Abstract
Free full text

Improved Retroviral Vectors for Gene Transfer and Expression
Abstract
We describe a set of murine retrovirus-based vectors that include unique cloning sites for insertion of cDNAs such that the cDNA can be driven by either the retroviral long terminal repeat, the immediate early promoter of human cytomegalovirus, or the simian virus 40 early promoter. The vectors carry the neomycin phosphotransferase gene expressed from an alternate promoter as a selectable marker. These vectors have been constructed to prevent viral protein synthesis from the remaining viral sequences, to yield high-titer virus stocks after introduction into retrovirus packaging cells, and to eliminate homologous overlap with viral DNAs present in retrovirus packaging cells in order to prevent helper virus production. Methods for generating high-titer virus are described.
INTRODUCTION
Retroviral vectors provide a highly efficient method for gene transfer into eukaryotic cells (12). This vector system can be divided into two components; the retroviral vector itself, which generally does not encode viral proteins, and the retrovirus packaging cell line, which provides the viral proteins necessary for vector transfer. Improvements in the design of both of these components have led to impressive gene transfer efficiencies, approaching 100% even in primary cells (17).
Improvements in retrovirus packaging cells have focused on reduction of the potential of these cell lines to produce replication-competent helper virus while still allowing the production of retroviral vectors at high titer (7,11,13). These goals have been accomplished by making multiple alterations in the replication-competent retrovirus used to make the packaging cells such that viral proteins are still made but viral RNA cannot be packaged into virions, reverse transcribed, or integrated.
A key improvement in the design of murine virus-based retroviral vectors was the discovery that the signal for packaging of viral RNA into virions extends into the gag region of the virus (1,2,4). Inclusion of this region in vectors provided about a 10-fold increase in vector titer and a corresponding increase in gene transfer efficiency compared with earlier vectors. However, homologous overlap of gag sequences in the retroviral vector and sequences present in packaging cells led to frequent generation of helper virus in early packaging cell lines (15), and can still lead to infrequent helper virus generation in improved packaging cells line (7, and see Results). In addition, these new vectors contained portions of viral coding regions that could lead to the production of proteins which are antigenic or have other unwanted properties.
To solve these problems, we have designed a set of retroviral vectors which cannot yield helper virus by homologous recombination with the retroviral genome present in the packaging cells, and include mutations to block viral protein synthesis. These alterations still allow the production of high-titer virus, over 107 cfu/ml. Unique cloning sites and strong viral promoters have been included to facilitate expression of inserted cDNAs.
MATERIALS AND METHODS
Cells and Culture Conditions
Cells were grown in Dulbecco modified Eagle medium with high glucose (4.5 g/l) supplemented with 10% fetal bovine serum. For some experiments, the cells were adapted to grow in iron-supplemented bovine calf serum (Hyclone, Logan, UT) by growing them for two days at moderate density in a 50:50 mixture of fetal and calf serum, followed by growing the cells in calf serum for about a week. Previously described cell lines include NIH 3T3 TK− (13) and PA317 amphotropic retrovirus packaging cells (13; American Type Culture Collection #CRL 9078). The ecotropic retrovirus packaging cell line PE501 used here contains a defective helper virus genome that is identical to that used to make PA317 cells, except that the amphotropic region of the virus was replaced with the ecotropic region from Moloney murine leukemia virus (MoMLV). Both PA317 and PE501 cells were derived from NIH 3T3 TK− cells by cotransfection of the defective viral DNA with the TK gene as a selectable marker. Cells were grown at 37°C in a humidified incubator with 10% CO2.
