Abstract
Free full text

Possible heterogeneity of initial pancreatic islet beta-cell autoimmunity heralding type 1 diabetes
Abstract
The etiology of type 1 diabetes foreshadows the pancreatic islet beta-cell autoimmune pathogenesis that heralds the clinical onset of type 1 diabetes. Standardized and harmonized tests of autoantibodies against insulin (IAA), glutamic acid decarboxylase (GADA), islet antigen-2 (IA-2A) and ZnT8 transporter (ZnT8A) allowed children to be followed from birth until the appearance of a first islet autoantibody. In the Environmental Determinants of Diabetes in the Young (TEDDY) study, a multicenter (Finland, Germany, Sweden and the US) observational study, children were identified at birth for the type 1 diabetes high risk HLA haplogenotypes DQ2/DQ8, DQ2/DQ2, DQ8/DQ8 and DQ4/DQ8. The TEDDY study was preceded by smaller studies in Finland, Germany, Colorado, Washington and Sweden. The aims were to follow children at increased genetic risk to identify environmental factors that trigger the first-appearing autoantibody (etiology) and progress to type 1 diabetes (pathogenesis). The larger TEDDY study found that the incidence rate of the first-appearing autoantibody was split into two patterns. IAA first peaked already during the first year of life and tapered off by 3–4 years of age. GADA first appeared by 2–3 years of age to reach a plateau by about 4 years. Prior to the first-appearing autoantibody, genetic variants were either common or unique to either pattern. A split was also observed in whole blood transcriptomics, metabolomics, dietary factors and exposures such as gestational life events and early infections associated with prolonged shedding of virus. An innate immune reaction prior to the adaptive response cannot be excluded. Clarifying the mechanisms by which autoimmunity is triggered to either insulin or GAD65 is key to uncovering the aetiology of autoimmune type 1 diabetes.
Introduction
Autoimmune type 1 diabetes (T1D) affects an increasing number of individuals throughout the world. An estimated 9 million individuals worldwide survive only through the daily administration of insulin [1]. Developing countries are witnessing an increasing number of patients. The disease is chronic, and insulin replacement therapy has been practiced for 100 years. There is no cure. Diffuse symptoms, including increased thirst, frequent urination and fatigue escalate over time prior to the diagnosis and lead over time to weight loss, worsening of polydipsia, polyuria, and finally to ketoacidosis, which is life threatening. The diagnosis at this very late stage is often made in the emergency room. Although the final clinical diagnosis may be dramatic, it is often not understood by the patient and the family that the diagnosis is the end of a prodrome, lasting months to years, of an autoimmune disease without symptoms specifically targeting only the pancreatic islet beta cells.
Already at the time of clinical diagnosis the beta-cell specific autoimmune disease is chronic. The prospect or hope of a cure is reduced, as a major proportion of the pancreatic islet beta cells have been destroyed by the immune system. After initiation of insulin replacement therapy, the residual beta cells are also lost. Beta cell replacement therapy with isolated pancreatic islets has met with limited success, often due to recurrence of disease [2, 3]. The immune system has a life-long memory of the beta cells as foreign. It is hard to imagine that a cure, let alone prevention, will be accomplished before the aetiology and pathogenesis are fully understood. Longitudinal research in children at increased genetic risk for T1D was therefore initiated at birth and conducted until the research participant was diagnosed with diabetes [4–9]. Studies initiated in Finland [4], Germany [5], Colorado [10], Sweden [7] and Washington [11] aimed to follow newborns at increased genetic risk for T1D but none of the studies were large enough and the protocols were not uniform. The TEDDY study, supported by the National Institutes of Health, used a common protocol and had enough statistical power to identify environmental factors that would trigger islet autoimmunity and show progression to the clinical onset of T1D [8, 12]. These longitudinal natural history studies have been possible because T1D biomarkers have evolved over time from research biomarkers into specialized enhancement biomarkers in clinical prevention trials [13, 14].
Pathogenesis – the origination and development of a disease (Merriam Webster)
The blood-based biomarkers for beta-cell autoimmunity include autoantibodies against four beta-cell proteins: insulin (IAA), glutamic acid decarboxylase 65 (GADA), islet antigen-2 (IA-2A) and ZnT8 transporter (ZnT8A). Investigator-initiated islet autoantibody standardization efforts effectively compare assay performance between laboratories— academic as well as industry—to develop common standards [15–18].The autoimmune character of the pathogenesis is well documented through the screening of first-degree relatives who have a distinct increased risk for T1D compared to the general population [19–22]. The pathogenesis has been well summarized in staging of the disease pathogenesis in three stages [23]. Stage 1 is presymptomatic and defined as the presence of two or more beta-cell autoantibodies with normoglycemia. Stage 2 is also presymptomatic and defined as the presence of two or more beta-cell autoantibodies with dysglycemia. Stage 3 is defined as onset of symptomatic disease.
