Abstract
Free full text

Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies.
Abstract
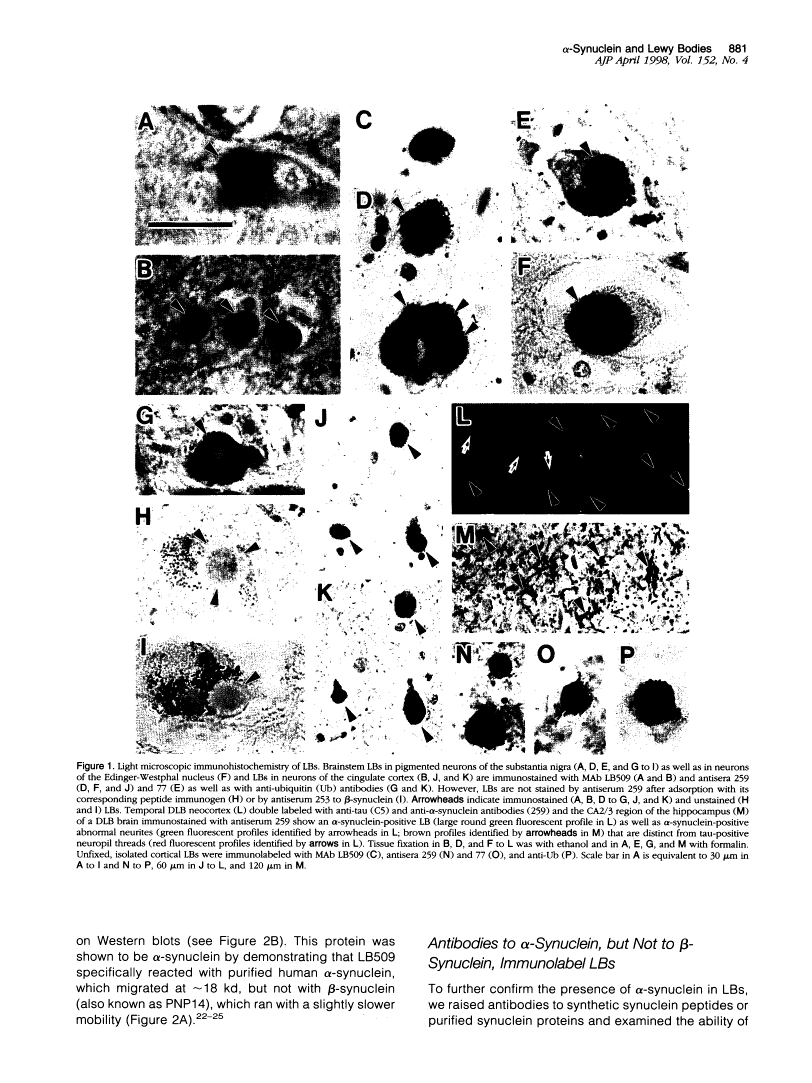
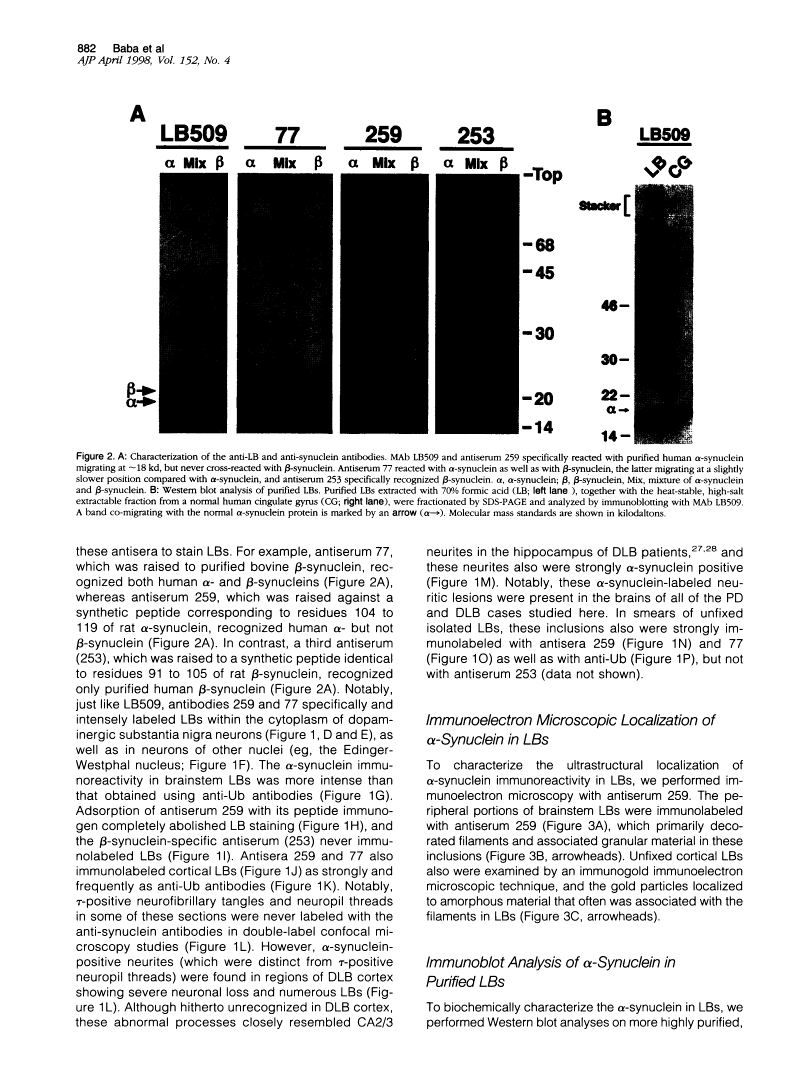
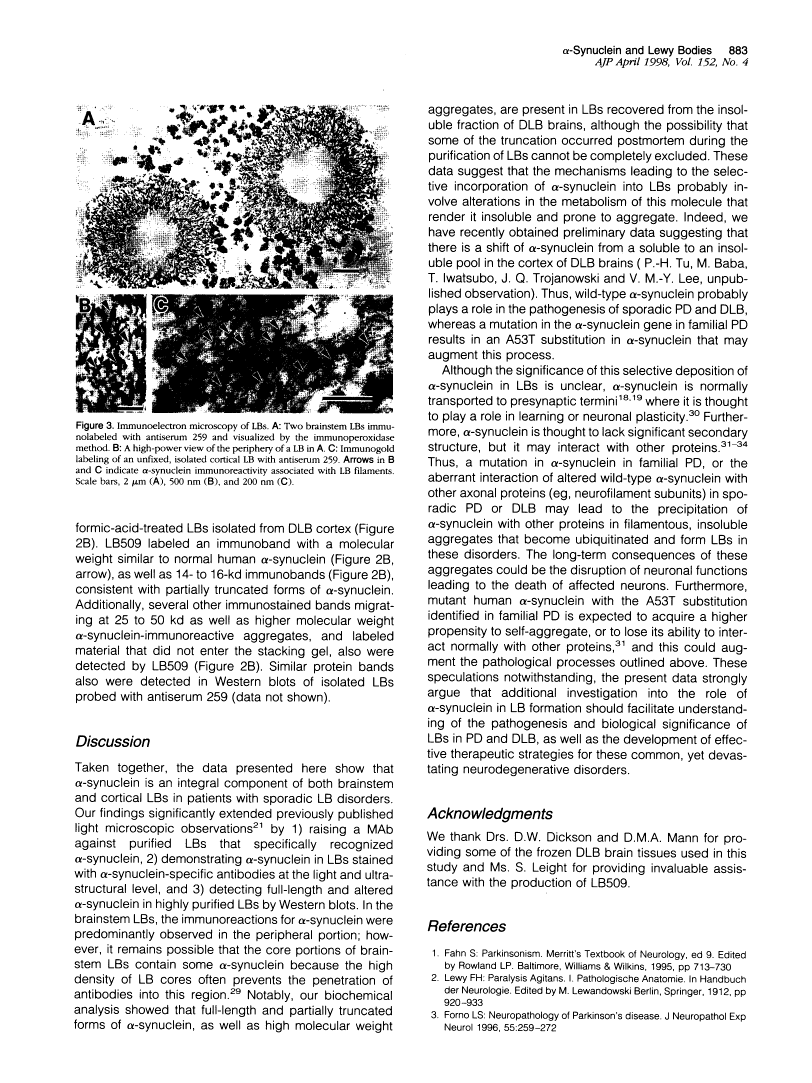
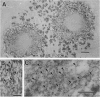
Lewy bodies (LBs) are hallmark lesions of degenerating neurons in the brains of patients with Parkinson's disease (PD) and dementia with Lewy bodies (DLB). Recently, a point mutation in the gene encoding the presynaptic alpha-synuclein protein was identified in some autosomal-dominantly inherited familial PD pedigrees, and light microscopic studies demonstrated alpha-synuclein immunoreactivity in LBs of sporadic PD and DLB. To characterize alpha-synuclein in LBs, we raised monoclonal antibodies (MAbs) to LBs purified from DLB brains and obtained a MAb specific for alpha-synuclein that intensely labeled LBs. Light and electron microscopic immunocytochemical studies performed with this MAb as well as other antibodies to alpha-and beta-synuclein showed that alpha-synuclein, but not beta-synuclein, is a component of LBs in sporadic PD and DLB. Western blot analyses of highly purified LBs from DLB brains showed that full-length as well as partially truncated and insoluble aggregates of alpha-synuclein are deposited in LBs. Thus, these data strongly implicate alpha-synuclein in the formation of LBs and the selective degeneration of neurons in sporadic PD and DLB.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996 Mar;55(3):259–272. [Abstract] [Google Scholar]
- Pollanen MS, Dickson DW, Bergeron C. Pathology and biology of the Lewy body. J Neuropathol Exp Neurol. 1993 May;52(3):183–191. [Abstract] [Google Scholar]
- Dickson DW, Davies P, Mayeux R, Crystal H, Horoupian DS, Thompson A, Goldman JE. Diffuse Lewy body disease. Neuropathological and biochemical studies of six patients. Acta Neuropathol. 1987;75(1):8–15. [Abstract] [Google Scholar]
- Trojanowski JQ, Schmidt ML, Shin RW, Bramblett GT, Rao D, Lee VM. Altered tau and neurofilament proteins in neuro-degenerative diseases: diagnostic implications for Alzheimer's disease and Lewy body dementias. Brain Pathol. 1993 Jan;3(1):45–54. [Abstract] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996 Nov;47(5):1113–1124. [Abstract] [Google Scholar]