Retroviral Vectors
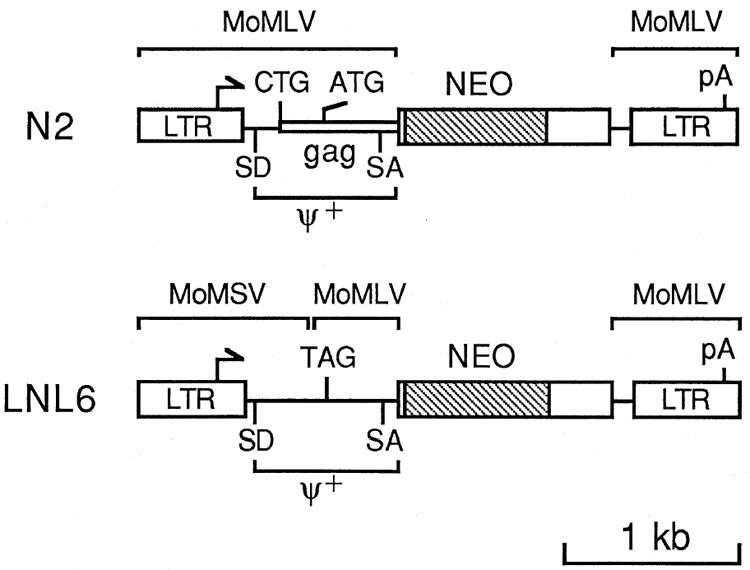
The construction of the retroviral vectors N2 (2) and LNL6 (4) has been described. Splice donor-deleted derivatives of these vectors were made by alteration of the consensus splice donor signals (Figure 1) from AGGT to AGGC. Vectors without env sequences were constructed by using a synthetic DNA fragment containing only the retroviral sequences after the env stop codon up to the Nhe I site in the LTR. Complete sequence information for the retroviral vector plasmids has been submitted to the GenBank/EMBL Databases.
Virus Production by Transient Transfection
Virus was generated from the plasmid forms of retroviral vectors by transient transfection of packaging cells. Plasmid DNA was purified before use by centrifugation in a cesium chloride gradient containing ethidium bromide. PA317 or PE501 retrovirus packaging cells were plated at 5 × 105 cells per 60-mm dish on day 1. On day 2 the culture medium was replaced with 4 ml fresh medium, and viral plasmid DNA was transfected onto the cells using the calcium phosphate precipitation procedure (6,15). For each plasmid sample, a DNA-CaCl2 solution was made by mixing 25 μl 2.0 M CaCl2, 10 μg plasmid DNA (in 10 mM Tris-Cl, pH 7.5) and water to make 200 μl total. Precipitation buffer was freshly prepared by mixing 100 μl 500 mM HEPES-NaOH (pH 7.1), 125 μl 2.0 M NaCl, 10 μl 150 mM Na2HPO4-NaH2PO4, (pH 7.0) and water to make 1 ml total. DNA-CaCl2 solution (200 μl) was added dropwise with constant agitation to 200 μl precipitation buffer. After 30 min at room temperature the resultant fine precipitate was added to a dish of cells. Cells were exposed to the DNA precipitate until day 3 when the medium was aspirated and fresh medium was added. On day 4 the virus-containing medium was removed, centrifuged at 3,000 × g for 5 min to remove cells and debris, and used to infect cells. After virus harvest, the transfected cells were trypsinized and split 1:20 into medium containing 1.5 mg/ml G418 (about 50% of which was active) to determine the transfection efficiency.
Generation of Stable Vector-Producing Cell Lines
Stable virus-producing cell lines were generated essentially as previously described (15). PE501 ecotropic packaging cells were plated at 5 × 105 per 6-cm dish on day 1. On day 2 the cells were transfected as described above. On day 3, the culture medium was replaced with fresh medium, and PA317 cells were plated at 105 per 6-cm dish. On day 4, the PA317 cells were fed with 4 ml fresh medium containing 4 μg/ml Polybrene (to facilitate virus infection). Virus was harvested from the PE501 cells, and 1 μl to 1 ml samples of virus-containing medium were used to infect the PA317 cells. On day 5 the PA317 cells were trypsinized and plated in 10-cm dishes in medium containing 1.5 mg/ml G418 (about 50% active). Dishes with small numbers of colonies were used for isolation of clones by using cloning rings. These clonal lines were then assayed for vector titer, possible helper virus contamination, and for the structure of the integrated virus by Southern analysis, and suitable clonal lines were used for further studies. Virus was harvested from virus-producing cells by adding fresh culture medium to confluent dishes of cells for 16 h, removing the medium, and subjecting the medium to centrifugation at 3,000 g for 5 min to remove cells and debris.
Virus Assay
For assay of virus carrying the neo selectable marker, recipient NIH 3T3 TK− cells were seeded at 5 × 105 per 60-mm dish on day one. On day 2 the medium was changed to medium containing 4 μg/ml Polybrene, and various amounts of test virus were added. On day 3 the cells were split 1:20 into medium containing 1.5 mg/ml G418 (about 50% active). Colonies were stained and counted on day 8. Virus titer in colony-forming units per ml (cfu/ml) was calculated by dividing the number of colonies by the volume (in ml) of virus used for infection and multiplying by 20 to correct for the 1:20 cell dilution. Helper virus was measured using the S+L− assay as previously described (14).