The staging has proven useful in prevention trials, either including both stage 1 and 2 [24] or only stage 2 [25] research participants. However, the pathogenesis is markedly heterogenous as Stage 3 risk varies with age, autoantibody type and metabolic status [26]. The pathogenesis of beta-cell autoimmunity is effectively being dissected in parallel with efforts to screen both populations and first-degree relatives. However, the aetiology of the autoimmune T1D syndrome is far from understood. The aim of the present review is to uncover recent observations in the TEDDY study and in the preceding smaller studies in Finland [4],Germany [5], Colorado [10], Washington [11], Skåne in Sweden [7], South East Sweden [27] and Norway [28] to uncover aetiological factors and mechanisms that may explain the development of islet autoimmunity using beta-cell specific autoantibodies as the end-point biomarker.
It is often asked if islet autoantibodies are mere biomarkers of an on-going pathogenesis without contributing to the loss of islet beta cells. There is no information in vivo in humans that any of the four autoantibodies may confer direct beta-cytotoxic effects. Both insulin and GAD65 are intracellular proteins, as are ZnT8 and IA-2A. ZnT8 is a transmembrane protein in the beta granule, and during exocytosis it is possible that the intra-granule portion of the protein may show up on the cell surface to be recognized by an autoantibody. The recent identification of ZnT8 extracellular component may represent a cell surface target of humoral autoimmunity [29].
Etiology – the cause of a disease (Merriam Webster)
It was realized in the late 1970s that etiology research efforts at the time of clinical diagnosis will reveal information about the genetic, but not the environmental, aetiology. First, the demonstration of a strong association between HLA and T1D [30, 31], which was later confirmed by PCR-based rapid genotyping [6, 32] to reveal that about 90% of newly diagnosed T1D children were positive for the HLA haplotypes DR3-DQ2, DR4-DQ8, or both [33, 34]. In high-risk countries such as Finland and Sweden, more than 25% of the population have one or both of these haplotypes. The fact that these HLA types are necessary but not sufficient for stage 3 autoimmune T1D made it possible to identify high risk children already at birth. Second, using an early islet autoantibody test, it was reported in 1981 that the initiation of pathogenesis may precede the clinical onset of diabetes by several years [35]. Research into the aetiology of autoimmune T1D was made possible through the rapid HLA typing at birth and standardized beta-cell specific autoantibodies. While the first was used to identify high-risk individuals at birth, the beta-cell specific autoantibodies were used as the endpoint to find environmental determinants that explain a first appearing autoantibody.
The autoantibodies are robust biomarkers of the fact that the immune system has been triggered to react with specific autoantigens in the pancreatic islet beta cells. The timeline of events from the exposure of a trigger such as a virus to the subsequent series of cellular events that eventually results in the appearance of a first islet autoantibody is short. Ideally, research participants should have been observed and blood samples obtained on a weekly or biweekly schedule to allow the dissection of the series of events that results in autoantibodies (Figure 1). The first step is antigen-presentation (several cell types such as dendritic cells and macrophages are able to do this) on HLA Class II proteins to T cell receptors on CD4+ T cells. These cells through cytokine secretion and cell-to-cell interactions initiate the development of CD8+ T cells (cytotoxic to virus infected cells expressing virus peptides on HLA Class I as well as beta cells expressing autoantigen peptides on HLA Class I) or B cells producing autoantibodies. The first appearance of an islet autoantibody therefore serves as a surrogate biomarker of a trigger or environmental determinant(s) that can be made responsible for the series of events that lead to the appearance of an islet autoantibody in individuals with the genetic propensity for autoimmune T1D.
TEDDY -The Environmental Determinants of Diabetes in the Young
TEDDY is a prospective cohort study funded by the National Institutes of Health with the primary goal to identify environmental causes of T1D. It includes six clinical research centers—three in the US: Colorado, Georgia/Florida, and Washington, and three in Europe: Finland, Germany, and Sweden. Detailed study design and methods have been previously published [8, 36]. Written informed consent was obtained for all study participants from a parent or primary caretaker separately for genetic screening and participation in prospective follow-up. The study was approved by local Institutional Review Boards and is monitored by External Advisory Board formed by the National Institutes of Health.
The primary outcome was the development of persistent confirmed islet autoimmunity, which was assessed every 3 months. Persistent autoimmunity was defined by the presence of confirmed islet autoantibody (GADA, IA-2A, IAA and later also ZnT8A) on two or more consecutive visits. Date of persistent autoimmunity was defined as the draw date of the first sample of the two consecutive samples which deemed the child persistent confirmed positive for a specific autoantibody (or any autoantibody).
Data assets, data sharing and data infrastructure are all available along with some 150 reports published so far by the TEDDY Study Group (https://teddy.epi.usf.edu/research/).
First appearing islet autoantibodies and HLA
It was not clear from earlier studies to what extent an autoantibody would appear alone or together with other autoantibodies. Early data from children born to mothers or fathers with T1D suggested that IAA was an early appearing autoantibody [5]. However, detecting it in children born to mothers with T1D is complicated by the fact that transplacental transfer of both insulin antibodies (present because of insulin treatment) and autoantibodies may be detected in the offspring up until at least 9 months of age, sometimes beyond. The data from the MIDIA [28], the DIPP [37] and the TEDDY [38] studies would not be complicated in this respect, as 90% of the research participants did not have first degree relatives with T1D. In 315 DIPP children followed until 5 years of age, IAA was the first positive autoantibody in 180 (57%), GADA was the first in 107 (34%), and IA-2A in 28 (9%) [37]. The first TEDDY report on the 6-year incidence rate of islet autoantibodies showed that 549/8503 (6.5%) children had developed an islet autoantibody. Autoantibodies at 3- and 6 months of age were rare (0.1% and 0.2%, respectively). Of the 549, 43.7% had IAA first, 37.7% had GADA first, 13.8% had both GADA and IAA, 1.6% had IA-2A first and 3.1% had other combinations [38] (Table 1).
