Abstract
Free full text

Vacuole Acidification Is Not Required for Survival of Salmonella enterica Serovar Typhimurium within Cultured Macrophages and Epithelial Cells
Abstract
Phagosome acidification is an important component of the microbicidal response by infected eukaryotic cells. Thus, intracellular pathogens that reside within phagosomes must either block phagosome acidification or be able to survive at low pH. In this work, we studied the effect of phagosomal acidification on the survival of intracellular Salmonella enterica serovar Typhimurium in different cell types. Bafilomycin A1, a specific inhibitor of the vacuolar proton-ATPases, was used to block acidification of salmonella-containing vacuoles. We found that in several epithelial cell lines, treatment with bafilomycin A1 had no effect on intracellular survival or replication. Furthermore, although acidification was essential for Salmonella intracellular survival in J774 cultured macrophages, as reported previously (13), it is not essential in other macrophage cell lines. These data suggest that vacuolar acidification may play a role in intracellular survival of salmonellae only under certain conditions and in specific cell types.
Salmonella spp. are gram-negative facultative intracellular pathogens which infect a wide variety of animals, including humans (6). Salmonella enterica serotype Typhimurium is one of the leading causes of food poisoning in human beings and of particular concern given the recent emergence of a multidrug-resistant strain, DT104 (1). In salmonellae, two distinct virulence-associated type III secretion systems are located within Salmonella pathogenicity islands 1 and 2 (SPI1 and SPI2) (7, 14). The type III systems translocate distinct sets of proteins into the host cell (10). These effectors confer on serovar Typhimurium the ability to invade mammalian cells and, once inside, to survive and replicate. Intracellular salmonellae remain in the phagosome, or salmonella-containing vacuole (SCV), the biogenesis of which is regulated by the bacteria so that interactions with the endocytic pathway are limited and fusion with the terminal lysosome is blocked (8, 11, 15). Despite these restrictions on biogenesis, the SCV can become acidified, and it has been shown that acidification is necessary for the survival of salmonellae inside cultured macrophages (13). Based on this finding, it was postulated that acidification was essential for intracellular induction of specific survival genes in salmonellae. Alternatively, secretion rather than expression may be regulated. Indeed, it has recently been shown that the secretion of at least one type III effector is pH dependent (3). In this study, we compared the effect of blocking vacuolar acidification in different cell lines to further investigate the role of acidification in intracellular survival.
Vacuolar proton-ATPase activity plays no role in intracellular survival of salmonellae in epithelial cell lines.
Previously it has been shown that vacuole acidification is required for survival of salmonellae in macrophages (13). However, invasion of epithelial cells also plays a vital role in salmonella pathogenesis, and we wanted to investigate whether there was a similar requirement for acidification in these cells. Invasion assays were carried out essentially as previously described (15). Briefly, the day before infection, cells were seeded in 24-well plates at 1 × 105 to 5 × 105 cells per well in Earle's minimal essential medium supplemented with 10% fetal bovine serum. To block endosome acidification, cells were treated with 1 μM bafilomycin A1 (BAF) (Kamiya Biomedical Company, Seattle, Wash.) for 30 min prior to invasion. Virulent S. enterica serovar Typhimurium strain 14028s was grown in Luria-Bertani (LB) broth with shaking overnight at 37°C and then subcultured (1:33 dilution) for 2.5 h. Bacteria were then washed by centrifugation, resuspended in Earle's buffered salt solution, and added to cells at a multiplicity of infection of ~10 for 10 min. Extracellular bacteria were then removed by washing three times with phosphate-buffered saline (PBS). Gentamicin (50 μg/ml) was added 20 min after invasion; when necessary, the concentration was decreased to 5 μg/ml at 2 h postinfection. At the indicated times, cells were washed twice with PBS and lysed in 1 ml of 1% Triton X-100–0.1% sodium dodecyl sulfate (SDS) in PBS. The lysate was serially diluted in PBS and plated onto LB plates, and colonies were counted the next day. Invasion assay results are expressed relative to the number of CFU recovered at the earliest time point in untreated cells. Three epithelial cell lines, HeLa, MDCK, and Henle (Intestine-407), were used in these experiments. Bacterial survival was followed for 6 h in all experiments to allow bacterial replication, which typically occurred after an initial lag phase of approximately 4 h (Fig. (Fig.1).1). While BAF treatment appears to slightly increase the number of intracellular bacteria in MDCK and Henle cells at 6 h, the differences are not significant (Henle, t = 1.03, P = 0.36; MDCK, t = 1.84, P = 0.13). Thus, acidification apparently plays no role in either invasion or intracellular survival or replication in these cell lines.