Marker Rescue Assay
NIH 3T3 TK− cells were infected with helper-free N2 virus (13), selected in G418, and drug resistant colonies were pooled to make 3T3/N2 cells. These cells harbor but do not produce the N2 virus. For the marker rescue assay, 3T3/N2 cells were infected with test virus, passaged for two weeks to allow possible helper virus to spread in the culture, and assayed for release of virus that conferred G418 resistance. As a positive control, 3T3/N2 cells were infected in parallel with a small amount of helper virus, which always resulted in production of >106 G418-resistant cfu/ml after passage of the cells for 2 weeks.
RESULTS
Vector Modification to Prevent Viral Protein Synthesis
The basis for the vectors described here is the N2 vector (Figure 1) that has been used successfully to infect a variety of different cell types (2). The N2 vector contains the extended packaging signal, denoted ψ+, which allows production of the vector at high titer. However, the ψ+ region also contains the start codons for the gag proteins of the parental helper virus (MoMLV), Pr65gag and a larger glycosylated protein, gPr85 gag, that initiates at an unusual CTG codon upstream of and in frame with the ATG codon of Pr65 (Figure 1). Thus we modified the N2 vector to make LNL6 (Figure 1) by inserting a stop codon in place of the Pr65 gag start codon, to prevent synthesis of Pr65 gag, and by replacing the upstream region of the vector with the homologous region from Moloney murine sarcoma virus (MoMSV), which is very similar to MoMLV but does not make the glycosylated gag protein. These alterations should prevent synthesis of viral proteins from the LNL6 vector.
The neo gene is located downstream of and out of frame with gag sequences in the N2 vector. RNA splicing between the normal MoMLV splice donor and a splice acceptor just upstream of neo (Figure 1) is required to generate an mRNA which can be translated to yield the neo polypeptide (2). Northern analysis of cells infected with N2 generally reveals two bands of similar intensity; one is full-length viral mRNA and the other is the spliced mRNA. However, in cells infected with LNL6, the full-length mRNA is by far the most abundant species (not shown). Thus, we examined the dependence of neo expression from these two vectors as a function of splicing by mutational inactivation of the splice donor in the two vectors. Inactivation of the splice donor in N2 led to a 30-fold reduction in transfection efficiency of the vector and a 2500-fold reduction in the yield of viruses that conferred G418-resistance following transient transfection of packaging cells (Table 1). In addition, colonies formed after transfection or infection by the splice donor-inactivated N2 vector were smaller than those of the other vectors shown in Table 1, again suggesting that neo expression was suboptimal. In contrast, inactivation of the splice donor in LNL6 did not change the transfection efficiency of the vector, the yield of viruses that conferred G418-resistance following transfection of packaging cells, nor the size of the drug-resistant colonies. Thus, while splicing is required for efficient neo-protein expression with the N2 vector, splicing is not required for expression with LNL6. This may provide an advantage for the LNL6 vector in cells that for some reason do not efficiently splice the full-length viral mRNA.
Table 1
Effects of Splice Donor Inactivation on Vector Transfection and Infection Efficiencies
| Vector | Transfection Efficiency (colonies/μg DNA/106cells) | Vector Titer (cfu/ml) |
|---|---|---|
| N2 (SD+) | 3 × 103 | 5 × 105 |
| N2 (SD−) | 1 × 102* | 2 × 102* |
| LNL6 (SD+) | 3 × 103 | 6 × 105 |
| LNL6 (SD−) | 3 × 103 | 6 × 105 |
Vector titers were measured following transient transfection of PA317 cells as described in Materials and Methods. Following virus harvest, the transfected packaging cells were split 1:20 into medium containing G418. The transfection efficiency was calculated as the number of drug resistant colonies/μg DNA/106 transfected cells, assuming that about 106 cells were present at the time of transfection.