Table 1
Incidence rates of first appearing islet autoantibodies at follow-up in the TEDDY study.
| Autoantibodies | 2014 6 years n=8,503 [38] | 2016 6 years n=8,503 [42] | 2017 9 years n= 7,777 [42] | 2021 10 years n=8,502 [47] | 2022 12 years n= 8072 |
|---|---|---|---|---|---|
| Total | 549 (100%) | 598 (100%) | 736 (100%) | 499 (100%) | 888 (100%) |
| IAA first | 240 (44%) | 252 (43%) | 281 (38%) | 174 (34%) | 320 (36%) |
| GADA first | 207 (38%) | 226 (38%) | 316 (43%) | 272 (55%) | 394 (45%) |
| IA-2A first | 9 (2%) | 9 (2%) | 17 (2%) | 11 (2%) | 27 (3%) |
| ZnT8A first | TBD | TBD | TBD | TBD | TBD |
| GADA - IAA first | 76 (13%) | 81 (13%) | 104 (14%) | 29 (6%) | 101 (11%) |
| Other combinations | 17 (3%) | 21 (4%) | 18 (2%) | 13 (3%) | 46 (5%) |
TBD is to be determined.
The data indicate that the longer the core group of children (n=8676 were initially recruited between 2004–2010 and about 30% have withdrawn) have been followed, the stronger the pattern of IAA first being different from GADA first has remained. This is also illustrated by the incidence rate of respective autoantibody (Figure 2). The incidence rate suggests possible age dependence with an early appearance of IAA first that peaked at 1 year of age and declined sharply thereafter. GADA first began to appear at about 1 year of age to reach a plateau at 3–4 years of age and remaining elevated thereafter. Simultaneous IAA-GADA had the highest incidence rate at 1–3 years of age. Based on the pattern that emerged during follow up for an additional 6 years (Table 1), the data suggest that GADA first was becoming more prevalent compared to IAA first. The simultaneous detection of IAA-GADA increased as the children got older, indicating the shortcoming of the quarterly sampling in relation to the expected development of IgG antibodies after the initial trigger (Figure 1).
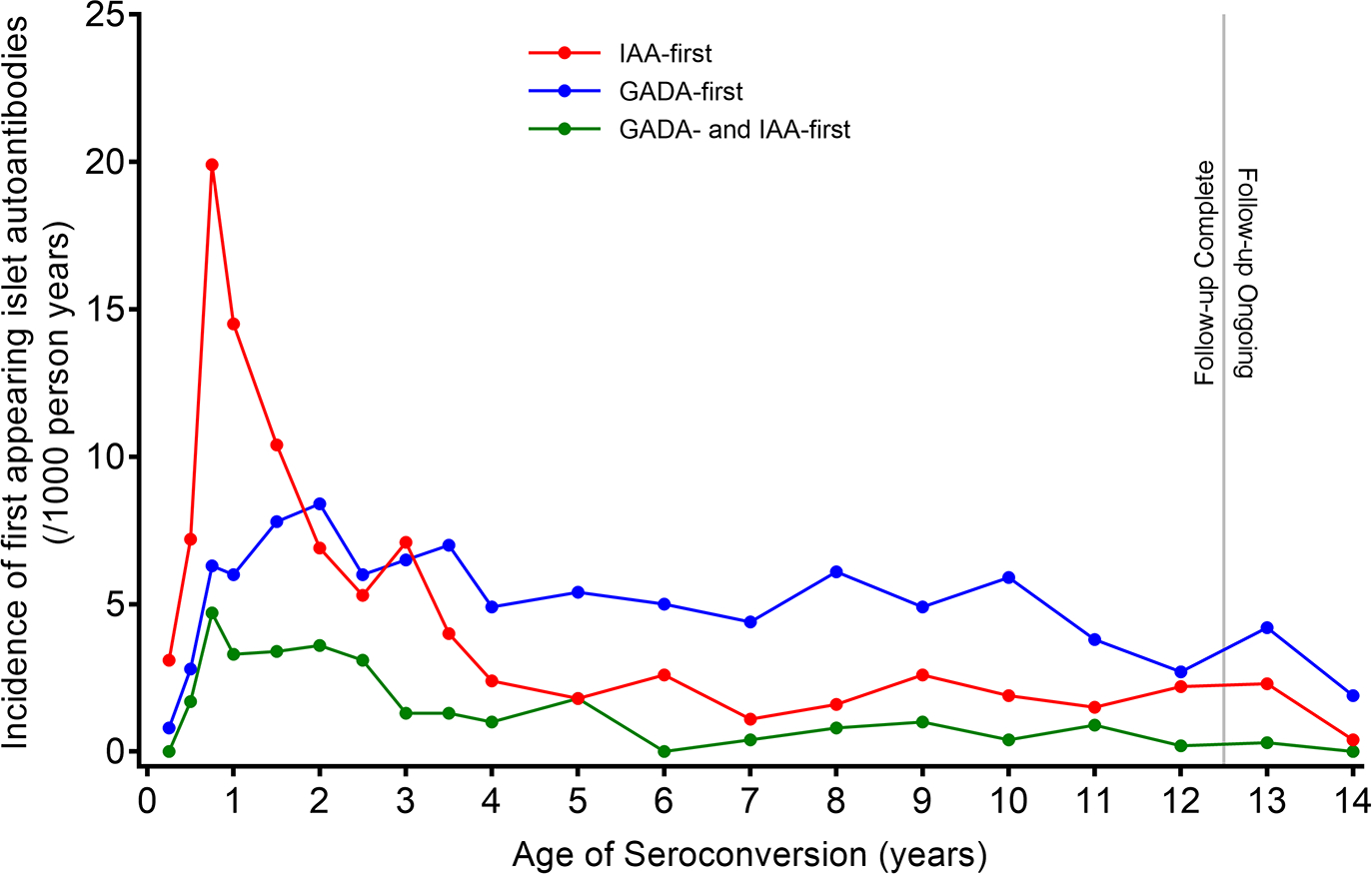
Incidence rate (new autoantibody/1000 person years) of IAA first (red), GADA first (blue) and IAA-GADA simultaneous (green).
