of follow‐up. Follow‐up patient‐years were calculated from time of enrolment until the occurrence of the respective outcome event. Patients were censored at their last observation or at the focal outcome events. Kaplan–Meier curves were constructed for the main outcomes, and log‐rank tests were performed.
We used Cox proportional hazards models to determine the association of BMP10 and NT‐proBNP concentrations with adverse outcomes. Results are presented as hazard ratio (HR) and 95% CI. We calculated the cause‐specific hazard for MACE. As a sensitivity analysis, we recalculated the main model using subdistribution hazard regression as described by Fine and Gray, in order to take into account, the competing risk of noncardiovascular death for the primary outcome MACE. Initial models were adjusted for age and sex. Multivariable models were additionally adjusted for a predefined set of risk factors, consisting of body mass index, heart rate, systolic blood pressure, rhythm at baseline, current smoking, history of diabetes, history of coronary artery disease, history of hypertension, history of heart failure, history of stroke/transient ischemic attack, oral anticoagulation, antiplatelet therapy, and estimated glomerular filtration rate. As a sensitivity analysis, the multivariable model for the primary outcomes was additionally adjusted for NT‐proBNP and for the study center. The covariates were selected based on clinical plausibility, expert knowledge, and availability in our cohort.
Because only a few patients had missing covariate values, we removed these from the multivariable analysis. A sensitivity analysis for the age‐ and sex‐adjusted models using only the complete cases of the multivariable model showed no relevant difference in estimates or conclusions (results not shown).
Using the Schoenfeld residuals, we found no strong violation of the proportional hazards assumption visually. Testing between time and residuals, we found a nonsignificant relationship (all P>0.05) for all models of the primary outcomes. Regarding the secondary outcomes, only for myocardial infarction, there was a suggestion of nonproportional hazards of NT‐proBNP (P=0.037), which could result in reduced power to detect effects.
We compared the multivariable adjusted Cox proportional hazards models using BMP10 and NT‐proBNP continuously with the concordance statistic (C‐index) and Akaike information criterion (AIC). A higher C‐index indicates a better discriminating power and a lower AIC indicates a better model fit. We provide 95% bootstrapped percentile CI of the C‐index from 1999 bootstrap rounds.
The correlation between BMP10 and NT‐proBNP was assessed by means of the Spearman's correlation coefficient. In addition, we constructed receiver operating characteristic curves and calculated the area under the curve (AUC) for BMP10 and NT‐proBNP separately and for both biomarkers combined using multivariable adjusted logistic regression models. A higher AUC indicates a better discriminating power. Bootstrapping was used to calculate 95% CIs.
Patients were further divided into low and high BMP10 groups according to the median of BMP10 (2.247 ng/mL). Based on the stratification using the 3 NT‐proBNP categories, 6 groups of patients were generated. We calculated the incidence rates per 100 patient‐years of follow‐up for these 6 groups, constructed Kaplan–Meier curves, and compared with the log‐rank test. We used Cox proportional hazard models adjusting for the same factors indicated to analyze the effect of BMP10 low/high in the 3 different categories of NT‐proBNP low, intermediate, and high (<300, 300–900, >900
ng/mL). Based on the stratification using the 3 NT‐proBNP categories, 6 groups of patients were generated. We calculated the incidence rates per 100 patient‐years of follow‐up for these 6 groups, constructed Kaplan–Meier curves, and compared with the log‐rank test. We used Cox proportional hazard models adjusting for the same factors indicated to analyze the effect of BMP10 low/high in the 3 different categories of NT‐proBNP low, intermediate, and high (<300, 300–900, >900 ng/L).
ng/L).
For the primary outcomes, we performed a subgroup analysis of the multivariable Cox proportional hazard model of BMP10 with specified variables (age, sex, rhythm at baseline, AF type, history of heart failure, coronary artery disease, stroke/transient ischemic attack, diabetes, hypertension, renal failure) and tested for interactions.
For BMP10, there are currently no established cutoffs. Therefore, we used BMP10 quartiles for a balanced analysis. For NT‐proBNP, we used the existing clinically used cutoffs in patients with AF and heart failure. 40 , 41 Histograms of BMP10 and NT‐proBNP are presented in Figures S1 and S2. NT‐proBNP was log‐transformed owing to the skewed distribution. All presented P values are 2‐sided. Considering the exploratory nature of the analysis, we performed no correction for multiple testing and interpreted P values as a continuous variable that adds to the evidence against the relevant null hypothesis. We did not set a threshold for significance. All analyses were performed using R version 4.1.0 (2021‐05‐18, R Core Team).
RESULTS
Participants
A total of 2219 patients were included in this analysis. Table 1 shows the overall baseline characteristics and the characteristics stratified by the individual BMP10 quartiles. Mean age (±SD) of patients was 73±9 years, and 73% of them were male. The highest BMP10 quartile contained older patients (QI 69±9 versus QIV 77±7
years, and 73% of them were male. The highest BMP10 quartile contained older patients (QI 69±9 versus QIV 77±7 years) and more female patients (QI 16% versus QIV 42%). Patients had more comorbidities, predominantly heart failure (QI 19% versus QIV 38%) and renal failure (QI 12% versus QIV 37%), and had a higher CHA2DS2‐VASc score (QI 2.8±1.7 versus QIV 4.2±1.5). The prevalence of nonparoxysmal AF was higher (QI 41% versus QIV 70%), and AF was more frequent in the baseline ECG (QI 17% versus QIV 69%) in patients in the highest BMP10 quartile. Accordingly, BMP10 concentration was higher in nonparoxysmal AF compared with paroxysmal AF, and in AF compared with sinus rhythm at baseline ECG (P<0.001, Figure S3).
years) and more female patients (QI 16% versus QIV 42%). Patients had more comorbidities, predominantly heart failure (QI 19% versus QIV 38%) and renal failure (QI 12% versus QIV 37%), and had a higher CHA2DS2‐VASc score (QI 2.8±1.7 versus QIV 4.2±1.5). The prevalence of nonparoxysmal AF was higher (QI 41% versus QIV 70%), and AF was more frequent in the baseline ECG (QI 17% versus QIV 69%) in patients in the highest BMP10 quartile. Accordingly, BMP10 concentration was higher in nonparoxysmal AF compared with paroxysmal AF, and in AF compared with sinus rhythm at baseline ECG (P<0.001, Figure S3).