Deletion of env Sequences
For many applications of retroviral vectors, it is crucial to generate virus in the absence of contaminating replication-competent retrovirus, or helper virus, to prevent virus spread in cells infected with the vector. This problem has been largely solved by the development of retrovirus packaging cell lines; however, helper virus production is still infrequently observed. Homologous overlap between viral sequences in the packaging cells and in the vector contributes to the possibility of helper virus production (15). Unfortunately, the high-titer vectors and packaging lines currently available all contain such overlap so that helper virus can potentially be generated by homologous recombination (12). To solve this problem, all env sequences have been removed from the vectors described below. The defective helper virus used to make PA317 cells was truncated immediately after env; thus, it is not possible to generate helper virus by homologous recombination between the vector and the defective helper virus used to make the PA317 packaging cells (Figure 2).
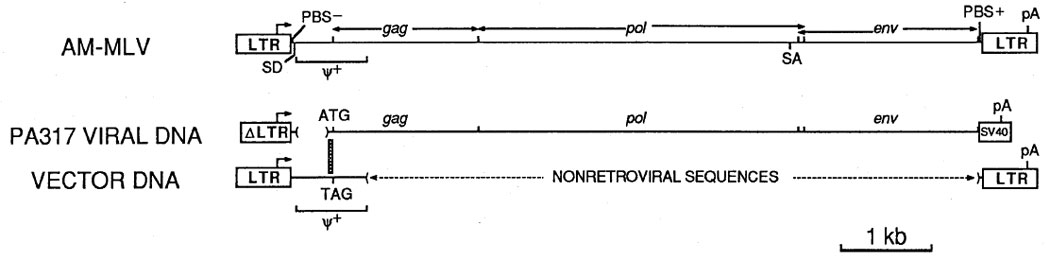
At top is a diagram of the replication-competent murine virus AM-MLV, which was made from MoMLV by replacing the env and part of the pol region of MoMLV with that of amphotropic virus 4070A (14). Sequences in the PA317 cells and in the vectors are aligned with the helper virus to show their origin. The vertical crosshatched box shows a 59-bp region of homology between the viral DNA in PA317 cells and the retroviral vectors where a homologous recombination event could add functional 5′ retroviral sequences to the defective helper virus DNA in PA317 cells. While additional homology exists at the 3′ end of ψ+, a recombination in this area would add 5′ viral sequences with a stop codon where the gag start codon should be, and thus would be non-functional. No homologous overlap exists at the 3′ end. Abbreviations are PBS− and PBS+, minus and plus strand primer binding sites respectively; pA, polyadenylation signal; SV40, the late polyadenylation signal from SV40; LTR, the retroviral long terminal repeat; ΔLTR, LTR with a deletion at the 5′ end; ψ+, retroviral packaging signal. Arrows indicate transcriptional start sites and direction of transcription.
Figure 3 depicts the retroviral vectors that incorporate the improvements described above and contain unique cloning sites for cDNA insertion. The vector LN contains only the neo gene, and is identical to LNL6 except that all of the env and some non-coding regions of neo have been removed. The strategy for expressing two genes in the other vectors is to express one gene from the retroviral LTR and the other gene from an internal promoter, either the human cytomegalovirus (CMV) immediate early promoter or the simian virus 40 (SV40) early promoter.
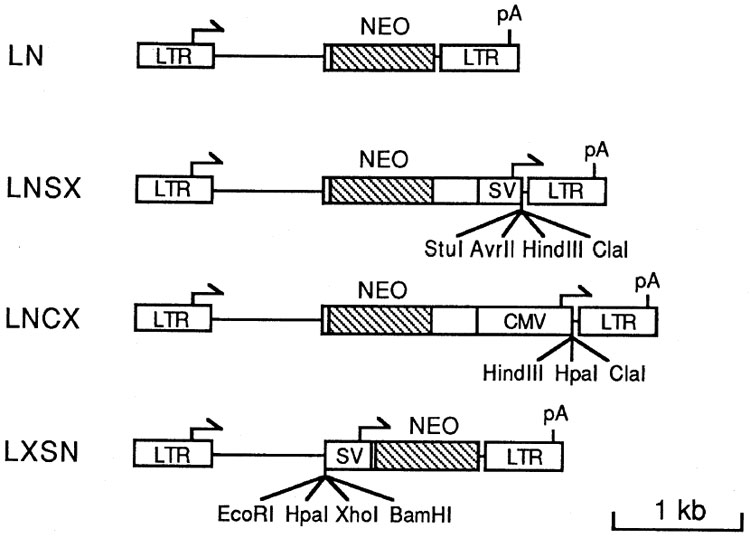
Arrows indicate transcriptional start sites and direction of transcription, pA indicates polyadenylation signals. Unique cloning sites for cDNA insertion are shown. The sequences in all vectors consist of the 5′ LTR and sequences through base 541 from MoMSV, base 566 to 1038 from MoMLV, non-retroviral sequences, and base 7774 through the 3′ LTR from MoMLV. Retroviral sequence numbers are as described (21). The neo sequences were from transposon Tn5 (3) and the protein-encoding region is crosshatched. The bacterial promoter was removed prior to insertion. SV indicates a Pvu II to Hind III fragment from SV40 containing the early promoter (8,20). CMV indicates a Bal I to Xma III fragment from human cytomegalovirus which contains the immediate early promoter (5). Unique BamH I sites separate the SV40 and CMV promoters from the neo gene sequences in LNSX and LNCX, respectively, allowing simple replacement of these promoters with other sequences.
