Abstract
Free full text

Specific Sindbis virus-coded function for minus-strand RNA synthesis.
Abstract
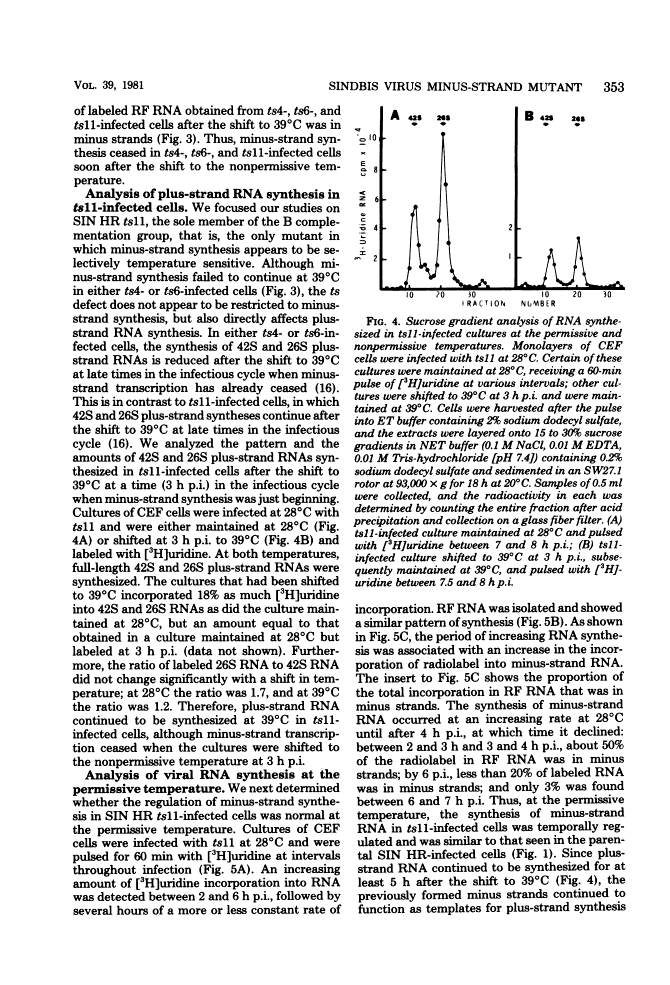
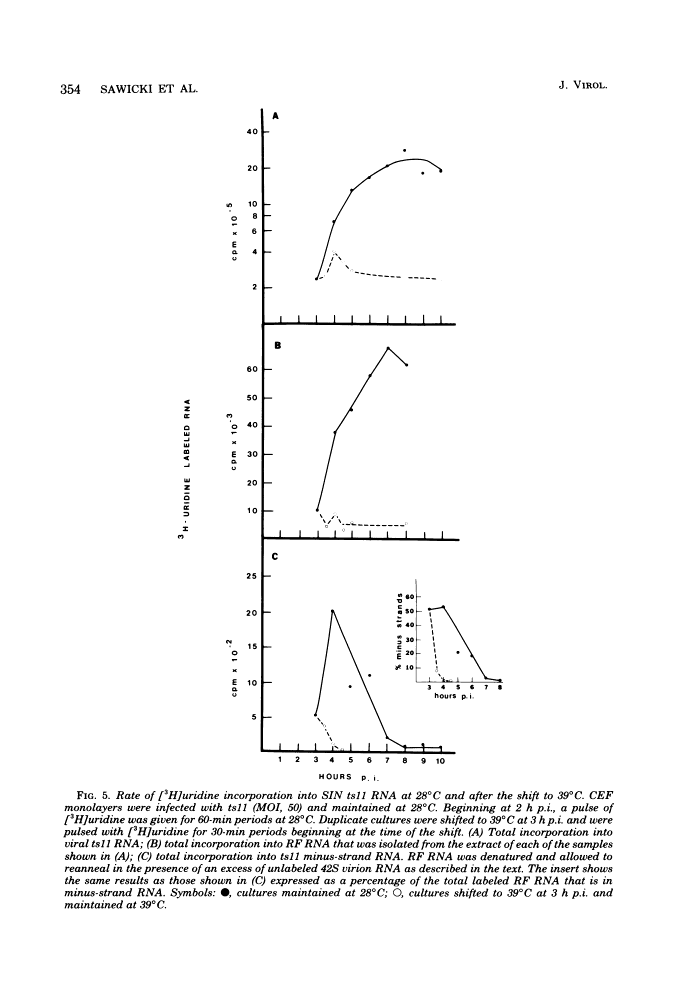
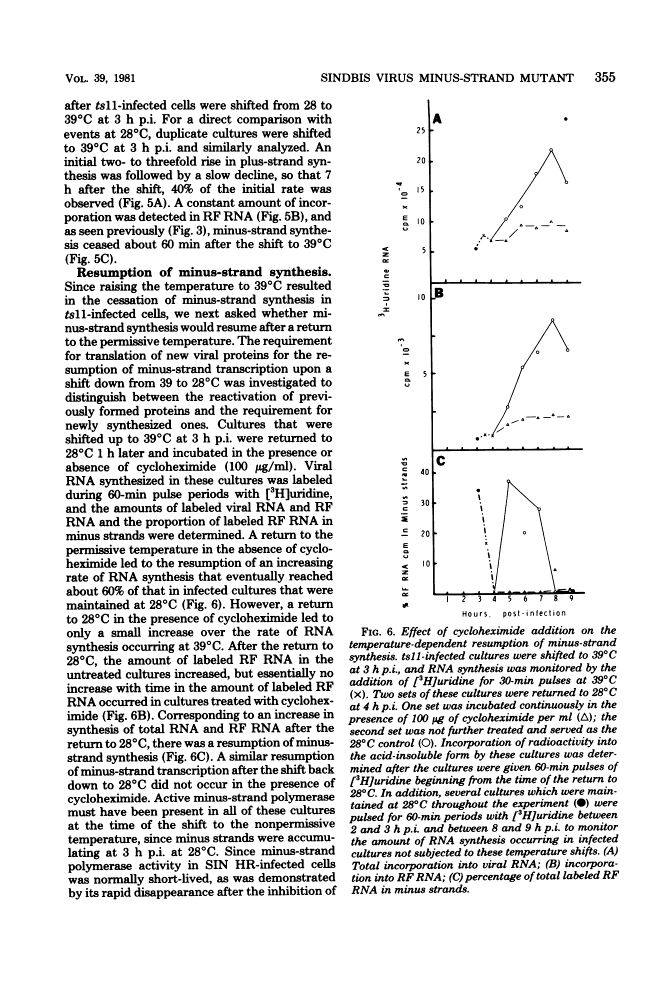
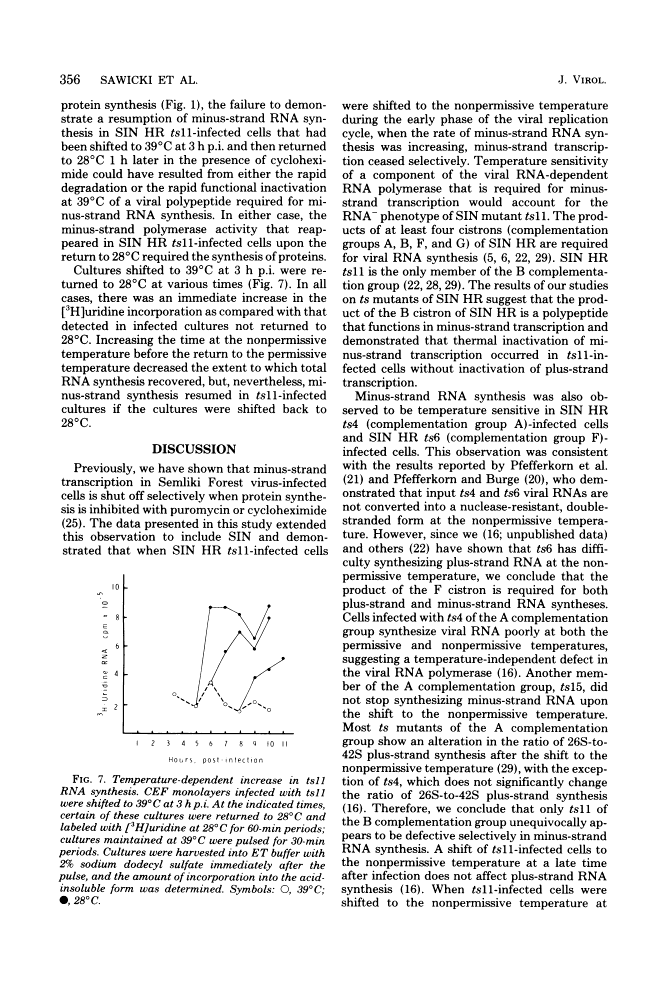
The synthesis of minus-strand RNA was studied in cell cultures infected with the heat-resistant strain of Sindbis virus and with temperature-sensitive (ts) belonging to complementation groups A, B, F, and G, all of which exhibited an RNA-negative (RNA-) phenotype when infection was initiated and maintained at 39 degrees C, the nonpermissive temperature. When infected cultures were shifted from 28 degrees C (the permissive temperature) to 39 degrees C at 3 h postinfection, the synthesis of viral minus-strand RNA ceased in cultures infected with ts mutants of complementation groups B and F, but continued in cultures infected with the parental virus and mutans of complementation groups A and G. In cultures infected with ts11 of complementation group B, the synthesis of viral minus-strand RNA ceased, whereas the synthesis of 42S and 26S plus-strand RNAs continued for at least 5 h after the shift to 39 degrees C. However, when ts11-infected cultures were returned to 28 degrees C 1 h after the shift to 39 degrees C, the synthesis of viral minus-strand RNA resumed, and the rate of viral RNA synthesis increased. The recovery of minus-strand synthesis translation of new proteins. We conclude that at least one viral function is required for alphavirus minus-strand synthesis that is not required for plus-strand synthesis. In cultures infected with ts6 of complementation group F, the syntheses of both viral plus-strand and minus-strand RNAs were drastically reduced after the shift to 39 degrees C. Since ts6 failed to synthesize both plus-strand and minus-strand RNAs after the shift to 39 degrees C, at least one common viral component appears to be required for the synthesis of both minus-strand and plus-strand RNAs.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal T, Carmichael GG. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. [Abstract] [Google Scholar]