Is it possible that an environmental trigger is able to initiate simultaneously an immune response to two different autoantigens? The data so far suggest two different conclusions: 1) GADA first is more common than IAA first in children with the HLA DR3-DQ2/DR3-DQ2 genotype [38, 39] and 2) GADA first is less common in DR4-DQ8/DR8-DQ4 children compared to IAA first [38–40]. It can therefore not be excluded that the well-known association between T1D and HLA DR3-DQ2, DR4-DQ8, or both, is secondary to a primary association, with the first-appearing islet autoantibody triggered by a yet unknown environmental factor. However, caution should be taken not to overinterpret these associations as it was found that autoantibodies against truncated GAD65 were associated with DR4-DQ8/DR4-DQ8 homozygosity [41]. It may contribute to the observation that GADA incidence rose with age and remained constant (Figure 2) and significant in those with HLA-DR4, but not in those withDR3/3 [42]. It will be crucial to uncover the mechanisms by which an environmental trigger will be restricted by HLA to induce an immune reaction to either insulin or GAD65, as it is well established that HLA DR-DQ-DP Class II molecules are key factors regulating the immune response to viruses, microbes, and parasites.
First appearing autoantibody in relation to non-HLA variants
The observation that IAA- and GADA first differ in incidence rate and in the association with HLA DR-DQ made it possible to test whether any of the many single nucleotide polymorphisms (SNP) known to be associated with autoimmune T1D [43, 44] were associated with the first appearing islet autoantibody [38, 45]. The susceptible A allele of the INS SNP was associated with 36% IAA first [38, 46, 47]. ERBB3 and SH2B3 were associated with the risk for both IAA- and GADA first (Table 2). ERBB3 is a member of the family of intracellular receptors of protein tyrosine kinases that control cell survival and proliferation. SH2B3 is a member of the SH2B family of adaptor proteins that influences signaling pathways mediated by Janus kinase and receptor tyrosine kinases important to lymphocyte differentiation moderating growth factor and cytokine receptor-mediated signaling [48].
Table 2.
Non-HLA gene polymorphisms associated with first appearing islet autoantibody
| Gene | Chromosome | rs ID | Coding | IAA first | GADA first | Reference |
|---|---|---|---|---|---|---|
| SH2B3 | 12q24.12 | rs3184504 | R262W | YES | YES | [38, 45] |
| PTPN22 | 1p13.2 | rs6679677 rs2476601 | coding | YES | YES | [38, 45, 46] |
| ERBB3 | 12q13.2 | rs2292239 | coding | YES | YES | [38, 45] |
| COBL | 7p12.1 | rs4948088 | intron | YES | NO | [47] |
| PRDM16 | 1p36.3 | rs28600853 | coding | YES | NO | [46] |
| INS | 11p15.5 | rs3842727 rs689 rs1004446 | Intergenic | YES | NO | [45–47, 53] |
| RBFOX1 | 16p13.3 | rs9934817 | coding | NO | YES | [46] |
| CLEC16A | 16p13.3 | rs12708716 | coding | NO | YES | [47] |
| IKZF4 | 12q13.2 | rs1701704 | coding | NO | YES | [53] |
PTPN22 was associated with an increased risk for both IAA- and GADA first [38], while COBL with a decreased risk for IAA first [45]. PTPN22—a member of the protein tyrosine phosphatase (PTP) superfamily—downregulates T cell signaling and proliferation [49], while COBL is thought to be important for B lymphocyte development. PRDM16—a zinc finger transcription factor gene on chromosome 1—was associated with IAA first, and RBFOX1 on chromosome 16 coding for an RNA binding protein—FOX1 homolog—was associated with GADA first [46]. CLEC16A was also associated with GADA first [47]. This gene codes for C-type lectin domain containing 16A and was found to be co-localized with an endoplasmic reticulum marker, but did not seem to be involved in T cell co-stimulation [50]. Similar to the genetic factors listed in Table 2, the mechanisms by which these factors contribute to the appearance of either IAA first, GADA first, or both, remains elusive.
