Abstract
Free full text

One cell–one immunoglobin. Origin of limited heterogeneity of myeloma proteins
Abstract
Plasma-cell tumour 5563 forms a single molecular species of immunoglobulin IgG2a, i.e. one variant of heavy chain and one variant of light chain. The molecules formed are labile and undergo alterations in charge properties, which rapidly lead to heterogeneity of the myeloma protein after synthesis. The single immunoglobulin species originally formed is found only after the shortest time-intervals tested, i.e. 10min incubation. Two types of changes in charge properties take place: (1) The originally formed protein (component o) is converted via an intermediate o′ into the most basic form of 5563 myeloma protein found in serum (component a). Charge differences between these components are suppressed at pH8.9, but can be studied by chromatography at pH6.5 or by analysis of isoelectric points by isoelectric focusing in polyacrylamide gel. The conversion of components o and o′ into component a apparently commences soon after assembly of the molecules and proceeds to completion extracellularly. (2) The second type of charge difference that distinguishes components a, b, c and d is exhibited over the pH range 6.0–8.9, but not at acid pH, and has been studied by electrophoresis at pH8.9, by chromatography and by isoelectric focusing. Conversion of component a into components b, c, d and e is only partial so that all five components can be found at decreasing concentrations in serum. Both types of charge alteration can be effected in vitro in the presence of serum, with optimum pH8.0. None of the charge differences could be attributed to the secretion process, since a component with the same isoelectric point as component o was found in secreted myeloma protein (1h). We have found no evidence to support the idea that the first type of change from component o to component a is due to ring formation of N-terminal [14C]glutamine into pyrrolid-2-one-5-carboxylic acid; however, our findings do not exclude this process happening very rapidly to a precursor of component o, possibly the polypeptide chain during or immediately after synthesis. In studying this point we noted that not only the heavy chains but also the κ-type light chain of mouse 5563 myeloma protein have a blocked N-terminus.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
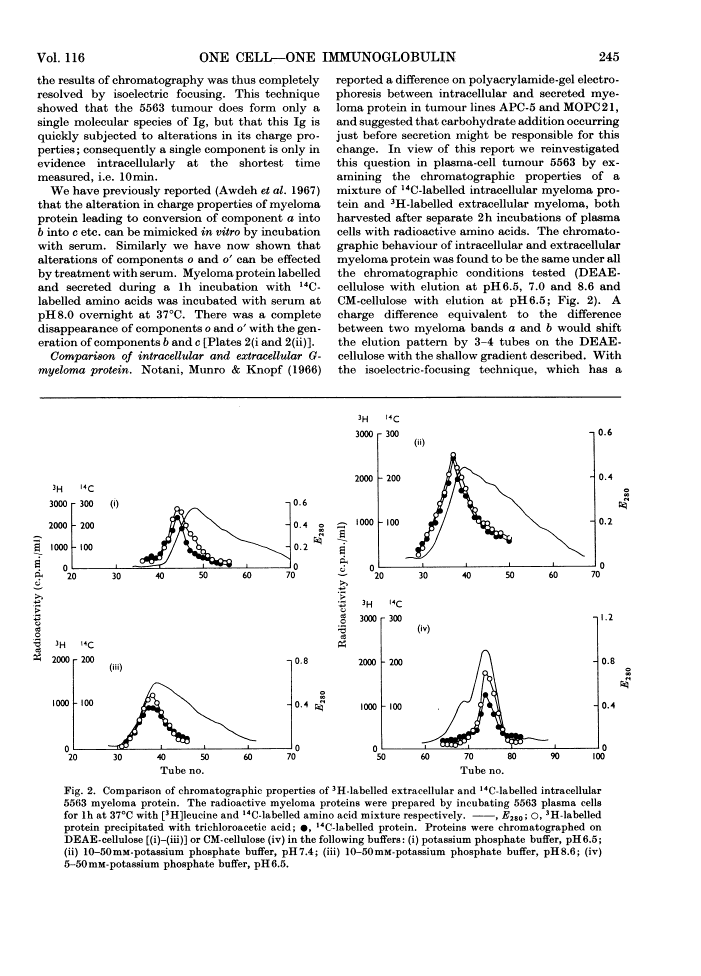
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS BA. A study on globulin formation by plasma-cell neoplasm (5563) transplantable in mice. Biochem J. 1961 Apr;79:33–43. [Europe PMC free article] [Abstract] [Google Scholar]
- Awdeh ZL, Askonas BA, Williamson AR. The homogeneous-gamma-G-immunoglobulin produced by mouse plasmacytoma 5563 and its subsequent heterogeneity in serum. Biochem J. 1967 Feb;102(2):548–553. [Europe PMC free article] [Abstract] [Google Scholar]
- Awdeh ZL, Williamson AR, Askonas BA. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. [Abstract] [Google Scholar]
- Awdeh ZL, Williamson AR, Askonas BA. Heavy and light chains of a homogeneous immunoglobulin-G. FEBS Lett. 1969 Nov 29;5(4):275–278. [Abstract] [Google Scholar]
- Bernfield MR, Nestor L. The enzymatic conversion of glutaminyl-tRNA to pyrrolidone carboxylate-tRNA. Biochem Biophys Res Commun. 1968 Dec 9;33(5):843–849. [Abstract] [Google Scholar]
- Cebra JJ, Colberg JE, Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. [Europe PMC free article] [Abstract] [Google Scholar]
- Chrambach A, Reisfeld RA, Wyckoff M, Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. [Abstract] [Google Scholar]
- Feinstein A. Use of charged thiol reagents in interpreting the electrophoretic patterns of immune globulin chains and fragments. Nature. 1966 Apr 9;210(5032):135–137. [Abstract] [Google Scholar]