- Bracha M, Leone A, Schlesinger MJ. Formation of a Sindbis virus nonstructural protein and its relation of 42S mRNA function. J Virol. 1976 Dec;20(3):612–620. [Europe PMC free article] [Abstract] [Google Scholar]
- Brzeski H, Kennedy SI. Synthesis of Sindbis virus nonstructural polypeptides in chicken embryo fibroblasts. J Virol. 1977 May;22(2):420–429. [Europe PMC free article] [Abstract] [Google Scholar]
- Burge BW, Pfefferkorn ER. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. [Abstract] [Google Scholar]
- Burge BW, Pfefferkorn ER. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. [Abstract] [Google Scholar]
- Dasgupta A, Zabel P, Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. [Abstract] [Google Scholar]
- Dmitrieva TM, Shcheglova MV, Agol VI. Inhibition of activity of encephalomyocarditis virus-induced RNA polymerase by antibodies against cellular components. Virology. 1979 Jan 30;92(2):271–277. [Abstract] [Google Scholar]
- Dubin DT, Timko K, Gillies S, Stollar V. The extreme 5'-terminal sequences of sindbis virus 26 and 42 S RNA. Virology. 1979 Oct 15;98(1):131–141. [Abstract] [Google Scholar]
- Flanegan JB, Petterson RF, Ambros V, Hewlett NJ, Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. [Europe PMC free article] [Abstract] [Google Scholar]
- Franklin RM. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. [Europe PMC free article] [Abstract] [Google Scholar]
- Frey TK, Strauss JH. Replication of Sindbis virus. VI. Poly(A) and poly(U) in virus-specific RNA species. Virology. 1978 May 15;86(2):494–506. [Abstract] [Google Scholar]
- Friedman RM, Grimley PM. Inhibition of arbovirus assembly by cycloheximide. J Virol. 1969 Sep;4(3):292–299. [Europe PMC free article] [Abstract] [Google Scholar]
- Fuller FJ, Marcus PI. Sindbis virus. I. Gene order of translation in vivo. Virology. 1980 Dec;107(2):441–451. [Abstract] [Google Scholar]
- Käriäinen L, Söderlund H. Structure and replication of alpha-viruses. Curr Top Microbiol Immunol. 1978;82:15–69. [Abstract] [Google Scholar]
- Keränen S, Käriäinen L. Functional defects of RNA-negative temperature-sensitive mutants of Sindbis and Semliki Forest viruses. J Virol. 1979 Oct;32(1):19–29. [Europe PMC free article] [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Nomoto A, Detjen B, Pozzatti R, Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. [Abstract] [Google Scholar]