It is noted that telomere length, important to cell survival, was not associated with either IAA- or GADA first [51]. In analyzing 3277 children diagnosed before 10 years of age in the Finnish Pediatric Diabetes register and deducing which autoantibody was first to appear, IKZF4 and ERBB3 were associated with GADA first, and INS, PTPN22 and PTPN2 with IAA first [52, 53].
Non-HLA genetic factors should prove useful when studying environmental exposures both during the first year of life with the high risk of IAA first, but also during childhood and adolescence associated with the high risk of GADA first (Figure 2). Similar dynamics of IAA-and GADA first were observed in the Finnish DIPP study [54, 55].
Linkage and association between the CTLA-4 gene on chromosome 2q33 and T1D were reported prior to genome-wide association studies [56]. The CTLA-4 T cell protein when bound to B-7 on antigen-presenting cells blocks T cells from killing other cells. The CTLA-4 T cell protein when bound to B-7 on antigen-presenting cells blocks T cells from killing other cells. Linkage and association between the CTLA-4 gene on chromosome 2q33 and T1D were reported prior to genome-wide association studies [57]. CTLA-4 has achieved clinical success as an immune regulator due to monoclonal antibodies targeting the CTLA-4 protein to restore anti-tumor T cell immunity. At the same time, the immune check point inhibitors induce immune-related adverse events including T1D [58]. CTLA-4 may contribute to the genetic etiology as the minor G allele of the CTLA-4 SNP was associated with risk of GADA first [38]. In an attempt to test whether environmental factors interact with non-HLA genetic factors and the risk for a first autoantibody, information about gestational respiratory infections was obtained from the TEDDY mothers at the very first visit [39]. About 45% of the mothers reported gestational respiratory infection. When gestational respiratory infections were not reported, HLA DR4-DQ8/DR8-DQ4 children had the highest incidence of IAA first across the HLA-DR-DQ genotypes. In contrast, gestational respiratory infections reduced risk of IAA first among HLA DR4-DQ8/DR8-DQ4 and CTLA-4 (AG, GG) children [39]. Similarly, gestational respiratory infection were associated with a moderately lower risk of GADA first but only in HLA DR3-DQ2/DR3-DQ2 children. The dichotomy of IAA first being increased without and decreased with gestational respiratory infection suggest prenatal in marking of the postnatal immune system development. Further investigations of the perinatal immune response to infections will be needed to disentangle the early development of autoimmunity.
Transcriptomics prior to the first appearing islet autoantibody
Using a predefined set of 225 IFN signature genes, expression of the IFN signature was increased in at-risk children before a first appearing autoantibody, but not in stage 3 T1D [59]. The expression was transient, temporally related to upper respiratory tract infections and by CD169 expression on CD14+ monocytes [59]. Peripheral blood collected at the quarterly TEDDY visits to extract total RNA [8] used purified RNA for complementary RNA (cRNA) and hydridization to Illumina Human HT-12 Expression Bead Chips [60]. Deconvolution to leukocyte subsets was used, and it was tested if expression signatures were unique to either IAA- or GADA first [60]. A dominant signature involving NK cells prior to seroconversion was common to both IAA- and GADA first, and there was a strong enrichment for B (naïve) cell-specific transcripts of kinases and transcription factors. In a preliminary study, blood mononuclear cell samples longitudinally collected from seven children who developed islet autoantibodies at a young age, transcripts—such as that of IL-32—were upregulated before the first autoantibody appeared. High IL-32 was contributed mainly by activated T cells and NK cells [61]. Further studies using RNAseq results in these TEDDY samples and extended deconvolution may provide further insights into gene expression signatures prior to seroconversion to either IAA- or GADA first.
Single-cell gene expression in islet autoantigen reactive CD4+ T cells before a first-appearing islet autoantibody showed expression of CCR6, IL21, TBX21, TNF, RORC, EGR2, TGFB1 and ICOS, in the absence of FOXP3, IL17 and other cytokines [62]. It was suggested that pre-T follicular helper cells developed prior to beta cell autoimmunity [62]. Further studies in infancy are needed to investigate the environmental and genetic etiological factors responsible for these CD4+ T cells. The NIDDK Central repository lists 243,007 frozen PBMC samples from TEDDY children in follow up available for studies (https://repository.niddk.nih.gov/studies/teddy/) to dissect environmental triggers eventually resulting in a first-appearing autoantibody and to discover novel biomarkers of antigen-specific autoimmune responses.
Metabolomics prior to the first-appearing islet autoantibody
Changes to metabolic plasma or serum profiles over time may imply responses to exposures and signal development of the first-appearing islet autoantibody. Metabolomics may uncover biomarkers of exposure as well as an immune response to exposures such as virus infections. At the time of seroconversion, and independent of age, autoantibody-positive children had higher levels of odd-chain triglycerides and polyunsaturated fatty acid-containing phospholipids along with low levels of methionine [63]. Serum metabolite profiles were analyzed prior to the appearance of IAA and GADA. These autoantibodies were preceded by diminished ketoleucine [63] and methionine [64], and elevated glutamic acid [64]. Lipidomics of cord blood from children who later in life developed T1D showed decreased levels of phosphatidylcholines and phosphatidylethanolamines [65, 66]. More important was that gestational infection during the first trimester was associated with lower cord-blood total lysophosphatidylcholines in all children examined [65]. More extensive analyses were carried out on 10,522 quarterly collected samples from 3 months until 4 years of age in 1648 TEDDY children in a nested case-control design (414 with a first-appearing autoantibody) [67, 68].
