Abstract
Free full text

Determination of the pKa of glucuronic acid and the carboxy groups of heparin by 13C-nuclear-magnetic-resonance spectroscopy.
Abstract
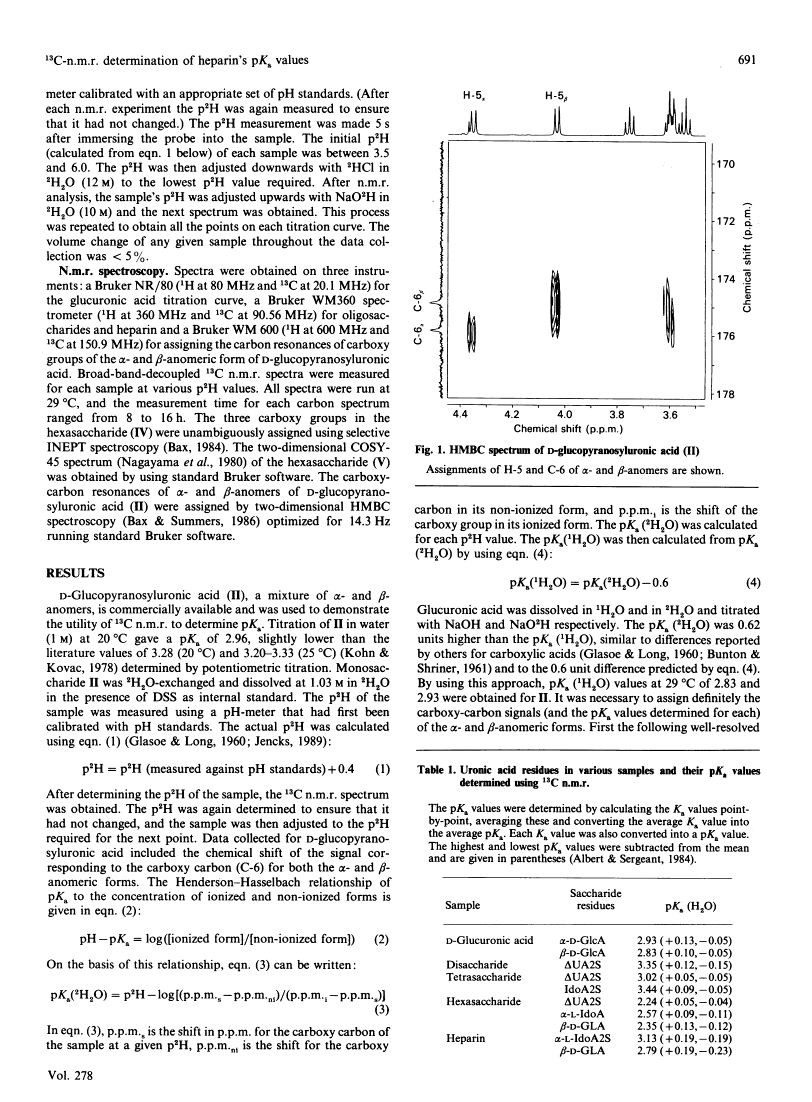
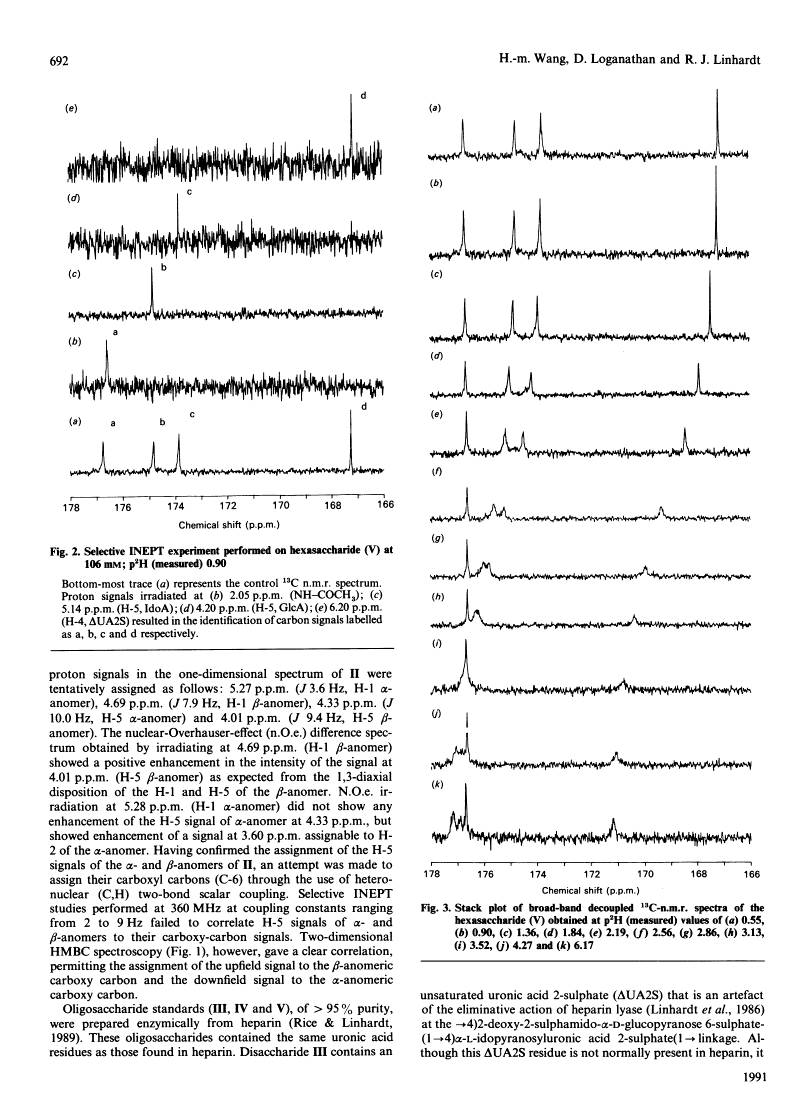
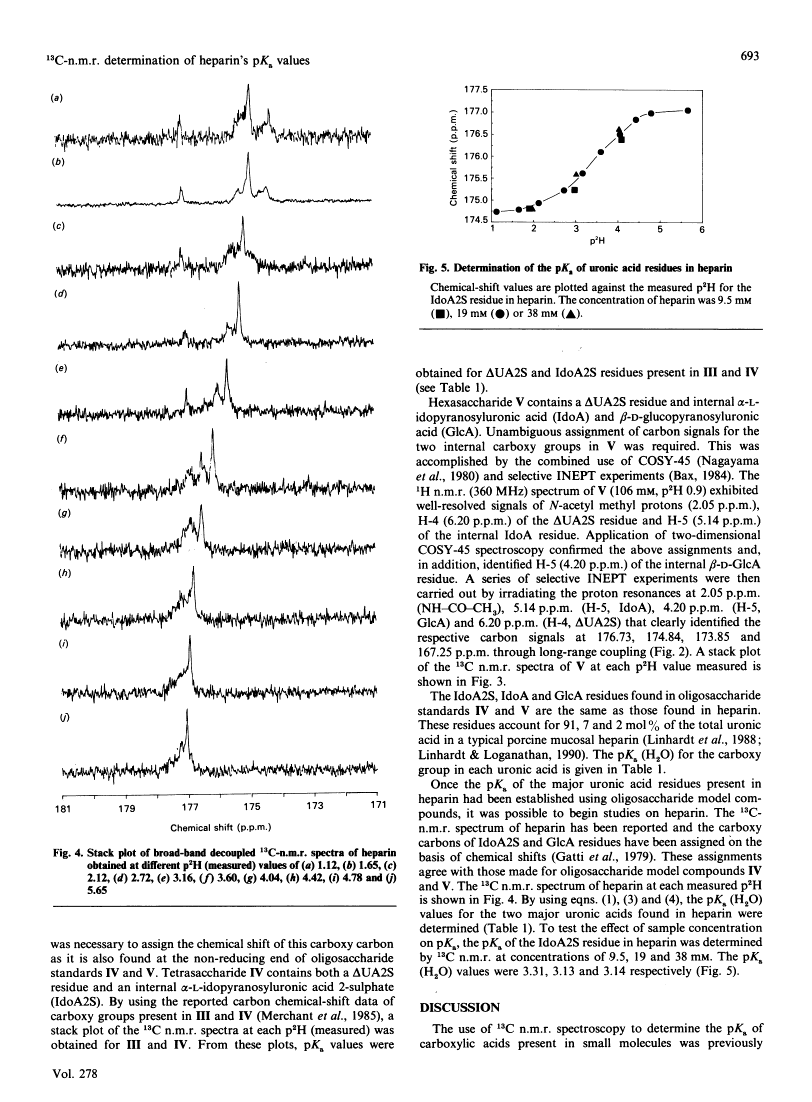
As part of our continuing studies on heparin, the present paper uses 13C-n.m.r. spectroscopy to examine the acidity of heparin's uronic acid carboxylate groups. Heparin contains three different uronic acids. In porcine mucosal heparin these account for approx. 91, 7 and 2 mol% of the total uronic acid residues. These are alpha-L-idopyranosyluronic acid 2-sulphate, beta-D-glucopyranosyluronic acid and alpha-L-idopyranosyluronic acid. The pKa values of their carboxylate groups were determined as 3.13 (using heparin), 2.79 (using heparin) and 3.0 (predicted by using model compounds) respectively. 18C-n.m.r. spectroscopy, performed at various pH values, provided a convenient method of simultaneously determining the pKa of multiple carboxylate groups, of similar acidity, within heparin D-Glucopyranosyluronic acid and heparin-derived di-, tetra- and hexa-saccharides were used as model compounds to determine pKa values of the different carboxy groups. The results suggested that molecular size had an effect on pKa. Unambiguous assignment of carboxy carbon resonances were accomplished through the use of two-dimensional n.m.r. spectroscopy. Finally, application of this method to the simplest model compound, D-glucopyranosyluronic acid, permitted the determination of the pKa of both its alpha- and beta-anomers.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atha DH, Stephens AW, Rosenberg RD. Evaluation of critical groups required for the binding of heparin to antithrombin. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1030–1034. [Europe PMC free article] [Abstract] [Google Scholar]
- Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. [Abstract] [Google Scholar]
- Casu B. Structure and biological activity of heparin. Adv Carbohydr Chem Biochem. 1985;43:51–134. [Abstract] [Google Scholar]
- Casu B, Gennaro U. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr Res. 1975 Jan;39(1):168–176. [Abstract] [Google Scholar]
- Ferro DR, Provasoli A, Ragazzi M, Casu B, Torri G, Bossennec V, Perly B, Sinaÿ P, Petitou M, Choay J. Conformer populations of L-iduronic acid residues in glycosaminoglycan sequences. Carbohydr Res. 1990 Jan 15;195(2):157–167. [Abstract] [Google Scholar]
- Fransson LA, Huckerby TN, Nieduszynski IA. alpha-L-iduronate ring conformations in heparin and heparin derivatives. 13-C Nuclear-magnetic-resonance analysis and titration data for variously desulphated and periodate-oxidized heparins. Biochem J. 1978 Oct 1;175(1):299–309. [Europe PMC free article] [Abstract] [Google Scholar]
- Laurent TC, Tengblad A, Thunberg L, Hök M, Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. [Europe PMC free article] [Abstract] [Google Scholar]
- Lindahl U, Bäckström G, Thunberg L. The antithrombin-binding sequence in heparin. Identification of an essential 6-O-sulfate group. J Biol Chem. 1983 Aug 25;258(16):9826–9830. [Abstract] [Google Scholar]
- Linhardt RJ, Cooney CL, Tapper D, Zannetos CA, Larsen AK, Langer R. An immobilized microbial heparinase for blood deheparinization. Appl Biochem Biotechnol. 1984 Feb;9(1):41–55. [Abstract] [Google Scholar]
- Linhardt RJ, Galliher PM, Cooney CL. Polysaccharide lyases. Appl Biochem Biotechnol. 1986 Apr;12(2):135–176. [Abstract] [Google Scholar]
- Linhardt RJ, Rice KG, Kim YS, Lohse DL, Wang HM, Loganathan D. Mapping and quantification of the major oligosaccharide components of heparin. Biochem J. 1988 Sep 15;254(3):781–787. [Europe PMC free article] [Abstract] [Google Scholar]