- Pettersson RF, Söderlund H, Käriäinen L. The nucleotide sequences of the 5'-terminal T1 oligonucleotides of Semliki-Forest-virus 42-S and 26-S RNAs are different. Eur J Biochem. 1980 Apr;105(3):435–443. [Abstract] [Google Scholar]
- Sawicki DL, Gomatos PJ. Replication of semliki forest virus: polyadenylate in plus-strand RNA and polyuridylate in minus-strand RNA. J Virol. 1976 Nov;20(2):446–464. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawicki DL, Kaariainen L, Lambek C, Gomatos PJ. Mechanism for control of synthesis of Semliki Forest virus 26S and 42s RNA. J Virol. 1978 Jan;25(1):19–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Sawicki DL, Sawicki SG. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. [Europe PMC free article] [Abstract] [Google Scholar]
- Scheele CM, Pfefferkorn ER. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. [Europe PMC free article] [Abstract] [Google Scholar]
- Spector DH, Baltimore D. Polyadenylic acid on poliovirus RNA IV. Poly(U) in replicative intermediate and double-stranded RNA. Virology. 1975 Oct;67(2):498–505. [Abstract] [Google Scholar]
- Strauss EG, Lenches EM, Strauss JH. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. [Abstract] [Google Scholar]
- Waite MR. Protein synthesis directed by an RNA temperature-sensitive mutant of Sindbis virus. J Virol. 1973 Feb;11(2):198–206. [Europe PMC free article] [Abstract] [Google Scholar]
- Wengler G. Studies on the synthesis of viral RNA-polymerase-template complexes in BHK 21 cells infected with Semliki Forest virus. Virology. 1975 Jul;66(1):322–326. [Abstract] [Google Scholar]
- Wengler G, Wengler G, Gross HS. Replicative form of Semliki Forest virus RNA contains an unpaired guanosine. Nature. 1979 Dec 13;282(5740):754–756. [Abstract] [Google Scholar]
- Yogo Y, Teng MH, Wimmer E. Poly(U) in poliovirus minus RNA is 5'-terminal. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1101–1109. [Abstract] [Google Scholar]
- Yogo Y, Wimmer E. Poly (A) and poly (U) in poliovirus double stranded RNA. Nat New Biol. 1973 Apr 11;242(119):171–174. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.39.2.348-358.1981