The metabolome-wide profile revealed 144 known primary metabolites and 213 known complex lipids. After correcting for age-dependent pattern, the following observations were made: IAA first was preceded by increased levels of lactate and GABA, and decreased levels of isoleucine, valine and leucine. GADA first was preceded by increased levels glutamate, and decreased levels of pyruvate, alanine, a-ketoglutarate and proline [67, 68]. Longitudinal analysis in children seroconverting after two years of age but not before two years of age showed decreased levels of isoleucine and valine for IAA first and increased levels of piperidone and decreased levels of proline for GADA first [67]. An interesting observation was made that dehydroascorbic acid was increased in children later developing either IAA first or GADA first [67, 68]. Dehydroascorbic acid is abundant in the human diet and may be reduced to ascorbic acid in the gastrointestinal tract and taken up to circulate in the blood [69]. Indeed, plasma ascorbic acid in the TEDDY children were inversely associated with IAA first [68, 70]. The data in Table 3 is a summary of decreased metabolites or complex lipids at risk for either IAA- or GADA first. Taken together, the longitudinal metabolomics analysis of the TEDDY children suggests that IAA- and GADA first differ in the way that metabolites and lipids precede seroconversion. These changes need to be dissected in relation to an exposure associated with autoimmunity or the consequence of the immune response (Figure 1) leading to a first-appearing autoantibody. It is yet to be clarified whether any of the metabolomics data in Table 3 were related to the weight growth rate in infancy associated with GADA first [71].
Table 3.
Decreased metabolite or complex lipid levels at risk for a first appearing autoantibody.
| Compound cluster | Cluster size | Key compounds | IAA first | GADA first |
|---|---|---|---|---|
| Unsaturated triglycerides (TG) | 53 | TG 52.4 and TG 62:1 | YES | YES |
| Phosphatidyl ethanolamines | 13 | Lyso-PE 18:2 and PE 38:2 | YES | YES |
| Unsaturated phosphatidylcholine | 40 | PC 31:2 and PC 38:5 | YES | YES |
| Sugar alcohols | 11 | Glycerol | YES | NO |
| Butyrates | 4 | GABA | YES | NO |
| Plasmalogens | 8 | Plasmenyl-PE 36:6 | NO | YES |
| Sphingomyelins | 14 | SM (d18:1/18:1) | NO | YES |
| Unsaturated diglycerides | 7 | DG 38:5 | NO | YES |
Associations between erythrocyte fatty acids and the risk of IAA- and GADA first autoantibodies in the TEDDY children were detected [72]. Docosapentaenoic acid (DPA 22:5n − 3) at 3 months showed a protective association for IAA first; conversely, a high ratio of polyunsaturated fatty acids at 6 months was associated with a higher risk for IAA first. In GADA first, a protective association was observed for arachidonic acid (AA) and adrenic acid at 6 months, while myristic acid (14:0) at 6 months was associated with an increased risk for GADA first. The erythrocyte fatty acids also need to be analyzed in relation to the metabolomics data to better understand the dichotomy between IAA- compared to GADA first.
First appearing autoantibody in relation to dietary factors
Later introduction of gluten-containing cereals during the first year of life was associated with increased risk of IAA first [73]. Early probiotic supplementation (at the age of 0–27 days) decreased the risk of IAA first when compared with probiotic supplementation after 27 days or no probiotic supplementation. The association was accounted for by children with the DR3/4 genotype [74].
The possibility that either IAA- or GADA first may be triggered by, or the subsequent autoimmune response affected by, dietary factors needs further investigation. Food consumption was collected by 3-day food records, biannually provided by the parents from the age of 12 months [8]. The food composition databases in the US, Sweden, Finland and Germany have been harmonized, and values for energy of protein, fat and carbohydrates are comparable [75]. Recent analyses suggest a relationship between GADA first and protein intake either as kcal/day or energy percentage. Hence, a diet with increased energy from protein may increase the risk for GADA first [75].
First-appearing autoantibody and exposures
Genetic sex apparently needs to be taken into account, as IAA first was less frequent in girls than boys and the risk for GADA first was not affected by sex. At the first visit (at 3–4.5 months of age), 8676 TEDDY mothers were asked about life events during their pregnancy, and 65% of the mothers reported disease or injury (25%), serious interpersonal (28%) and job-related (25%) life events. Serious interpersonal life events correlated with increased risk of GADA first only showing an additive interaction between HLA DR3-DQ2 and the BACH2-T allele. Independently, job-related life events were also associated with increased risk of GADA first among HLA DR3-DQ2/DR4-DQ8 children. Job-related life events were associated with reduced risk of IAA first especially in children with the BTNL2-GG allele [76]. These observations underscore the importance of taking into account maternal phenomena including reported gestational infections [39, 65] when considering factors that may affect the developing immune system in the child.
Vaccination is an important exposure. The possible effect of the mandatory vaccination practices in the four TEDDY countries is yet to be analysed. Children who received the Pandemrix® vaccine during the A/H1N1 2009 pandemic had no increased risk of islet autoimmunity. Indeed, the vaccinated children under 2 years of age had reduced risk for IAA first in Finland [77].