High-Titer Virus Production
To test the ability of the vectors to generate high-titer virus, we measured virus production following transient transfection of the vectors into PA317 cells. Vector titers ranged from 105 to 8 × 105 (Table 2) and thus are as high as the N2 or LNL6 vectors (Table 1). We also generated stable vector-producing cell lines containing the LN vector. Randomly chosen G418-resistant clones produced LN vector at up to 4 × 107 cfu/ml (Table 3) and contained unrearranged vector sequences by DNA blot analysis (not shown). Similar experiments involving the high-titer vectors N2 or LNL6 yield titers of up to 2 × 107 cfu/ml (4,13); thus, the titers of the LN vector are comparable to earlier high-titer vectors. These results show that modifications and deletions of viral sequences made in the construction of the LN vector do not adversely affect virus titers. In particular, deletion of the entire env region does not affect the plus-strand primer binding site located adjacent to the 3′ LTR.
Table 2
Retrovirus Vector Titers Produced after Transient Transfection of PA317 Retrovirus Packaging Cells
| Vector | Transfection Efficiency (colonies/μg DNA/106cells) | Vector Titer (cfu/ml) |
|---|---|---|
| LN | 4 × 103 | 8 × 105 |
| LNSX | 4 × 103 | 8 × 105 |
| LNCX | 2 × 103 | 2 × 105 |
| LXSN | 6 × 102 | 1 × 105 |
Vector titer and transfection efficiency for the indicated vectors was measured as described in Table 1.
Table 3
Titer of LN Vector Produced by Clonal PA317 Cell Lines Infected with the LN Vector
| Clone | Titer(cfu/ml) | Clone | Titer (cfu/ml) |
|---|---|---|---|
| 1 | 3 × 106 | 7 | 3 × 107 |
| 2 | <2 × 105 | 8 | <2 × 105 |
| 3 | 6 × 106 | 9 | 9 × 106 |
| 4 | 4 × 105 | 10 | 8 × 106 |
| 5 | 3 × 107 | 11 | 4 × 107 |
| 6 | 1 × 107 | 12 | 2 × 107 |
Stable PA317 clonal cell lines producing the LN vector were generated as described in Materials and Methods. Virus titers produced by 12 randomly-chosen clones are shown. All clones contained proviruses of the correct size by Southern analysis and none produced helper virus.
Assay for Potential Helper Virus Production
The PA317 clones that produced the highest titer of LN vector (clones 5,7, and 11) were assayed for helper virus production by using the S+L− assay (14), and no helper virus was found (<1 ffu/ml of medium exposed to the cells for 16 h). Concurrent assay of a stock of amphotropic helper virus (AM-MLV, Figure 2) indicated a helper virus concentration of >106/ml, showing that the assay was capable of detecting helper virus. We have passaged PA317 cells that produce a derivative of the LNSX virus containing an adenosine deaminase cDNA insert (LNSA, Reference 9) for over 7 months without generating helper virus. Two samples of this cell line were independently passaged every 3–5 days and were assayed at several time points for helper virus production by both the S+L− assay and a marker rescue assay. Helper virus was not detected by either of these sensitive assays.
Another method for evaluating the potential for helper virus production from packaging cells containing a retroviral vector is to screen for helper virus during sequential passage of the virus between packaging cells (7). This procedure allows many chances for recombination and amplification of such events. Reinfection of packaging cells with virus generated by the same packaging cells is not efficient, because the viral env protein synthesized by the packaging cells interferes with entry of virus bearing the same env protein. However, virus can be passaged between ecotropic and amphotropic packaging cells because virions made by these cells use different receptors for entry into cells.