- FLEISCHMAN JB, PAIN RH, PORTER RR. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [Abstract] [Google Scholar]
- LEVY AL. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. [Abstract] [Google Scholar]
- Moav B, Harris TN. Pyrrolid-2-one-5 carboxylic acid involvement in the biosynthesis of rabbit immunoglobulin. Biochem Biophys Res Commun. 1967 Dec 15;29(5):773–776. [Abstract] [Google Scholar]
- Notani GW, Munro AJ, Knopf PM. A charge difference between an intracellular and secreted mouse myeloma globulin. Biochem Biophys Res Commun. 1966 Nov 22;25(4):395–401. [Abstract] [Google Scholar]
- Perham R, Appella E, Potter M. Light chains of mouse myeloma proteins: partial amino acid sequence. Science. 1966 Oct 21;154(3747):391–393. [Abstract] [Google Scholar]
- Pernis B, Chiappino G, Kelus AS, Gell PG. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. [Europe PMC free article] [Abstract] [Google Scholar]
- Press EM, Piggot PJ, Porter RR. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. [Europe PMC free article] [Abstract] [Google Scholar]
- Robert B, Bockman RS. Studies on the proteolytic activity of gamma-globulin preparations. Biochem J. 1967 Feb;102(2):554–563. [Europe PMC free article] [Abstract] [Google Scholar]
- Rüde E, Givol D. A blocked N-terminal residue in the light chain of rabbit immunoglobulin G. Biochem J. 1968 Apr;107(4):449–453. [Europe PMC free article] [Abstract] [Google Scholar]
- Williamson AR, Askonas BA. Biosynthesis of immunoglobulins: the separate classes of polyribosomes synthesizing heavy and light chains. J Mol Biol. 1967 Jan 28;23(2):201–216. [Abstract] [Google Scholar]
Associated Data
Articles from Biochemical Journal are provided here courtesy of The Biochemical Society
Full text links
Read article at publisher's site: https://doi.org/10.1042/bj1160241
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1185352?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1042/bj1160241
Article citations
Risk-Based Control Strategies of Recombinant Monoclonal Antibody Charge Variants.
Antibodies (Basel), 11(4):73, 20 Nov 2022
Cited by: 8 articles | PMID: 36412839 | PMCID: PMC9703962
Review Free full text in Europe PMC
Novel polyol-responsive monoclonal antibodies against extracellular β-D-glucans from Pleurotus ostreatus.
Biotechnol Prog, 32(1):116-125, 07 Dec 2015
Cited by: 2 articles | PMID: 26580487
Discovery of a chemical modification by citric acid in a recombinant monoclonal antibody.
Anal Chem, 86(18):8932-8936, 27 Aug 2014
Cited by: 15 articles | PMID: 25136741 | PMCID: PMC4165448
Arginine modifications by methylglyoxal: discovery in a recombinant monoclonal antibody and contribution to acidic species.
Anal Chem, 85(23):11401-11409, 18 Nov 2013
Cited by: 39 articles | PMID: 24168114 | PMCID: PMC3869466
NK cells--versatile tools for viral defense and cancer treatment.
Eur J Immunol, 43(4):860-863, 01 Apr 2013
Cited by: 1 article | PMID: 23592381
Go to all (134) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The formation of pyrrolid-2-one-5-carboxylic acid at the N-terminus of immunoglobulin G heavy chain.
Biochem J, 128(5):1221-1227, 01 Aug 1972
Cited by: 16 articles | PMID: 4674626 | PMCID: PMC1174010
The homogeneous-gamma-G-immunoglobulin produced by mouse plasmacytoma 5563 and its subsequent heterogeneity in serum.
Biochem J, 102(2):548-553, 01 Feb 1967
Cited by: 24 articles | PMID: 4166041 | PMCID: PMC1270279
Interchain disulphide-bond formation in the assembly of immunoglobulin G. Heavy-chain dimer as an intermediate.
Biochem J, 109(4):637-643, 01 Oct 1968
Cited by: 21 articles | PMID: 5683511 | PMCID: PMC1186949
A protein related to immunoglobulin light chain detected in mouse myeloma cells.
Biochem Biophys Res Commun, 51(1):88-93, 01 Mar 1973
Cited by: 2 articles | PMID: 4699568