- Goldman JE, Yen SH, Chiu FC, Peress NS. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science. 1983 Sep 9;221(4615):1082–1084. [Abstract] [Google Scholar]
- Schmidt ML, Murray J, Lee VM, Hill WD, Wertkin A, Trojanowski JQ. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991 Jul;139(1):53–65. [Europe PMC free article] [Abstract] [Google Scholar]
- Pollanen MS, Bergeron C, Weyer L. Detergent-insoluble cortical Lewy body fibrils share epitopes with neurofilament and tau. J Neurochem. 1992 May;58(5):1953–1956. [Abstract] [Google Scholar]
- Pollanen MS, Bergeron C, Weyer L. Deposition of detergent-resistant neurofilaments into Lewy body fibrils. Brain Res. 1993 Feb 12;603(1):121–124. [Abstract] [Google Scholar]
- Pollanen MS, Bergeron C, Weyer L. Characterization of a shared epitope in cortical Lewy body fibrils and Alzheimer paired helical filaments. Acta Neuropathol. 1994;88(1):1–6. [Abstract] [Google Scholar]
- Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y. Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol. 1988;75(4):345–353. [Abstract] [Google Scholar]
- Galloway PG, Grundke-Iqbal I, Iqbal K, Perry G. Lewy bodies contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles. J Neuropathol Exp Neurol. 1988 Nov;47(6):654–663. [Abstract] [Google Scholar]
- Love S, Saitoh T, Quijada S, Cole GM, Terry RD. Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol. 1988 Jul;47(4):393–405. [Abstract] [Google Scholar]
- Manetto V, Perry G, Tabaton M, Mulvihill P, Fried VA, Smith HT, Gambetti P, Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. [Europe PMC free article] [Abstract] [Google Scholar]
- Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski JQ, Lee VM. Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol. 1996 May;148(5):1517–1529. [Europe PMC free article] [Abstract] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994 May 23;345(1):27–32. [Abstract] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995 Feb;14(2):467–475. [Abstract] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997 Jun 27;276(5321):2045–2047. [Abstract] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997 Aug 28;388(6645):839–840. [Abstract] [Google Scholar]