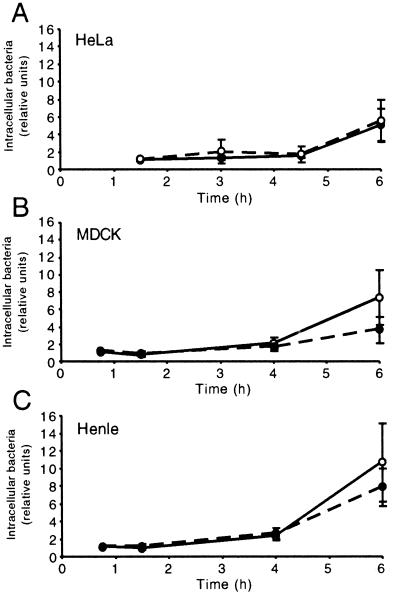
Intracellular survival of salmonellae in epithelial cells is acidification independent. Epithelial cells were incubated in the presence of salmonellae for 10 min and then further incubated in the presence of gentamicin as indicated. Intracellular survival is expressed relative to the number of CFU recovered from untreated infected cells at the earliest time point. Solid circles, untreated cells: open circles, BAF-treated cells. The standard deviations obtained from three independent experiments are indicated.
Vacuolar proton-ATPase activity is not necessarily essential for intracellular survival of salmonellae in macrophage cell lines.
The above results indicate that vacuole acidification does not play a role in survival of salmonellae in epithelial cells. In order to directly compare our results with those of Rathman et al. (13), we next carried out similar experiments in three different mouse macrophage cell lines, J774A.1, RAW264.7, and LM-1. LM-1 is a stable mouse macrophage cell line which has recently been described (G. Forget, K. A. Siminovitch, S. Brochu, S. Rivest, D. Radzioch, and M. Olivier, submitted for publication). All these cells were grown in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum, and invasion was carried out as described above. In J774A.1, intracellular bacteria did not replicate over the time course of the experiment (up to 6 h); indeed, the CFU recovered decreased with time (Fig. (Fig.2A,2A, inset). As expected, we found that inhibition of vacuolar acidification by BAF treatment significantly reduced the survival of intracellular bacteria (at 6 h, t = 5.68, P = 0.015) (Fig. (Fig.2A).2A). To confirm that the BAF effect was due to reduced survival of intracellular bacteria rather than decreased uptake, we performed an experiment in which the cells were solubilized immediately after 10 min of invasion (Fig. (Fig.2B).2B). BAF had no significant effect on invasion efficiency. This result was confirmed using a previously described immunofluorescent assay (15) which allows differentiation of intracellular and extracellular bacteria (not shown).
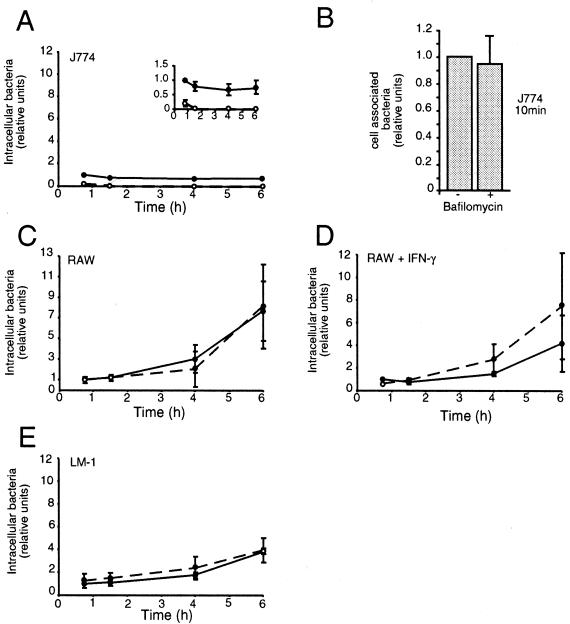
Intracellular survival of salmonellae in cultured macrophages. J774 cells (A and B), RAW cells (C), RAW cells treated with gamma interferon (IFN-γ) (D), or LM-1 cells (E) were incubated in the presence of salmonellae for 10 min and then further incubated without extracellular bacteria as indicated. Intracellular survival is expressed relative to the number of CFU recovered from untreated infected cells at the earliest time point. Solid circles, untreated cells; open circles, BAF-treated cells. The standard deviations obtained from three independent experiments are indicated.
Surprisingly, when similar experiments were carried out in RAW264.7 (Fig. (Fig.2C),2C), no requirement for acidification could be detected (at 6 h, t = 0.14, P = 0.54). This is in direct contrast to the findings of Rathman et al. (13), and we considered that a possible reason for this was the state of activation of the cells. For this reason, we repeated the experiment using RAW cells which had been activated with 200 U of recombinant mouse gamma interferon (MG-FIN; Genzyme Diagnostics, Cambridge, Mass.) for 18 to 24 h prior to infection (Fig. (Fig.2D).2D). However, we could still detect no significant difference in the intracellular survival of salmonellae (at 6 h, t = 1.07, P = 0.82). To further investigate the requirement for acidification in macrophages, we next repeated the experiments using LM-1 cells (Fig. (Fig.2E).2E). The results in these cells were very similar to those in the RAW cells, and there was no significant difference in intracellular survival in treated versus untreated cells (at 6 h, t = 0, P = 0.5).
BAF treatment abolishes vacuolar acidification in infected cells.