Table 1
Overall Baseline Characteristics and Stratified by BMP10 Quartiles
BMP10 ng/mL (range) ng/mL (range) | Overall (1.18–6.99) | Quartile I (1.18–1.92) | Quartile II (1.92–2.25) | Quartile III (2.25–2.65) | Quartile IV (2.65–6.99) | P value |
|---|---|---|---|---|---|---|
| Number of patients | 2219 | 554 | 554 | 556 | 555 | |
| Age, y | 73.2±8.5 | 69.3±9.0 | 72.3±8.2 | 74.2±7.3 | 77.1±7.4 | <0.001 |
| Male sex | 1618 (72.9) | 466 (84.1) | 435 (78.5) | 394 (70.9) | 323 (58.2) | <0.001 |
| Body mass index, kg/m2 | 27.7±4.8 | 28.6±4.6 | 28.1±4.9 | 27.6±4.9 | 26.5±4.4 | <0.001 |
| Current smoker | 159 (7.2) | 48 (8.7) | 53 (9.6) | 35 (6.3) | 23 (4.1) | 0.002 |
| Atrial fibrillation‐related symptoms | 1364 (61.6) | 374 (67.5) | 336 (61.0) | 333 (59.9) | 321 (57.8) | 0.007 |
| Heart rate, beats/min | 66 [59, 76] | 63 [57, 71] | 65 [58, 75] | 68 [60, 78] | 70 [60, 80] | <0.001 |
Blood pressure, mm Hg Hg | ||||||
| Systolic | 134.3±18.7 | 133.9±17.7 | 134.4±17.8 | 134.4±18.0 | 134.7±21.2 | 0.92 |
| Diastolic | 77.7±11.8 | 78.6±10.6 | 78.1±11.0 | 77.6±12.5 | 76.7±12.8 | 0.04 |
| Atrial fibrillation type | <0.001 | |||||
| Paroxysmal | 982 (44.3) | 325 (58.7) | 275 (49.6) | 217 (39.0) | 165 (29.7) | |
| Nonparoxysmal | 1237 (55.7) | 229 (41.3) | 279 (50.4) | 339 (61.0) | 390 (70.3) | |
| Rhythm at baseline | <0.001 | |||||
| Sinus rhythm | 1106 (50.1) | 428 (77.4) | 310 (56.5) | 233 (42.1) | 135 (24.4) | |
| Atrial fibrillation | 953 (43.2) | 92 (16.6) | 193 (35.2) | 288 (52.1) | 380 (68.7) | |
| Other | 149 (6.7) | 33 (6.0) | 46 (8.4) | 32 (5.8) | 38 (6.9) | |
| CHA2DS2‐VASc score | 3.5±1.7 | 2.8±1.7 | 3.3±1.6 | 3.7±1.6 | 4.2±1.5 | <0.001 |
| History of pulmonary vein isolation | 444 (20.0) | 193 (34.8) | 118 (21.3) | 86 (15.5) | 47 (8.5) | <0.001 |
| History of atrial flutter | 470 (21.2) | 127 (22.9) | 120 (21.7) | 127 (22.8) | 96 (17.3) | 0.07 |
| History of coronary artery disease | 665 (30.0) | 158 (28.5) | 160 (28.9) | 175 (31.5) | 172 (31.0) | 0.63 |
| History of stroke/transient ischemic attack | 436 (19.7) | 79 (14.3) | 113 (20.4) | 115 (20.7) | 129 (23.2) | 0.002 |
| History of systemic embolism | 120 (5.4) | 21 (3.8) | 28 (5.1) | 25 (4.5) | 46 (8.3) | 0.005 |
| History of hypertension | 1555 (70.1) | 353 (63.7) | 390 (70.4) | 400 (71.9) | 412 (74.2) | 0.001 |
| History of heart failure | 584 (26.3) | 103 (18.6) | 116 (20.9) | 152 (27.3) | 213 (38.4) | <0.001 |
| History of diabetes | 398 (17.9) | 77 (13.9) | 99 (17.9) | 112 (20.1) | 110 (19.8) | 0.025 |
| History of renal failure | 466 (21.0) | 64 (11.6) | 86 (15.5) | 114 (20.5) | 202 (36.5) | <0.001 |
| Estimated glomerular filtration rate, mL/min per 1.7 | 59 [47, 72] | 67 [57, 78] | 61 [51, 73] | 60 [47, 72] | 49 [36, 60] | <0.001 |
| N‐terminal prohormone of B‐type natriuretic peptide, ng/L | 648 [220, 1470] | 194 [88, 480] | 470 [194, 995] | 824 [329, 1495] | 1656 [962, 2922] | <0.001 |
| Antiarrhythmic agents | ||||||
| Class IC | 87 (3.9) | 44 (7.9) | 18 (3.2) | 18 (3.2) | 7 (1.3) | <0.001 |
| Class II | 1568 (70.7) | 379 (68.4) | 388 (70.0) | 411 (73.9) | 390 (70.3) | 0.23 |
| Class III | 406 (18.3) | 108 (19.5) | 108 (19.5) | 109 (19.6) | 81 (14.6) | 0.08 |
| Oral anticoagulation | 2008 (90.5) | 487 (87.9) | 491 (88.6) | 514 (92.4) | 516 (93.0) | 0.005 |
| Vitamin K antagonist | 886 (39.9) | 146 (26.4) | 200 (36.1) | 238 (42.8) | 302 (54.4) | <0.001 |
| Direct oral anticoagulants | 1121 (50.5) | 341 (61.6) | 290 (52.3) | 276 (49.6) | 214 (38.6) |
<0.001 |
| Antiplatelet therapy | 436 (19.7) | 112 (20.2) | 105 (19.0) | 107 (19.3) | 112 (20.3) | 0.93 |
Values are given as mean±SD, median [interquartile range], or number (percentage). BMP10 indicates bone morphogenetic protein 10.
BMP10 and Adverse Outcomes
During a median follow‐up time of 4.3 years (interquartile range 3.9, 5.1), 395 patients died. The incidence rate per 100 patient‐years of follow‐up increased with rising BMP10 quartiles from 1.61 in QI to 8.04 in QIV (Table 2). Figure 1A shows the cumulative incidence for all‐cause death stratified by BMP10 quartiles. In the age‐ and sex‐adjusted Cox proportional hazard model (Table 2), the HR of BMP10 (continuous) for all‐cause death was 1.94 (95% CI, 1.71–2.21), which means that per an increase of 1
years (interquartile range 3.9, 5.1), 395 patients died. The incidence rate per 100 patient‐years of follow‐up increased with rising BMP10 quartiles from 1.61 in QI to 8.04 in QIV (Table 2). Figure 1A shows the cumulative incidence for all‐cause death stratified by BMP10 quartiles. In the age‐ and sex‐adjusted Cox proportional hazard model (Table 2), the HR of BMP10 (continuous) for all‐cause death was 1.94 (95% CI, 1.71–2.21), which means that per an increase of 1 ng/mL of BMP10, the hazard increased by 94%. There was a stepwise increase across BMP10 quartiles (P<0.001 for linear trend). After multivariable adjustment, the HR was 1.60 (95% CI, 1.37–1.87), and evidence for the linear trend across BMP10 quartiles remained strong (P<0.001). When the multivariable model was additionally adjusted for NT‐proBNP or the study center, the HR remained elevated for all‐cause death (Tables S2 and S3).
ng/mL of BMP10, the hazard increased by 94%. There was a stepwise increase across BMP10 quartiles (P<0.001 for linear trend). After multivariable adjustment, the HR was 1.60 (95% CI, 1.37–1.87), and evidence for the linear trend across BMP10 quartiles remained strong (P<0.001). When the multivariable model was additionally adjusted for NT‐proBNP or the study center, the HR remained elevated for all‐cause death (Tables S2 and S3).