The development of the microbiome was extensively analysed in the first TEDDY nested case-control study [78, 79] and in a smaller study of 22 children and matched controls prior to the appearance of a first islet autoantibody [80]. Neither the pattern of development nor individual bacteria was clearly related to the appearance of a first islet autoantibody [78, 79]. However, it cannot be excluded that a targeted analysis would provide more detailed information. Fungal taxa were not related to first islet autoantibodies [81]. Metagenome-assembled genomes (MAGs) in 887 TEDDY children followed longitudinally revealed 883 species, including 176 previously undescribed human microbiome species [82]. Metagenome assemblies based on protein families were examined for association with the appearance of islet autoantibodies. Results showed that 2373 MAGs were positively and 1549 negatively associated with islet autoimmunity [82]. A possible MAGs dichotomy of IAA- and GADA first remains to be determined.
Viruses as candidate exposures to trigger islet autoimmunity were recently reviewed [83]. Parents in the TEDDY study reported infections at their regular visits [84], and respiratory infections preceded first-appearing autoantibodies. The effect size did not markedly differ between IAA- or GADA first [85]. In blood samples from Norwegian children, there was no association between enterovirus RNA and a first islet autoantibody [86]. A higher frequency of enterovirus was detected in the first islet autoantibody-positive sample (16%) compared with the corresponding time point in matched controls [86]. In the Finnish DIPP study, Coxsackievirus B1 infections were associated with IAA first but not with GADA first [87].
In the large-scale TEDDY study of known eukaryotic DNA and RNA viruses in stools from children, it was found that prolonged enterovirus B infection, primarily Coxsackievirus B4, was associated with islet autoimmunity [88]. In children with stool Enterovirus B at 3–6 months of age, the appearance of a first autoantibody was more likely to be IAA first rather than GADA first (Figure 3). In addition, the minor allele of the CXADR (rs6517774) polymorphism independently correlated with islet autoimmunity. The CXADR gene encodes the Coxsackievirus and adenovirus receptor and as this receptor is expressed on the islet beta cells, direct virus infection needs to be taken into account for the development of either IAA-, GADA first, or both. Moreover, fewer early-life human mastadenovirus C infections were negatively associated with first appearing islet autoantibodies autoimmunity [88]. Further analyses are needed to relate first appearing islet autoantibody to virus present in the blood samples which have been collected at every TEDDY visit. Analysis of neutralizing serotype specific virus antibodies should also prove to be useful to explore virus exposure prior to the first appearing autoantibody similar to that reported in the Finnish DIPP children [87]. A recent meta-analysis concluded that virome analyses have demonstrated associations between enteroviruses and islet autoimmunity that may be clinically significant [89]. As has been pointed out, it will be important in future analyses to take analyses from pregnancy into account and more frequent sampling to uncover to what extent virus exposure early in life contributes to the appearance of either IAA- or GADA first.
Conclusion and future directions
Current studies of the first appearance of an islet autoantibody are carried out in so-called high-risk children based on the frequency of HLA DR-DQ haplogenotypes at the time of clinical onset of T1D. In the TEDDY study, the genotypes selected would have eventually revealed T1D in less than 50% of the enrolled infants. Despite this shortcoming, the TEDDY study offers the advantage of investigating the highest risk genotype, DR3-DQ2/DR4-DQ8, as well as high-risk DR4-DQ8/DR4-DQ8 and DR3-DQ2/DR3-DQ2. These genotypes represent as many as 7.5% of newborn children in Sweden, 5.5% in Finland and 4% or less in the US and Germany. An important conclusion is that these HLA genotypes are not primarily associated with T1D; the primary association is with the first appearing islet autoantibody.
Future directions may include analysis of the molecular structure of the HLA heterodimer which determines which peptides are able to form the trimolecular complex that is the ligand for the T cell receptor. The longitudinal studies of TEDDY, DIPP and similar investigations underscore that “timing is everything” [90]. The risk for a first-appearing autoantibody may be related to the number of variable amino acid residues in the groove of the HLA Class II heterodimer, be it DQ [91, 92] or DR [93, 94]. Analyses will be needed to relate the DR and DQ heterodimer polymorphisms to the first appearing autoantibody incidence (Figure 2) rather than the frequency at the time of T1D clinical diagnosis. Peptides, autoantigens, as well as virus or other infectious agents able to bind to the predicted DR and DQ risk structure may allow a better understanding of the immune response (Figure 1) that takes place and results in autoantibodies of the IgG type—the current endpoint measure of either IAA- or GADA first.
The immune response to common infant infections in high-risk children, perhaps identified at birth based on genetic risk scores that take both HLA and non-HLA variants into account [95, 96] and followed biweekly for common childhood infections—including measures of exposures to common viruses affecting children—are needed to better understand why some children but not all show prolonged shedding. The latter phenomenon may allow autoimmunity to develop. It is an attractive hypothesis that children dysfunctional in generating neutralising virus antibodies are sensitized to autoimmunity. With poor neutralizing virus antibodies, there may be enough time for a virus to infect beta cells to allow beta-cell damage and the development of a cellular immune response—controlled by HLA DR-DQ—to either IAA or GADA. Not only one virus, but several, may be a trigger. HLA DR3-DQ2 and DR4-DQ8 are likely involved in sorting out whether it will be GADA- or IAA first, or perhaps also IA-2A-first? The magnitude of the cellular immune response is likely to reflect the severity of the subsequent pathogenesis and the rate by which a second, third, or fourth autoantibody appears, as the number of autoantibodies is related to the progression to clinical onset of T1D.