- Linhardt RJ, Turnbull JE, Wang HM, Loganathan D, Gallagher JT. Examination of the substrate specificity of heparin and heparan sulfate lyases. Biochemistry. 1990 Mar 13;29(10):2611–2617. [Abstract] [Google Scholar]
- Linker A, Hovingh P. Structural studies on heparin. Tetrasaccharides obtained by heparinase degradation. Carbohydr Res. 1984 Apr 2;127(1):75–94. [Abstract] [Google Scholar]
- Loganathan D, Wang HM, Mallis LM, Linhardt RJ. Structural variation in the antithrombin III binding site region and its occurrence in heparin from different sources. Biochemistry. 1990 May 8;29(18):4362–4368. [Abstract] [Google Scholar]
- Mallis LM, Wang HM, Loganathan D, Linhardt RJ. Sequence analysis of highly sulfated, heparin-derived oligosaccharides using fast atom bombardment mass spectrometry. Anal Chem. 1989 Jul 1;61(13):1453–1458. [Abstract] [Google Scholar]
- Merchant ZM, Kim YS, Rice KG, Linhardt RJ. Structure of heparin-derived tetrasaccharides. Biochem J. 1985 Jul 15;229(2):369–377. [Europe PMC free article] [Abstract] [Google Scholar]
- Park JW, Chakrabarti B. Acid-base and optical properties of heparin. Biochem Biophys Res Commun. 1977 Sep 23;78(2):604–608. [Abstract] [Google Scholar]
- Rice KG, Linhardt RJ. Study of structurally defined oligosaccharide substrates of heparin and heparan monosulfate lyases. Carbohydr Res. 1989 Jul 15;190(2):219–233. [Abstract] [Google Scholar]
- Rice KG, Rottink MK, Linhardt RJ. Fractionation of heparin-derived oligosaccharides by gradient polyacrylamide-gel electrophoresis. Biochem J. 1987 Jun 15;244(3):515–522. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang VC, Linhardt RJ, Bernstein H, Cooney CL, Langer R. Purification and characterization of heparinase from Flavobacterium heparinum. J Biol Chem. 1985 Feb 10;260(3):1849–1857. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj2780689
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1151401?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Bioinspired, Carbohydrate-Containing Polymers Efficiently and Reversibly Sequester Heavy Metals.