Table 2
Association of BMP10 Concentration and Adverse Outcomes
| Adverse outcomes | BMP10 ng/mL ng/mL | Number of events | Patient‐years | Incidence rate per 100 patient‐years | Age‐ and sex‐adjusted model HR (95% CI) | Multivariable adjusted model* HR (95% CI) |
|---|---|---|---|---|---|---|
| All‐cause death | Continuous | 395 | 9618 | 4.11 | 1.94 (1.71–2.21), P<0.001 | 1.60 (1.37–1.87), P<0.001 |
| Quartile I | 41 | 2546 | 1.61 | Reference | Reference | |
| Quartile II | 76 | 2451 | 3.10 | 1.64 (1.12–2.40) | 1.49 (1.00–2.20) | |
| Quartile III | 100 | 2408 | 4.15 | 2.00 (1.39–2.90) | 1.73 (1.17–2.55) | |
| Quartile IV | 178 | 2213 | 8.04 | 3.60 (2.52–5.13) | 2.53 (1.71–3.74) | |
| P linear trend | <0.001 | <0.001 | ||||
| P quadratic trend | 0.69 | 0.94 | ||||
| P cubic trend | 0.17 | 0.34 | ||||
| Major adverse cardiovascular events | Continuous | 605 | 8648 | 7.00 | 1.78 (1.59–1.99), P<0.001 | 1.54 (1.35–1.76), P<0.001 |
| Quartile I | 79 | 2397 | 3.30 | Reference | Reference | |
| Quartile II | 127 | 2238 | 5.67 | 1.48 (1.11–1.96) | 1.35 (1.01–1.80) | |
| Quartile III | 158 | 2162 | 7.31 | 1.79 (1.36–2.35) | 1.58 (1.19–2.11) | |
| Quartile IV | 241 | 1851 | 13.02 | 2.85 (2.18–3.73) | 2.18 (1.62–2.94) | |
| P linear trend | <0.001 | <0.001 | ||||
| P quadratic trend | 0.67 | 0.90 | ||||
| P cubic trend | 0.21 | 0.45 | ||||
| Hospitalization for heart failure | Continuous | 362 | 8931 | 4.05 | 1.94 (1.69–2.23), P<0.001 | 1.62 (1.37–1.91), P<0.001 |
| Quartile I | 43 | 2451 | 1.75 | Reference | Reference | |
| Quartile II | 67 | 2302 | 2.91 | 1.44 (0.98–2.11) | 1.30 (0.88–1.92) | |
| Quartile III | 93 | 2241 | 4.15 | 1.91 (1.32–2.75) | 1.64 (1.12–2.40) | |
| Quartile IV | 159 | 1937 | 8.21 | 3.33 (2.33–4.75) | 2.41 (1.63–3.56) | |
| P linear trend | <0.001 | <0.001 | ||||
| P quadratic trend | 0.41 | 0.60 | ||||
| P cubic trend | 0.49 | 0.74 | ||||
| Cardiovascular death | Continuous | 254 | 9618 | 2.64 | 1.87 (1.59–2.20), P<0.001 | 1.47 (1.21–1.79), P<0.001 |
| Quartile I | 24 | 2546 | 0.94 | Reference | Reference | |
| Quartile II | 53 | 2451 | 2.16 | 1.89 (1.17–3.07) | 1.73 (1.04–2.87) | |
| Quartile III | 61 | 2408 | 2.53 | 2.01 (1.24–3.23) | 1.69 (1.02–2.81) | |
| Quartile IV | 116 | 2213 | 5.24 | 3.81 (2.42–6.02) | 2.57 (1.55–4.29) | |
| P linear trend | <0.001 | <0.001 | ||||
| P quadratic trend | 0.99 | 0.67 | ||||
| P cubic trend | 0.06 | 0.10 | ||||
| Stroke and systemic embolism | Continuous | 114 | 9406 | 1.21 | 1.36 (1.03–1.80), P=0.03 | 1.13 (0.80–1.59), P=0.49 |
| Quartile I | 21 | 2517 | 0.83 | Reference | Reference | |
| Quartile II | 23 | 2400 | 0.96 | 1.01 (0.56–1.84) | 0.81 (0.44–1.50) | |
| Quartile III | 32 | 2334 | 1.37 | 1.36 (0.78–2.38) | 1.00 (0.55–1.81) | |
| Quartile IV | 38 | 2155 | 1.76 | 1.59 (0.90–2.81) | 1.03 (0.54–1.96) | |
| P linear trend | 0.07 | 0.79 | ||||
| P quadratic trend | 0.71 | 0.55 | ||||
| P cubic trend | 0.63 | 0.51 | ||||
| Stroke | Continuous | 107 | 9425 | 1.14 | 1.39 (1.04–1.85), P=0.02 | 1.16 (0.82–1.66), P=0.40 |
| Quartile I | 20 | 2518 | 0.79 | Reference | Reference | |
| Quartile II | 22 | 2402 | 0.92 | 1.02 (0.55–1.87) | 0.82 (0.44–1.53) | |
| Quartile III | 28 | 2346 | 1.19 | 1.23 (0.68–2.20) | 0.88 (0.47–1.64) | |
| Quartile IV | 37 | 2158 | 1.71 | 1.60 (0.89–2.85) | 1.02 (0.53–1.98) | |
| P linear trend | 0.09 | 0.90 | ||||
| P quadratic trend | 0.53 | 0.39 | ||||
| P cubic trend | 0.91 | 0.83 | ||||
| Myocardial infarction | Continuous | 81 | 9459 | 0.86 | 1.17 (0.82–1.67), P=0.38 | 1.27 (0.85–1.90), P=0.25 |
| Quartile I | 15 | 2512 | 0.60 | Reference | Reference | |
| Quartile II | 16 | 2421 | 0.66 | 0.97 (0.48–1.97) | 0.95 (0.46–1.97) | |
| Quartile III | 24 | 2374 | 1.01 | 1.40 (0.72–2.70) | 1.59 (0.80–3.17) | |
| Quartile IV | 26 | 2152 | 1.21 | 1.50 (0.76–2.96) | 1.80 (0.85–3.82) | |
| P linear trend | 0.15 | 0.06 | ||||
| P quadratic trend | 0.81 | 0.71 | ||||
| P cubic trend | 0.50 | 0.36 |
n=2219 (Quartile I n=554; Quartile II n=554; Quartile III n=556; Quartile IV n=555). BMP10 indicates bone morphogenetic protein 10; and HR, hazard ratio.