To control for abolishment of vacuolar acidification under the conditions used in these experiments, we used acridine orange, a cell-permeating probe for acidified organelles (12, 13). For these experiments cells were grown on coverslips (12 mm), stained for 5 min with 5 μg (16.5 μM) of acridine orange hydrochloride (Sigma, St. Louis, Mo.) per ml, and visualized for fluorescence using a longpass fluorescein isothiocyanate filter (excitation, 450 to 490 nm; emission, ≥520 nm). The fluorescence emitted after excitation at 450 to 490 nm indicated the pH of the vacuoles. Figure Figure33 illustrates results for J774A.1 (A and B), RAW264.7 (C and D), and Henle (E and F) cells. As expected, control cells emitted a red-orange fluorescence due to the naturally acidic nature of intracellular vacuoles, including endosomes and lysosomes. In BAF-treated cells, an intense green fluorescence was detected due to the lack of acidified intracellular organelles, confirming the effectiveness of vacuolar ATPase inhibition. Similar results were obtained for the other cell lines tested (data not shown).
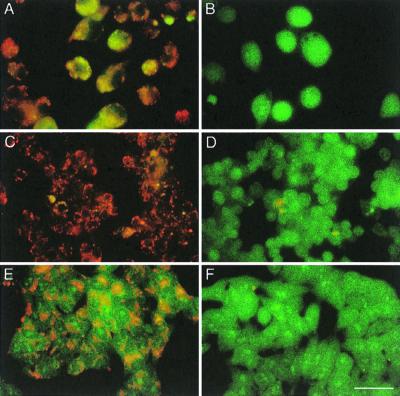
Effect of BAF on vacuolar acidification after staining with acridine orange. BAF-treated (B, D, and F) or control untreated cells (A, C, and E) were incubated for 5 min with acridine orange (5 μg/ml) in PBS and then visualized using a Zeiss Axiophot microscope with an ×65 oil immersion lens. (A and B) J774 cells. (C and D) RAW cells. (E and F) Henle cells. Bar, 20 μm.
Our results indicate that vacuole acidification is not essential for intracellular survival of salmonellae in all cell types. Certainly in epithelial cells we could detect no requirement for acidification, although in macrophage cells the results were more ambiguous. It is now clear that, as previously suggested (13), survival within host cells requires the induction of specific survival genes (2, 4, 9, 16). However, these genes may also be induced extracellularly under certain conditions, thus bypassing the need for intracellular induction. Slight differences in the growth and preparation of bacteria may account for the differences seen in our experiments. For example, it has been shown that the activity of both type III secretion systems of S. enterica is affected by pH (3, 5). Our results clearly show that vacuole acidification is not required per se for intracellular survival of salmonellae and illustrate the complexity of the interactions that occur between host cell and pathogen. Further studies, including comparison of bacterial genes induced by low pH or intracellularly, will be required to dissect the real role of acidification in intracellular survival of salmonellae.
Acknowledgments
This work was supported by the Medical Research Council of Canada (grant MT10551). O.S.-M. was supported by an EMBO postdoctoral fellowship. B.B.F. is an MRC Scientist and a Howard Hughes International Research Scholar.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.68.9.5401-5404.2000
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/68/9/5401.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
How the PhoP/PhoQ System Controls Virulence and Mg2+ Homeostasis: Lessons in Signal Transduction, Pathogenesis, Physiology, and Evolution.
Microbiol Mol Biol Rev, 85(3):e0017620, 30 Jun 2021
Cited by: 49 articles | PMID: 34191587 | PMCID: PMC8483708
Review Free full text in Europe PMC
Phenotypic and Genetic Comparison of a Plant-Internalized and an Animal-Isolated Salmonella Choleraesuis Strain.
Microorganisms, 9(8):1554, 21 Jul 2021
Cited by: 1 article | PMID: 34442630 | PMCID: PMC8398053
Gene expression profiling in live attenuated Edwardsiella tarda vaccine immunized and challenged zebrafish: insights into the basic mechanisms of protection seen in immunized fish.
Dev Comp Immunol, 40(2):132-141, 19 Feb 2013
Cited by: 26 articles | PMID: 23434462
Global transcription analysis of vaccinated channel catfish following challenge with virulent Edwardsiella ictaluri.
Vet Immunol Immunopathol, 146(1):53-61, 06 Feb 2012
Cited by: 3 articles | PMID: 22365332
Human genome-wide RNAi screen for host factors that modulate intracellular Salmonella growth.
PLoS One, 7(6):e38097, 11 Jun 2012
Cited by: 12 articles | PMID: 22701604 | PMCID: PMC3372477
Go to all (28) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Acidification of phagosomes containing Salmonella typhimurium in murine macrophages.
Infect Immun, 64(7):2765-2773, 01 Jul 1996
Cited by: 176 articles | PMID: 8698506 | PMCID: PMC174137
Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages.
J Biol Chem, 265(34):21099-21107, 01 Dec 1990
Cited by: 123 articles | PMID: 2147429
Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages.
Infect Immun, 67(8):4041-4047, 01 Aug 1999
Cited by: 158 articles | PMID: 10417172 | PMCID: PMC96697
Salmonella enterica: living a double life in epithelial cells.
Curr Opin Microbiol, 23:23-31, 11 Nov 2014
Cited by: 54 articles | PMID: 25461569
Review