We tested three different clones of PA317 cells containing the LN vector and one PA317 clone containing the N2 vector by this method (Table 4). All cell lines were negative for helper virus at the start of the experiment; however, after 2 passages of the N2 virus, helper virus was detected. In contrast, the LN virus remained negative for helper virus during 8 sequential passages, both by S+L− and marker rescue assays. At several steps in the experiment, neo-virus transfer efficiency was determined by plating the infected packaging cells at low density in the presence and absence of G418. Greater than 30% of the cells were converted to G418 resistance at each step, showing that the vector was not being lost during sequential passage. Virus taken from PA317 cells after 8 sequential passages contained a high titer of neo-virus, again showing that the neo-viruses were being sequentially transferred and were not lost for some reason. Thus, while we cannot prove that PA317 cells containing the vectors described here will be helper free under all conditions, these results and the lack of homologous overlap between the vector and viral DNA in the PA317 packaging cells suggest that helper virus will not be produced.
Table 4
Measurement of Helper Virus Following Sequential Passage of Virus Between Packaging Cell Lines
| Helper Virus (ffu/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PA317 Clone | PA317→ | PE501→ | PA317→ | PE501→ | PA317→ | PE501→ | PA317→ | PE501→ | PA317→ | Marker Rescue Assay for Helper Virus |
| LN c5 | 0 | nd | nd | nd | 0 | nd | nd | nd | 0 | − |
| LN c7 | 0 | nd | nd | nd | 0 | nd | nd | nd | 0 | − |
| LN cl1 | 0 | nd | nd | nd | 0 | nd | nd | nd | 0 | − |
| N2 c11 | 0 | nd | 200 | nd | nd | nd | >103 | ++++ | ||
Helper virus was measured by using the S+L− assay (14). The sensitivity of the assay was 1 ffu/ml. All of the clonal PA317 cell lines containing the indicated vectors were negative for helper virus initially (column 2). Virus from the clones was passaged sequentially between PE501 and PA317 cells, and at several points virus from the PA317 cells was assayed for helper virus (columns 4,6,8,10). At the end of the experiment (8 passages for the LN vector, 6 for N2), virus-containing medium was also assayed for helper virus by a marker rescue assay as described in Materials and Methods. nd - not determined.
DISCUSSION
We have designed a set of retroviral vectors which facilitate cDNA transfer and expression. We have inserted several cDNAs into each of these vectors, including cDNAs encoding purine nucleoside phosphorylase (16), factor IX (18), and adenosine deaminase (9). The titers of virus obtainable from PA317 cells producing the cDNA-containing vectors ranged from 8 × 105 to 2 × 107 cfu/ml. In general, the SV40 promoter has proven the weakest promoter for cDNA expression, the LTR is the best and the CMV promoter is intermediate. We have found that the relative activity of these promoters depends on the infected cell type and on the particular cDNA inserted into the vectors (9,16,18).
It is interesting to note that, while there are no translation initiation codons between the SV40 or CMV transcription initiation sites and the cDNA cloning sites in the vectors LNCX and LNSX, there are 10 AUG codons between the LTR transcription initiation site and the cloning sites in LXSN. These additional AUGs have little effect on translation of a cDNA inserted downstream, because movement of the cDNA to a position between the LTR transcriptional initiation site and the first AUG does not markedly increase production of the protein encoded by the cDNA (W.R.A. Osborne and A.D.M., unpublished results). In contrast, the presence of the normal gag start codon in this region had a severe deleterious effect on neo expression in the N2 vector with an inactivated splice donor (Table 1). This result is in general agreement with the scanning model for translation (10), which states that the 40S ribosome subunit binds at the capped 5′ end of mRNA, migrates downstream, and begins translation at the first AUG in a favorable context. The gag AUG is in a favorable context, and initiation of gag translation would inhibit neo translation. After removal of the gag AUG codon, 9 of the remaining AUGs are in poor contexts, and the one AUG in a favorable context is followed by a stop codon only 4 codons downstream, which would allow reinitiation of translation at the downstream neo start codon.