ACS Cent Sci, 10(9):1782-1788, 11 Sep 2024
Cited by: 0 articles | PMID: 39345813 | PMCID: PMC11428261
The sugar donor specificity of plant family 1 glycosyltransferases.
Front Bioeng Biotechnol, 12:1396268, 02 May 2024
Cited by: 1 article | PMID: 38756413 | PMCID: PMC11096472
Review Free full text in Europe PMC
Strategies for the biological synthesis of D-glucuronic acid and its derivatives.
World J Microbiol Biotechnol, 40(3):94, 13 Feb 2024
Cited by: 0 articles | PMID: 38349469
Review
Use of Real-World Data and Physiologically-Based Pharmacokinetic Modeling to Characterize Enoxaparin Disposition in Children With Obesity.
Clin Pharmacol Ther, 112(2):391-403, 18 May 2022
Cited by: 7 articles | PMID: 35451072 | PMCID: PMC9504927
Complexation of CXCL12, FGF-2 and VEGF with Heparin Modulates the Protein Release from Alginate Microbeads.
Int J Mol Sci, 22(21):11666, 28 Oct 2021
Cited by: 2 articles | PMID: 34769095 | PMCID: PMC8583835
Go to all (47) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Identification of N-sulphated disaccharide units in heparin-like polysaccharides.
Biochem J, 179(1):77-87, 01 Apr 1979
Cited by: 34 articles | PMID: 157737 | PMCID: PMC1186597
1H-N.m.r. spectral assignments for two series of heparin-derived oligosaccharides.
Carbohydr Res, 225(1):43-57, 01 Feb 1992
Cited by: 18 articles | PMID: 1633604
One- and two-dimensional 13C-n.m.r. characterization of two series of oligosaccharides derived from porcine intestinal mucosal heparin by degradation with heparinase.
Carbohydr Res, 223:81-98, 01 Jan 1992
Cited by: 13 articles | PMID: 1596934
13C-NMR relation study of heparin-disaccharide interactions with tripeptides GRG and GKG.
Biochem J, 315 ( Pt 2):447-454, 01 Apr 1996
Cited by: 7 articles | PMID: 8615813 | PMCID: PMC1217216
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: HL 29797
NIGMS NIH HHS (1)
Grant ID: GM 38060