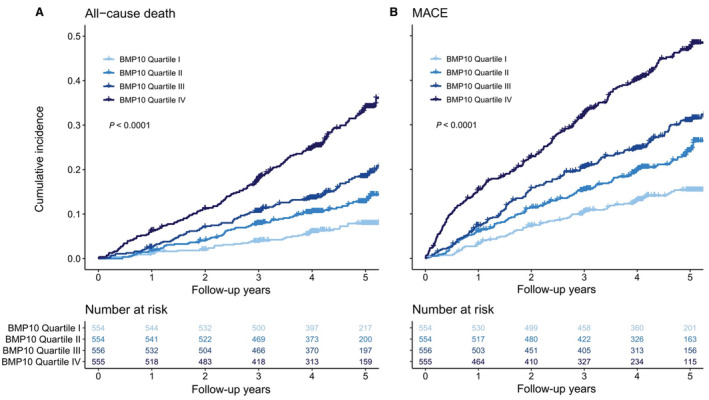
Cumulative incidence per follow‐up years for all‐cause death (A) and major adverse cardiovascular events (MACE; B) stratified by BMP10 quartiles. MACE is a composite of heart failure hospitalization, cardiovascular death, stroke, systemic embolism, and myocardial infarction. P values were calculated by log‐rank test. BMP10 indicates bone morphogenetic protein 10.
A total of 605 MACE occurred during the study. The incidence rate per 100 patient‐years of follow‐up increased across BMP10 quartiles from 3.30 in QI to 13.02 in QIV. Figure 1B highlights the cumulative incidence per BMP10 quartiles for MACE. The HR for the age‐ and sex‐adjusted Cox proportional hazard model (Table 2) was 1.78 (95% CI, 1.59–1.99), and the HR increased across BMP10 quartiles (P<0.001 for linear trend). In the multivariable adjusted model, the HR was 1.54 (95% CI, 1.35–1.76) with a strong linear trend across BMP10 quartiles (P<0.001). When the multivariable model was additionally adjusted for NT‐proBNP or the study center, the HR remained elevated for MACE (Tables S2 and S3). The competing risk model (Fine and Gray) taking into account the competing risk of noncardiovascular death for MACE revealed similar findings. For the age‐ and sex‐adjusted model, the subdistribution HR of BMP10 was 1.73 (95% CI, 1.53–1.96, P<0.001) for MACE. For the multivariable model, the subdistribution HR of BMP10 was 1.52 (95% CI, 1.31–1.75, P<0.001) for MACE.
For all secondary outcomes, we observed an increasing incidence per 100 patient‐years across BMP10 quartiles (Table 2). After multivariable adjustment, HRs were estimated at 1.62 (95% CI, 1.37–1.91) for hospitalization for heart failure and at 1.47 (95% CI, 1.21–1.79) for cardiovascular death. Evidence of an association of BMP10 with stroke/systemic embolism or myocardial infarction was weak after multivariable adjustment.
In the subgroup analysis (Figure 2), we found no strong support for interactions for the primary outcomes with age, sex, AF type, history of heart failure, coronary artery disease, stroke/transient ischemic attack, hypertension, and renal failure. Only for history of diabetes, there was an interaction with all‐cause death (P=0.002) but not with MACE (P=0.58). For rhythm at baseline (AF versus sinus rhythm), there was an interaction with MACE (P=0.04) but not with all‐cause death (P=0.82).
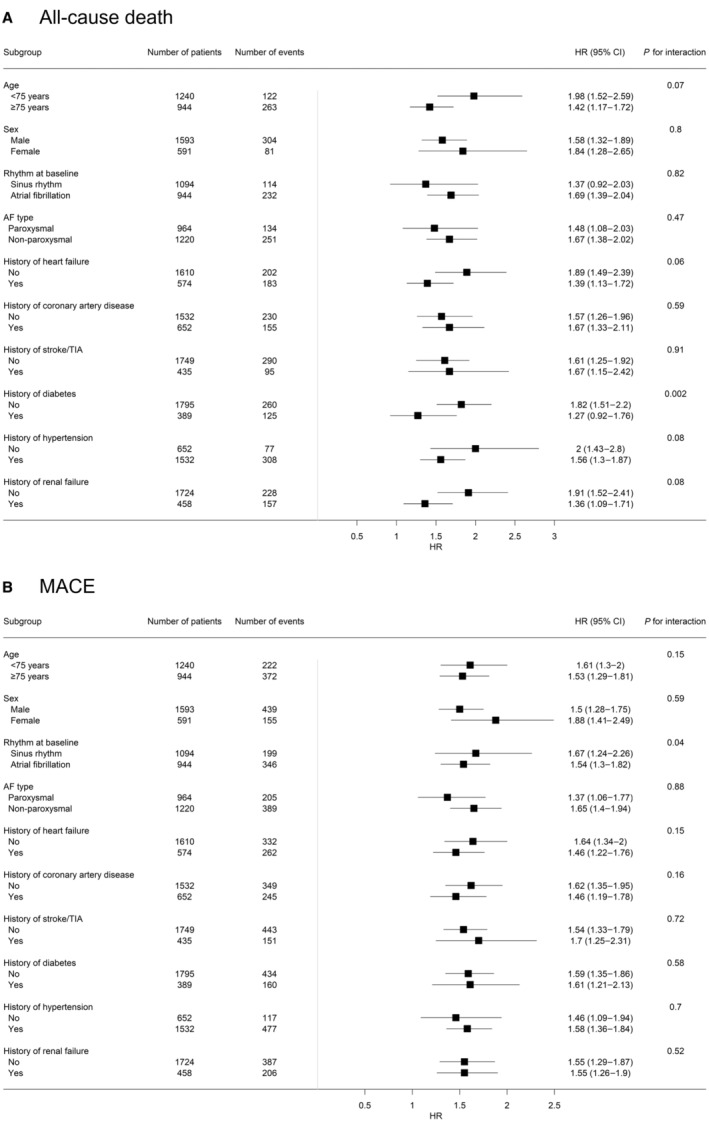
Association of BMP10 (bone morphogenetic protein 10) with all‐cause death (A) and major adverse cardiovascular events (MACE; B) across various subgroups. Hazard ratio (HR) and 95% CIs for BMP10 were calculated using multivariable adjusted Cox proportional hazard models. AF indicates atrial fibrillation; and TIA, transient ischemic attack.
NT‐proBNP and Adverse Outcomes
Table S4 shows the association between NT‐proBNP categories and adverse outcomes. For all‐cause death, there were increasing incidence rates per 100 patient‐years across the different NT‐proBNP categories. In the age‐ and sex‐adjusted Cox proportional hazard model, we found a HR of 1.80 (95% CI, 1.63–1.98), and after multivariable adjustment a HR of 1.59 (95% CI, 1.40–1.79) for all‐cause death. There was a strong linear trend across increasing NT‐proBNP categories (P<0.001).