Virus titers obtained following transient transfection are quite high, up to 8 × 105 per ml (Tables 1 and and2).2). The use of cesium gradient-purified DNA seems to be important to obtain high virus titer. The protocol that we employ for transfection is an older protocol, and we have not evaluated newer protocols that are reported to provide higher transfection efficiencies. Regardless, such high virus titers following transient transfection provides a rapid method for producing virus from plasmid constructions and should facilitate approaches to cDNA cloning in retroviral vectors.
We have been able to generate stable PA317 cell lines that produce the LN vector at over 107 cfu/ml. Several factors are important to generate such high titers. First, prolonged passage of retrovirus packaging cell lines can lead to poor packaging function, presumably by loss or inactivation of the transfected retroviral DNA used to make these cell lines. Reselection of the cells in selective agents used to transfect the viral DNA into the cells helps to restore packaging function. In the case of PA317 cells, selection in HAT medium will restore packaging function (4). Second, it is important to infect rather than transfect the packaging cells to make stable lines. Third, the medium used to grow the cells is important. Most batches of fetal bovine serum work well, but we have found some that result in low virus titers. We have been able to adapt the clonal cell lines to growth in calf serum while maintaining high-titer virus production, but not all serum lots work.
While helper virus cannot be generated by homologous recombination between the improved vectors described here and the defective helper virus used to make PA317 or PE501 cells, this is not true of other recently described packaging cell lines (7,11). Unfortunately, the viral DNAs used to make these cell lines were truncated in the 3′ LTR; thus, there is unavoidable overlap between the 3′ ends of the vectors described here and the packaging DNA. However, three homologous recombination events would be required to generate helper virus in these cases, suggesting that helper virus production would be infrequent.
Virus produced from PA317 cells containing the LNL6 vector has recently been used to mark tumor-infiltrating lymphocytes in order to follow these cells after their reintroduction into patients with cancer. Tests required prior to approval for use in humans included extensive tests for the presence of helper virus and other possible viral contaminants, all of which have been negative. Many liters of virus-containing medium have been produced without detection of helper virus, suggesting that for practical purposes the problem of helper virus production has been solved, and that the vectors described here will be useful for the treatment of human disease.
Acknowledgments
We thank Jay Morgenstern and Hartmut Land for providing the env− sequences adjacent to the 3′ LTRs of these vectors, which were chemically synthesized. This work was supported by Public Health Service grants from the National Heart, Lung and Blood Institute, National Institutes of Health.
References
Citations & impact
Impact metrics
Citations of article over time
Article citations
The oncogenic fusion protein EML4-NTRK3 requires three salt bridges for stability and biological activity.
Heliyon, 10(16):e36278, 13 Aug 2024
Cited by: 0 articles | PMID: 39253179 | PMCID: PMC11381775
Critical domains for NACC2-NTRK2 fusion protein activation.
PLoS One, 19(6):e0301730, 27 Jun 2024
Cited by: 0 articles | PMID: 38935636 | PMCID: PMC11210774
External Guide Sequence Effectively Suppresses the Gene Expression and Replication of Herpes Simplex Virus 2.
Molecules, 29(9):2052, 29 Apr 2024
Cited by: 0 articles | PMID: 38731543 | PMCID: PMC11085068
V3: an enigmatic isoform of the proteoglycan versican.
Am J Physiol Cell Physiol, 325(2):C519-C537, 03 Jul 2023
Cited by: 2 articles | PMID: 37399500 | PMCID: PMC10511178
Review Free full text in Europe PMC
Suppressing Kaposi's Sarcoma-Associated Herpesvirus Lytic Gene Expression and Replication by RNase P Ribozyme.
Molecules, 28(8):3619, 21 Apr 2023
Cited by: 3 articles | PMID: 37110852 | PMCID: PMC10142857
Go to all (1,221) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Production of retroviral vectors.
Curr Protoc Hum Genet, Chapter 12:Unit 12.5, 01 May 2001
Cited by: 1 article | PMID: 18428250
A convenient method for positive selection of retroviral producing cells generating vectors devoid of selectable markers.
J Virol Methods, 118(1):61-67, 01 Jun 2004
Cited by: 2 articles | PMID: 15158069
An improved method for generating retroviral producer clones for vectors lacking a selectable marker gene.
Blood Cells Mol Dis, 24(2):167-182, 01 Jun 1998
Cited by: 47 articles | PMID: 9642098
Retrovirus packaging cells.
Hum Gene Ther, 1(1):5-14, 01 Jan 1990
Cited by: 190 articles | PMID: 2081186
Review