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/39/2/348.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/39/2/348
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/39/2/348
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jvi.39.2.348-358.1981
Article citations
In Vitro and In Vivo Coinfection and Superinfection Dynamics of Mayaro and Zika Viruses in Mosquito and Vertebrate Backgrounds.
J Virol, 97(1):e0177822, 04 Jan 2023
Cited by: 7 articles | PMID: 36598200 | PMCID: PMC9888278
Sindbis Macrodomain Poly-ADP-Ribose Hydrolase Activity Is Important for Viral RNA Synthesis.
J Virol, 96(7):e0151621, 17 Mar 2022
Cited by: 3 articles | PMID: 35297669 | PMCID: PMC9006893
Sorting nexin 5 mediates virus-induced autophagy and immunity.
Nature, 589(7842):456-461, 16 Dec 2020
Cited by: 43 articles | PMID: 33328639 | PMCID: PMC7856200
Quantitative measurements of early alphaviral replication dynamics in single cells reveals the basis for superinfection exclusion.
Cell Syst, 12(3):210-219.e3, 29 Jan 2021
Cited by: 8 articles | PMID: 33515490 | PMCID: PMC9143976
Identification of Natural Molecular Determinants of Ross River Virus Type I Interferon Modulation.
J Virol, 94(8):e01788-19, 31 Mar 2020
Cited by: 5 articles | PMID: 31996431 | PMCID: PMC7108833
Go to all (73) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mechanism for control of synthesis of Semliki Forest virus 26S and 42s RNA.
J Virol, 25(1):19-27, 01 Jan 1978
Cited by: 30 articles | PMID: 621775 | PMCID: PMC353896
Sindbis virus RNA-negative mutants that fail to convert from minus-strand to plus-strand synthesis: role of the nsP2 protein.
J Virol, 70(5):2706-2719, 01 May 1996
Cited by: 33 articles | PMID: 8627744 | PMCID: PMC190127
The effect of loss of regulation of minus-strand RNA synthesis on Sindbis virus replication.
Virology, 151(2):339-349, 01 Jun 1986
Cited by: 15 articles | PMID: 3705466 | PMCID: PMC7131060
Demonstration in vitro of temperature-sensitive elongation of RNA in Sindbis virus mutant ts6.
J Virol, 62(10):3597-3602, 01 Oct 1988
Cited by: 55 articles | PMID: 3418782 | PMCID: PMC253499
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: 5S07-RR-05700-09
NIAID NIH HHS (1)
Grant ID: AI-15123