For MACE, the incidence rate per 100 patient‐years also increased across NT‐proBNP categories. For the age‐ and sex‐adjusted model, the HR was 1.73 (95% CI, 1.60–1.86), and after multivariable adjustment the HR was 1.64 (95% CI, 1.49–1.81). The linear trend was strong across NT‐proBNP categories (P<0.001). For all secondary outcomes, the HR remained strongly elevated after multivariable adjustment.
Comparison of BMP10 and NT‐proBNP
The Spearman's correlation coefficient between BMP10 and NT‐proBNP was 0.59 (Figure S4). Table 3 illustrates the C‐index and AIC of the different multivariable Cox proportional hazard models for the primary outcomes. For both all‐cause death and MACE, C‐index and AIC values indicated the best discriminative power and best fit for the model including both BMP10 and NT‐proBNP (C‐index all‐cause death, 0.789 [95% CI, 0.771–0.815], MACE, 0.750 [95% CI, 0.734–0.771]; AIC all‐cause death, 5198; MACE 8316). When the biomarkers were analyzed separately, BMP10 and NT‐proBNP had a comparable C‐index for all‐cause death (BMP10, 0.783 [95% CI, 0.763–0.809], NT‐proBNP 0.784 [95% CI, 0.765–0.810]), but BMP10 had a higher AIC (5230) compared with NT‐proBNP (5207). For MACE, NT‐proBNP had a higher C‐index (0.747 [95% CI, 0.731–0.768]) and a lower AIC (8322) compared with BMP10 (C‐index, 0.732 [95% CI, 0.715–0.754], AIC 8383).
Table 3
C‐Indices and Akaike Information Criterion for BMP10, NT‐proBNP, and Both Biomarkers Combined
| Adverse outcomes | Multivariable adjusted model* | C‐index (95% CI) | AIC |
|---|---|---|---|
| All‐cause death | BMP10 | 0.783 (0.763–0.809) | 5230 |
| NT‐proBNP† | 0.784 (0.765–0.810) | 5207 | |
| BMP10 and NT‐proBNP† | 0.789 (0.771–0.815) | 5198 | |
| Major adverse cardiovascular events | BMP10 | 0.732 (0.715–0.754) | 8383 |
| NT‐proBNP† | 0.747 (0.731–0.768) | 8322 | |
| BMP10 and NT‐proBNP† | 0.750 (0.734–0.771) | 8316 |
n=2184. AIC indicates Akaike information criterion; BMP10, bone morphogenetic protein 10, C‐index, concordance index; and NT‐proBNP, N‐terminal prohormone of B‐type natriuretic peptide.
The AUC for the multivariable logistic regression model for all‐cause death was 0.813 (95% CI, 0.785–0.840) for BMP10 and 0.815 (95% CI, 0.789–0.840) for NT‐proBNP (P=0.71, DeLong test; Figure 3A). Both biomarkers combined had an AUC of 0.820 (95% CI, 0.794–0.845). Figure 3B shows the receiver operating characteristic curve for the multivariable logistic regression models for MACE. BMP10 had an AUC of 0.762 (95% CI, 0.739–0.785), and NT‐proBNP had an AUC of 0.780 (95% CI, 0.758–0.802; P=0.003, DeLong test). Both biomarkers combined achieved an AUC of 0.783 (95% CI, 0.760–0.804).
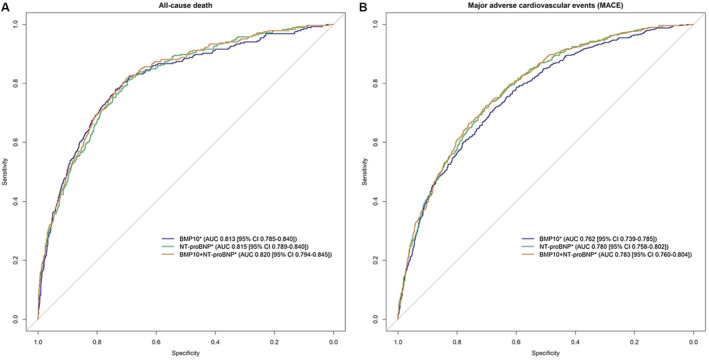
Area under the curve (AUC) and 95% CI for all‐cause death (A) and major adverse cardiovascular events (MACE; B) according to multivariable adjusted logistic regression models of BMP10 (bone morphogenetic protein 10), NT‐proBNP (N‐terminal prohormone of B‐type natriuretic peptide), and both biomarkers combined. MACE is a composite of heart failure hospitalization, cardiovascular death, stroke, systemic embolism, and myocardial infarction. *Multivariable models were adjusted for age, sex, body mass index, heart rate, systolic blood pressure, rhythm at baseline, current smoking, history of diabetes, coronary artery disease, hypertension, heart failure, stroke/transient ischemic attack, oral anticoagulation, antiplatelet therapy, and estimated glomerular filtration rate.
In Table 4, patients were categorized into 6 groups according to NT‐proBNP (low [<300 ng/L], intermediate [300–900
ng/L], intermediate [300–900 ng/L], high [>900
ng/L], high [>900 ng/L]) and BMP10 concentrations (low [<2.247
ng/L]) and BMP10 concentrations (low [<2.247 ng/mL], high [≥2.247
ng/mL], high [≥2.247 ng/mL]). For patients with low or high NT‐proBNP, BMP10 concentration higher than the median was associated with an increase in incidence rate per 100 patient‐years as well as an increase in the adjusted HR for both primary outcomes. For all‐cause death, the HR in the multivariable adjusted model increased to 2.28 (95% CI, 1.15–4.52) in the low NT‐proBNP and high BMP10 group compared with the reference group (low NT‐proBNP and low BMP10). When high NT‐proBNP and low BMP10 served as the reference group, the HR increased to 1.61 (95% CI, 1.14–2.26) in patients with high NT‐proBNP and high BMP10. For MACE, the HR increased to 1.88 (95% CI, 1.07–3.28) in the low NT‐proBNP and high BMP10 group compared with the reference group (low NT‐proBNP and low BMP10). When high NT‐proBNP and low BMP10 served as the reference group, the HR increased to 1.38 (95% CI, 1.07–1.80) in patients with high NT‐proBNP and high BMP10. The cumulative incidence of the 6 groups is shown in Figure 4 for all‐cause death and MACE.
ng/mL]). For patients with low or high NT‐proBNP, BMP10 concentration higher than the median was associated with an increase in incidence rate per 100 patient‐years as well as an increase in the adjusted HR for both primary outcomes. For all‐cause death, the HR in the multivariable adjusted model increased to 2.28 (95% CI, 1.15–4.52) in the low NT‐proBNP and high BMP10 group compared with the reference group (low NT‐proBNP and low BMP10). When high NT‐proBNP and low BMP10 served as the reference group, the HR increased to 1.61 (95% CI, 1.14–2.26) in patients with high NT‐proBNP and high BMP10. For MACE, the HR increased to 1.88 (95% CI, 1.07–3.28) in the low NT‐proBNP and high BMP10 group compared with the reference group (low NT‐proBNP and low BMP10). When high NT‐proBNP and low BMP10 served as the reference group, the HR increased to 1.38 (95% CI, 1.07–1.80) in patients with high NT‐proBNP and high BMP10. The cumulative incidence of the 6 groups is shown in Figure 4 for all‐cause death and MACE.