Acknowledgments
The TEDDY families and the TEDDY children are acknowledged for participating in the study from birth until 15 years of age. The illustrations in Figure 1 and the graphical abstract were created using BioRender®. The assistance by Sarah Austin-Gonzalez is greatly appreciated.
The TEDDY Study is funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483, U01 DK124166, U01 DK128847, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Centers for Disease Control and Prevention (CDC), and JDRF. This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR002535). ÅL was supported by the Swedish Research Council (2020-01537), Interreg European Regional Development Fund (NYPS 202003388), Vinnova (2021-02684), The Strategic Research Area Exodiab (Dnr 2009-1039) and The Swedish Foundation for Strategic Research (Dnr IRC15-0067). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
From the 103th Berzelius Symposium: Immunity and autoimmunity in early childhood
Data sharing
The datasets generated and analyzed are made available in the NIDDK Central Repository at https://repository.niddk.nih.gov/studies/teddy.
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/joim.13648
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/joim.13648
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/147578504
Article citations
Family History of Diabetes and Clinical Characteristics in Children at Diagnosis of Type 1 Diabetes-A Swedish Population-Based Study.
Diabetes Care, 47(11):2012-2016, 01 Nov 2024
Cited by: 0 articles | PMID: 39302847 | PMCID: PMC11502525
Consensus guidance for monitoring individuals with islet autoantibody-positive pre-stage 3 type 1 diabetes.
Diabetologia, 67(9):1731-1759, 01 Sep 2024
Cited by: 1 article | PMID: 38910151 | PMCID: PMC11410955
Consensus Guidance for Monitoring Individuals With Islet Autoantibody-Positive Pre-Stage 3 Type 1 Diabetes.
Diabetes Care, 47(8):1276-1298, 01 Aug 2024
Cited by: 1 article | PMID: 38912694
Review
Untangling the genetics of beta cell dysfunction and death in type 1 diabetes.
Mol Metab, 86:101973, 22 Jun 2024
Cited by: 0 articles | PMID: 38914291 | PMCID: PMC11283044
Review Free full text in Europe PMC
Go to all (7) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
SNPs (Showing 13 of 13)
- (1 citation) dbSNP - rs9934817
- (1 citation) dbSNP - rs1701704
- (1 citation) dbSNP - rs28600853
- (1 citation) dbSNP - rs4948088
- (1 citation) dbSNP - rs3184504
- (1 citation) dbSNP - rs2292239
- (1 citation) dbSNP - rs1004446
- (1 citation) dbSNP - rs6517774
- (1 citation) dbSNP - rs2476601
- (1 citation) dbSNP - rs6679677
- (1 citation) dbSNP - rs3842727
- (1 citation) dbSNP - rs689
- (1 citation) dbSNP - rs12708716
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dynamics of Islet Autoantibodies During Prospective Follow-Up From Birth to Age 15 Years.
J Clin Endocrinol Metab, 105(12):dgaa624, 01 Dec 2020
Cited by: 24 articles | PMID: 32882033 | PMCID: PMC7686032
HLA-DR-DQ haplotypes and specificity of the initial autoantibody in islet specific autoimmunity.
Pediatr Diabetes, 21(7):1218-1226, 23 Jul 2020
Cited by: 11 articles | PMID: 32613719
Tissue transglutaminase autoantibodies in children with newly diagnosed type 1 diabetes are related to human leukocyte antigen but not to islet autoantibodies: A Swedish nationwide prospective population-based cohort study.
Autoimmunity, 51(5):221-227, 01 Aug 2018
Cited by: 4 articles | PMID: 30444426
Islet Autoantibodies.
Curr Diab Rep, 16(6):53, 01 Jun 2016
Cited by: 46 articles | PMID: 27112957
Review
Funding
Funders who supported this work.
NCATS NIH HHS (2)
Grant ID: UL1 TR000064
Grant ID: UL1 TR002535
NIDDK NIH HHS (20)
Grant ID: U01 DK063829
Grant ID: U01 DK063863
Grant ID: UC4 DK063865
Grant ID: U01 DK063821
Grant ID: UC4 DK100238
Grant ID: UC4 DK117483
Grant ID: UC4 DK063836
Grant ID: U01 DK124166
Grant ID: UC4 DK063861
Grant ID: U01 DK063865
Grant ID: UC4 DK063821
Grant ID: UC4 DK063863
Grant ID: UC4 DK112243
Grant ID: U01 DK063836
Grant ID: U01 DK063861
Grant ID: U01 DK128847
Grant ID: UC4 DK063829
Grant ID: U01 DK063790
Grant ID: UC4 DK095300
Grant ID: UC4 DK106955
NLM NIH HHS (1)
Grant ID: HHSN267200700014C
University of Colorado (1)
Grant ID: UL1 TR002535
Vinnova (1)
Grant ID: 2021‐02684