- Nakajo S, Omata K, Aiuchi T, Shibayama T, Okahashi I, Ochiai H, Nakai Y, Nakaya K, Nakamura Y. Purification and characterization of a novel brain-specific 14-kDa protein. J Neurochem. 1990 Dec;55(6):2031–2038. [Abstract] [Google Scholar]
- Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993 Nov 1;217(3):1057–1063. [Abstract] [Google Scholar]
- Shibayama-Imazu T, Okahashi I, Omata K, Nakajo S, Ochiai H, Nakai Y, Hama T, Nakamura Y, Nakaya K. Cell and tissue distribution and developmental change of neuron specific 14 kDa protein (phosphoneuroprotein 14). Brain Res. 1993 Sep 17;622(1-2):17–25. [Abstract] [Google Scholar]
- Nakajo S, Tsukada K, Kameyama H, Furuyama Y, Nakaya K. Distribution of phosphoneuroprotein 14 (PNP 14) in vertebrates: its levels as determined by enzyme immunoassay. Brain Res. 1996 Nov 25;741(1-2):180–184. [Abstract] [Google Scholar]
- Iwatsubo T, Hasegawa M, Esaki Y, Ihara Y. Lack of ubiquitin immunoreactivities at both ends of neuropil threads. Possible bidirectional growth of neuropil threads. Am J Pathol. 1992 Feb;140(2):277–282. [Europe PMC free article] [Abstract] [Google Scholar]
- Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH. Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology. 1991 Sep;41(9):1402–1409. [Abstract] [Google Scholar]
- Dickson DW, Schmidt ML, Lee VM, Zhao ML, Yen SH, Trojanowski JQ. Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol. 1994;87(3):269–276. [Abstract] [Google Scholar]
- Galloway PG, Mulvihill P, Perry G. Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am J Pathol. 1992 Apr;140(4):809–822. [Europe PMC free article] [Abstract] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995 Aug;15(2):361–372. [Abstract] [Google Scholar]
- Goedert M. Familial Parkinson's disease. The awakening of alpha-synuclein. Nature. 1997 Jul 17;388(6639):232–233. [Abstract] [Google Scholar]
- Jensen PH, Hojrup P, Hager H, Nielsen MS, Jacobsen L, Olesen OF, Gliemann J, Jakes R. Binding of Abeta to alpha- and beta-synucleins: identification of segments in alpha-synuclein/NAC precursor that bind Abeta and NAC. Biochem J. 1997 Apr 15;323(Pt 2):539–546. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoshimoto M, Iwai A, Kang D, Otero DA, Xia Y, Saitoh T. NACP, the precursor protein of the non-amyloid beta/A4 protein (A beta) component of Alzheimer disease amyloid, binds A beta and stimulates A beta aggregation. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9141–9145. [Europe PMC free article] [Abstract] [Google Scholar]
- Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996 Oct 29;35(43):13709–13715. [Abstract] [Google Scholar]
Associated Data
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/152/4/879
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
β-synuclein regulates the phase transitions and amyloid conversion of α-synuclein.
Nat Commun, 15(1):8748, 09 Oct 2024
Cited by: 0 articles | PMID: 39384788 | PMCID: PMC11464764
Modeling Parkinson's disease pathology in human dopaminergic neurons by sequential exposure to α-synuclein fibrils and proinflammatory cytokines.
Nat Neurosci, 08 Oct 2024
Cited by: 0 articles | PMID: 39379564
Analysis and comparison of post-translational modifications of α-synuclein filaments in multiple system atrophy and dementia with Lewy bodies.
Sci Rep, 14(1):22892, 02 Oct 2024
Cited by: 0 articles | PMID: 39358446 | PMCID: PMC11446954
Targeting ferroptosis with the lipoxygenase inhibitor PTC-041 as a therapeutic strategy for the treatment of Parkinson's disease.
PLoS One, 19(9):e0309893, 18 Sep 2024
Cited by: 0 articles | PMID: 39292705 | PMCID: PMC11410249
Alpha-synuclein, autophagy-lysosomal pathway, and Lewy bodies: Mutations, propagation, aggregation, and the formation of inclusions.
J Biol Chem, 300(10):107742, 02 Sep 2024
Cited by: 0 articles | PMID: 39233232 | PMCID: PMC11460475
Review Free full text in Europe PMC
Go to all (990) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Parkinson's disease, dementia with Lewy bodies, multiple system atrophy and alpha-synuclein].
Rinsho Shinkeigaku, 39(12):1285-1286, 01 Dec 1999
Cited by: 2 articles | PMID: 10791099
Aggregation of alpha-synuclein in the pathogenesis of Parkinson's disease.
J Neurol, 250 Suppl 3:III11-4, 01 Oct 2003
Cited by: 37 articles | PMID: 14579119
[The mechanism of Lewy body formation in Parkinson's disease].
Nihon Rinsho, 58(10):2022-2027, 01 Oct 2000
Cited by: 0 articles | PMID: 11068441
Review
Alpha-synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution--a pilot study.
Acta Neuropathol, 106(3):191-201, 05 Jul 2003
Cited by: 103 articles | PMID: 12845452