Table 4
Association of BMP10 According to NT‐proBNP Categories With Adverse Outcomes
| Adverse outcomes | NT‐proBNP categories | BMP10 categories | Number of patients | Number of events | Patient‐years | Incidence rate per 100 patient‐years | Age‐ and sex‐adjusted model HR (95% CI) | Multivariable adjusted model* HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| All‐cause death | Low | Low | 545 | 23 | 2551 | 0.90 | Reference | Reference |
| High | 152 | 14 | 669 | 2.09 | 2.29 (1.17–4.47) | 2.28 (1.15–4.52) | ||
| Intermediate | Low | 347 | 49 | 1529 | 3.20 | Reference | Reference | |
| High | 270 | 38 | 1184 | 3.21 | 0.96 (0.63–1.47) | 0.95 (0.62–1.48) | ||
| High | Low | 216 | 45 | 917 | 4.91 | Reference | Reference | |
| High | 689 | 226 | 2769 | 8.16 | 1.62 (1.17–2.23) | 1.61 (1.14–2.26) | ||
| Major adverse cardiovascular events | Low | Low | 545 | 39 | 2481 | 1.57 | Reference | Reference |
| High | 152 | 19 | 643 | 2.95 | 1.76 (1.01–3.05) | 1.88 (1.07–3.28) | ||
| Intermediate | Low | 347 | 88 | 1383 | 6.36 | Reference | Reference | |
| High | 270 | 67 | 1058 | 6.33 | 0.95 (0.69–1.30) | 0.99 (0.71–1.37) | ||
| High | Low | 216 | 79 | 772 | 10.23 | Reference | Reference | |
| High | 689 | 313 | 2312 | 13.54 | 1.27 (0.99–1.63) | 1.38 (1.07–1.80) |
NT‐proBNP categories: low <300 ng/L, intermediate 300–900
ng/L, intermediate 300–900 ng/L, high >900
ng/L, high >900 ng/L; BMP10 categories according to median: low <2.247
ng/L; BMP10 categories according to median: low <2.247 ng/mL, high ≥2.247
ng/mL, high ≥2.247 ng/mL. n=2219. BMP10 indicates bone morphogenetic protein 10; HR, hazard ratio; and NT‐proBNP, N‐terminal prohormone of B‐type natriuretic peptide.
ng/mL. n=2219. BMP10 indicates bone morphogenetic protein 10; HR, hazard ratio; and NT‐proBNP, N‐terminal prohormone of B‐type natriuretic peptide.
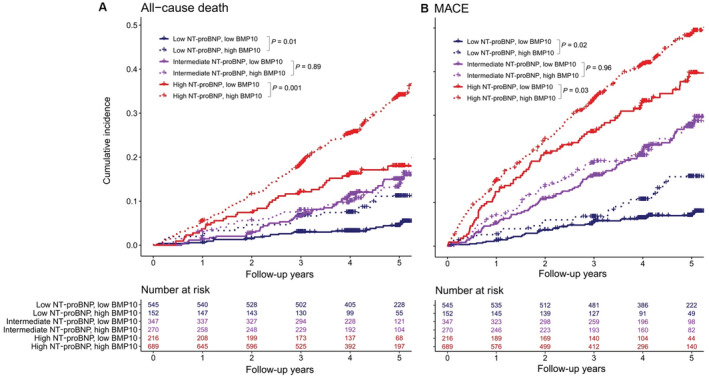
Cumulative incidence per follow‐up years for all‐cause death (A) and major adverse cardiovascular events (MACE; B) stratified by NT‐proBNP (N‐terminal prohormone of B‐type natriuretic peptide) and BMP10 (bone morphogenetic protein 10) categories. MACE is a composite of heart failure hospitalization, cardiovascular death, stroke, systemic embolism, and myocardial infarction. NT‐proBNP categories: low (<300 ng/L), intermediate (300–900
ng/L), intermediate (300–900 ng/L), high (>900
ng/L), high (>900 ng/L). BMP10 categories according to median: low (<2.247
ng/L). BMP10 categories according to median: low (<2.247 ng/mL), high (≥2.247
ng/mL), high (≥2.247 ng/mL). P values were calculated by log‐rank test.
ng/mL). P values were calculated by log‐rank test.
DISCUSSION
This is the first study to report the prognostic value of BMP10 for all‐cause death and MACE in patients with AF. The main findings of the analysis are the following: (1) in a large prospective cohort of well‐characterized and clinically stable patients with AF at study entry, BMP10 was strongly predictive of all‐cause death and MACE; (2) the predictive performance of BMP10 for all‐cause death and MACE was similar to that of the natriuretic peptide NT‐proBNP; and (3) however, BMP10 provided additional prognostic information in patients with AF and either low or high NT‐proBNP concentration. The latter could be helpful to specifically identify patients with AF at higher risk for worse outcomes.
Because of the increasing AF incidence and high risk for adverse outcomes, refined risk stratification tools are needed. 3 , 42 In addition to clinical risk stratification, a biomarker that is easily quantified in a simple blood test could substantially improve risk assessment in patients with AF. 3 , 9 , 10 , 11 , 12 Different biomarkers such as NT‐proBNP or cTn (cardiac troponin) were previously shown to improve the prediction of stroke and death in patients with AF. 13 , 43 , 44 , 45 Moreover, a biomarker score‐guided management of patients with AF for the reduction of stroke and death is currently evaluated in an ongoing randomized controlled trial (ABC‐AF [Age, Biomarkers, Clinical History‐AF] Study, NCT03753490).
In a meta‐analysis, elevated concentrations of the cardiac biomarkers cTnT (troponin‐T) and cTnI (troponin‐I) in patients with AF were shown to be independently associated with all‐cause death and MACE. 46 However, troponin is also influenced by other factors such as renal function, sepsis, blood pressure, and AF itself. 47 , 48
NT‐proBNP correlates with cardiac wall stress and is currently the preferred cardiac biomarker in patients with heart failure, both for diagnostic and prognostic use. 8 In a meta‐analysis, NT‐proBNP was associated with all‐cause death and MACE in patients with AF as well. 9 Our analysis in a cohort of patients with AF confirms this association of NT‐proBNP concentration with all‐cause death and MACE in multivariable adjusted Cox proportional hazard models. Nevertheless, NT‐proBNP is not atrial specific, and its performance to predict heart failure has been shown to be impaired in patients with AF compared with those in sinus rhythm. 8 , 49 , 50 Therefore, the concept of measuring an atrial‐specific biomarker for risk prediction in patients with AF is appealing.
BMP10 is a heart‐restricted biomarker with high atrial specificity. 19 , 20 , 21 , 22 , 23 , 51 Reyat et al illustrated the distinct relationship between BMP10 and PITX2 in patients with AF, and identified BMP10 as predictor of AF recurrence after ablation. 19 Common gene variants on chromosome 4q25, where PITX2 is located, are associated with AF and AF recurrence. 24 , 25 , 52 , 53 , 54 , 55 , 56 , 57 Using unbiased RNA sequencing, qualitative polymerase chain reaction, and Western blotting, the authors showed that BMP10 is a PITX2‐repressed protein essentially restricted to the atria. 19 They demonstrated that PITX2 and BMP10 were independently associated with AF recurrence. 19 Because PITX2 sampling requires cardiac tissue, BMP10 may serve as a suitable surrogate of PITX2 in the clinical routine, because it is a secreted protein measurable in peripheral blood samples. 19 , 35 , 58 However, little is known as to whether BMP10, by virtue of its high atrial specificity, may be used for refined risk prediction for other adverse outcomes in addition to AF recurrence after ablation. 19 , 34
In our study, a lower BMP10 concentration was associated with younger age, male sex, sinus rhythm, paroxysmal AF, and a lower CHA2DS2‐VASc score. In agreement with our results, a recent study found lower BMP10 concentration in sinus rhythm compared with AF as well. 59 In our multivariable adjusted Cox proportional hazard models, BMP10 remained strongly associated with all‐cause death, MACE, hospitalization for heart failure, and cardiovascular death. In line with our findings, a previous smaller analysis demonstrated an association of BMP10 and hospitalizations for cardiovascular causes in patients with AF. 34 We found no strong association of BMP10 with myocardial infarction. For stroke/systemic embolism, the HR of BMP10 was elevated in the age‐ and sex‐adjusted model, but this association did not persist after multivariable adjustment. Despite the atrial specificity of BMP10 and previous studies that found PITX2 to be associated with stroke, 60 , 61 , 62 , 63 , 64 , 65 BMP10 was not predictive of the AF‐specific outcome stroke/systemic embolism in our study. However, considering the high rate of oral anticoagulation and the very low number of events in our cohort, larger studies are needed to clarify this point.
Given the lack of interaction for the vast majority of the tested variables in our subgroup analysis, BMP10 appears to be a robust predictor for all‐cause death and MACE. The possible interaction with diabetes for all‐cause death needs further evaluation. In a small study, low BMP10 concentration was predictive of diabetes, but this association proved weak after adjustment for body mass index and sex. 35
To assess the additional value of BMP10 on top of a well‐established biomarker, we compared its prognostic performance for all‐cause death and MACE to that of NT‐proBNP and also analyzed the combination of both biomarkers. As single tests, the prognostic performance of BMP10 and NT‐proBNP was comparable and it remained similar when including both biomarkers in the same model. However, when grouping patients according to clinically used NT‐proBNP categories, there seems to be potential for better differentiation when based on BMP10.
In particular, the Kaplan–Meier curves in the low (<300 ng/L) and high (>900
ng/L) and high (>900 ng/L) NT‐proBNP groups split strongly between patients with high or low BMP10 concentration, whereas in patients with intermediate NT‐proBNP there is no such split. This suggests conditional value for BMP10 in refined risk stratification in groups of clinically stable patients with AF.
ng/L) NT‐proBNP groups split strongly between patients with high or low BMP10 concentration, whereas in patients with intermediate NT‐proBNP there is no such split. This suggests conditional value for BMP10 in refined risk stratification in groups of clinically stable patients with AF.
From a pathophysiological perspective, the different origin and function of BMP10 compared with NT‐proBNP also provide potential to differentiate the various AF phenotypes, with possibilities to guide disease‐ and stage‐specific therapy strategies. 66 For example, BMP10 has been described as a cardioprotective contributor to maintain normal vascular tone and endothelial function. 67 Specifically, the heterodimer of circulating BMP10 (atrium) with BMP9 (liver) act on vascular smooth muscle cells and thus the vascular tone and blood pressure. 67 BMP10 has also been described to preserve cardiac function by a dual activation of Smad and STAT3 (Signal transducer and activator of transcription 3) pathways and inhibition of cardiomyocyte death and cardiac fibrosis. 68 In a recent mouse model of experimental heart failure, the administration of recombinant BMP10 alleviated the cardiomyocyte disorder. 69
The role of BMP10 in AF and its complications has yet to be further determined. Research is needed to clarify the potential of BMP10 for targeted therapy approaches and whether the BMP10 pathway provides a safe opportunity to modulate vascular smooth muscle cell contractility and atrial remodeling in AF. Moreover, prospective trials using BMP10 for management and therapy decisions are needed to answer the question whether adverse outcomes in patients with AF predicted by BMP10 are preventable or could be reduced.
Limitations and Strengths
Our study had several limitations and strengths. We determined blood concentrations of BMP10 and NT‐proBNP only at study enrolment. Biomarker concentration immediately before and after the occurrence of an acute adverse event were not measured. Furthermore, no distinct BMP10 cutoff concentration is known so far, and the decision to allocate according to quartiles could be challenged. In our study population, there was a striking predominance of male patients. This might be partly explained as male sex is a known risk factor for AF. 3 Additionally, most study participants were of European origin. Both facts may limit the generalizability of our findings. In addition, our findings apply only to patients with AF and the predictive value of BMP10 in patients without AF remains to be addressed. Finally, this observational study provided only associative evidence, and residual confounding is possible despite multivariable adjustment.
A main strength of our study was the large sample size of patients with well‐characterized AF and clinical outcomes. Blood sampling was performed with simultaneous rhythm documentation (5 minutes 16‐lead ECG). This is important because BMP10 seems to be rhythm dependent and thus we were able to adjust for baseline rhythm in our models.
59
Blood concentrations of BMP10 and NT‐proBNP were analyzed centrally under equal conditions and using the same protocol. Furthermore, all clinical outcome events were adjudicated by an event committee using precise definitions.
minutes 16‐lead ECG). This is important because BMP10 seems to be rhythm dependent and thus we were able to adjust for baseline rhythm in our models.
59
Blood concentrations of BMP10 and NT‐proBNP were analyzed centrally under equal conditions and using the same protocol. Furthermore, all clinical outcome events were adjudicated by an event committee using precise definitions.
CONCLUSIONS
In clinically stable patients with AF, BMP10 is strongly associated with all‐cause death and MACE during long‐term follow‐up. In patients with low and high NT‐proBNP concentration, BMP10 seems to add to the prognostic performance of NT‐proBNP. Whether BMP10 may be useful to manage and guide therapies in patients with AF has to be shown in prospective clinical trials.
Sources of Funding
This work was supported by grants provided by the Swiss National Science Foundation (grant numbers 33CS30_148474, 33CS30_177520, 32473B_176178, and 32003B_197524), the Swiss Heart Foundation, the Foundation for Cardiovascular Research Basel, and the University of Basel. BMP10 and NT‐proBNP were measured free of charge by Roche Diagnostics.
Disclosures
J. H. Beer received grants from the Swiss National Foundation of Science, the Swiss Heart Foundation, and the Kardio foundation, and he received grant support and consultancy fees to the institution from Bayer, Sanofi, and Daichii. S. Blum reports research grants from the Swiss National Science Foundation, the Gottfried & Julia Bangerter‐Rhyner Foundation, and the Mach‐Gaensslen Foundation outside the submitted work. L. H. Bonati received grants from the Swiss National Science Foundation, Swiss Heart Foundation, and University of Basel; unrestricted research grant from AstraZeneca; consultancy or advisory board fees or speaker's honoraria from Amgen, Bayer, Bristol‐Myers Squibb, and Claret Medical; and travel grants from AstraZeneca and Bayer. M. Bossard received speaker and/or consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sanofi, Mundipharma, and Vifor. D. Conen received consultancy fees from Roche Diagnostics and speaker fees from Servier and BMS/Pfizer. G. Conte received a research grant from the Swiss National Science Foundation, speaker fees from Boston Scientific, and investigator‐initiated research grants from Boston Scientific. R. Kobza received institutional grants from Abbott, Biosense Webster, Boston, Biotronik, Medtronik, and Sis‐Medical. Consultant for Biosense Webster and Biotronik. M. Kühne reports personal fees from Bayer, Böhringer Ingelheim, Pfizer BMS, Daiichi Sankyo, Medtronic, Biotronik, Boston Scientific, Johnson & Johnson, and Roche and grants from Bayer, Pfizer, Boston Scientific, BMS, Biotronik, Daiichi Sankyo. C. Müller received research support from the Swiss National Science Foundation, Swiss Heart Foundation, KTI, University Hospital Basel, University of Basel, Abbott, Astra Zeneca, Beckman Coulter, Brahms, Idorsia, Novartis, LSI Medience Corporation, Ortho Clinical Diagnostics, Quidel, Roche, Siemens, Singulex, and Sphingotec, all outside the current work and paid to the institution, as well as speaker honoraria/consulting honoraria from Abbott, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, BMS, Idorsia, Novartis, Osler, Roche, Sanofi, and Singulex, all outside the current work and paid to the institution. S. Osswald received research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, Foundation for CardioVascular Research Basel, and Roche and educational and speaker office grants from Roche, Bayer, Novartis, Sanofi, AstraZeneca, DaiichiSankyo, and Pfizer. O. Pfister received speaker/consulting honoraria or travel support from Astra Zeneca, Bayer, Boehringer‐Ingelheim, Daiichi Sankyo, Novartis, Pfizer, Sanofi, and Vifor Pharma, all for work outside the submitted study. T. Reichlin received research grants from the Swiss National Science Foundation and the Swiss Heart Foundation, all for work outside the submitted study; speaker/consulting honoraria or travel support from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense‐Webster, Biotronik, Boston‐Scientific, Daiichi Sankyo, Medtronic, Pfizer‐BMS, and Roche, all for work outside the submitted study; and support for his institution's fellowship program from Abbott/SJM, Biosense‐Webster, Biotronik, Boston‐Scientific, and Medtronic for work outside the submitted study. C.S. Zuern reports advisory board and speaker fee from Vifor Pharma. The remaining authors have no disclosures to report.
Notes
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028255
For Sources of Funding and Disclosures, see page 13.
References
Articles from Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease are provided here courtesy of Wiley
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/144504046
Article citations
Translation of pathophysiological mechanisms of atrial fibrosis into new diagnostic and therapeutic approaches.
Nat Rev Cardiol, 23 Oct 2024
Cited by: 0 articles | PMID: 39443702
Review
BMP10 reflects pre-capillary pulmonary hemodynamics: association of biomarkers and hemodynamic parameters in pulmonary hypertension.
Clin Res Cardiol, 19 Sep 2024
Cited by: 0 articles | PMID: 39297942
Atrial cardiomyopathy revisited-evolution of a concept: a clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS).
Europace, 26(9):euae204, 01 Aug 2024
Cited by: 1 article | PMID: 39077825 | PMCID: PMC11431804
Atrial fibrillation burden: a new outcome predictor and therapeutic target.
Eur Heart J, 45(31):2824-2838, 01 Aug 2024
Cited by: 1 article | PMID: 38953776 | PMCID: PMC11328870
Review Free full text in Europe PMC
Blood-based cardiometabolic phenotypes in atrial fibrillation and their associated risk: EAST-AFNET 4 biomolecule study.
Cardiovasc Res, 120(8):855-868, 01 Jul 2024
Cited by: 0 articles | PMID: 38613511 | PMCID: PMC11218688
Go to all (9) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (2 citations) ClinicalTrials.gov - NCT02105844
- (1 citation) ClinicalTrials.gov - NCT03753490
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Total NT-proBNP, a novel biomarker related to recurrent atrial fibrillation.
BMC Cardiovasc Disord, 21(1):553, 19 Nov 2021
Cited by: 10 articles | PMID: 34798808 | PMCID: PMC8603582
Bone morphogenetic protein 10: a novel risk marker of ischaemic stroke in patients with atrial fibrillation.
Eur Heart J, 44(3):208-218, 01 Jan 2023
Cited by: 14 articles | PMID: 36380569 | PMCID: PMC9839419
Effects of Atrial Fibrillation Screening According to N-Terminal Pro-B-Type Natriuretic Peptide: A Secondary Analysis of the Randomized LOOP Study.
Circulation, 147(24):1788-1797, 15 Apr 2023
Cited by: 6 articles | PMID: 37061802 | PMCID: PMC10249603
The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all-cause mortality in patients who undergo noncardiac surgery.
Cochrane Database Syst Rev, 12:CD013139, 21 Dec 2021
Cited by: 20 articles | PMID: 34931303 | PMCID: PMC8689147
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Swiss National Science Foundation (1)
Swiss Atrial Fibrillation Cohort Study
Stefan Osswald, University of Basel
Grant ID: 148474

 1
,
2
,
*
and the Swiss‐AF Investigators
†
1
,
2
,
*
and the Swiss‐AF Investigators
†


