Abstract
Free full text

Manganese: Recent Advances in Understanding its Transport and Neurotoxicity
1. Introduction
Mn exists in a number of physical and chemical forms in the earth's crust, in water and in the atmosphere's particulate matter. Because its outer electron shell can donate up to 7 electrons (US EPA, 1984), Mn can assume 11 different oxidation states. Of environmental importance are Mn2+, Mn4+ and Mn7+. In living tissue, Mn has been found as Mn2+, Mn3+ and possibly as Mn4+ (Archibald and Tyree, 1987). Mn5+, Mn6+, Mn7+ and other complexes of Mn at higher oxidation states are generally unrecognized in biological materials (Keen, 1995). Mn plays an essential role as a cofactor in many enzymatic reactions in humans, but in excess quantities, can cause damage to the nervous system. Typically found in compounds with a coordination number of 6 and lacking octahedral coordination complexes, Mn tends to form very tight complexes with other substances (Keen, 1995). As a result, its free plasma and tissue concentrations tend to be extremely low (Cotzias et al., 1968)
Manganese (Mn) is an essential metal found in a variety of biological tissues and is necessary for normal functioning of a variety of physiological processes including amino acid, lipid, protein and carbohydrate metabolism (Erikson et al., 2005). Mn also plays an essential role in immune system functioning, regulation of cellular energy, bone and connective tissue growth and blood clotting (Erikson and Aschner, 2003). In the brain, Mn is an important cofactor for a variety of enzymes, including the anti-oxidant enzyme superoxide dismutase (Hurley and Keen, 1987), as well as enzymes involved in neurotransmitter synthesis and metabolism (Golub et al., 2005).
Despite its essentiality, Mn has been known to be a neurotoxicant for at least 150 years (ATSDR, 2000). Mn neurotoxicity was first identified as an extra-pyramidal syndrome in miners exposed to high concentrations of Mn ore (Barbeau et al., 1976; Couper, 1987; Mena et al., 1967). Exposure to excessive amounts of Mn is associated with a variety of psychiatric and motor disturbances (Chia et al., 1993; Calne et al., 1994; Pal, et al., 1999; Stredrick et al., 2004). The signs and symptoms from relatively high levels of exposure, as might occur in occupational settings, include postural instability, mood and psychiatric changes (i.e., depression, agitation, hallucinations) (Mena et al., 1967), and Parkinsonian symptoms such as bradykinesia, rigidity, tremor, gait disturbance, postural instability and dystonia and/or ataxia (Josephs et al., 2005). Cognitive deficits such as memory impairment, reduced learning capacity, decreased mental flexibility, cognitive slowing (Josephs et al., 2005) and difficulty with visuomotor and visuospatial information processing (Bowler et al., 2003) have also been reported. It has been suggested that the earliest signs of Mn intoxication may be subtle (Bowler et al., 2006) and that psychomotor testing, in particular, may be more sensitive than standardized neurological examinations in detecting central nervous system (CNS) defects in exposed workers (Roels et al., 1987; 1992).
The primary source of Mn intoxication in humans is due to occupational exposure in miners, smelters, welders and workers in dry-cell battery factories (Bowler et al., 2006; Chandra et al., 1981; Huang et al., 1989; Jiang et al., 2006a; Myers et al., 2003; Ono et al., 2002). Significant neurological dysfunction has been associated with the duration of Mn exposure (Roels et al., 1987). Other epidemiological studies of industrial workers have shown a positive correlation between neurological dysfunction and lifetime integrated or cumulative Mn exposure (Lucchini et al., 1995, 1999; Roels et al., 1992).
The present review is based on presentations from the meeting of the Society of Toxicology in San Diego, CA (March 2006). It addresses recent developments in the understanding of the transport of manganese (Mn) into the central nervous system (CNS), as well as brain imaging and neurocognitive studies in non-human primates aimed at improving our understanding of the mechanisms of Mn neurotoxicity. Finally, we discuss potential therapeutic modalities for treating Mn intoxication in humans.
2. Manganese (Mn) Transport in the Central Nervous System (CNS)
Mn enters the brain from the blood either across the cerebral capillaries and/or the cerebrospinal fluid (CSF). At normal plasma concentrations, Mn appears to enter into the CNS primarily across the capillary endothelium, whereas at high plasma concentrations, transport across the choroid plexus appears to predominate (Murphy et al., 1991; Rabin et al., 1993), consistent with observations on the rapid appearance and persistent elevation of Mn in this organ (London et al., 1989). Radioactive Mn injected into the blood stream is concentrated in the choroid plexus within 1 hour after injection; 3 days post-injection, it is localized to the dentate gyrus and CA3 of the hippocampus (Takeda et al., 1994). Manganism, a condition associated with exposure to excessive Mn levels, is associated with elevated brain levels of Mn, primarily in those areas known to contain high concentrations of non-heme iron (Fe), especially the caudate-putamen, globus pallidus, substantia nigra and subthalamic nuclei.
How and in what chemical form Mn is transported across the BBB has come to light in a series of studies conducted over the last two decades. It appears that facilitated diffusion (Rabin et al., 1993), active transport (Murphy et al., 1991; Aschner and Gannon, 1993; Rabin et al., 1993), divalent metal transport 1 (DMT-1; also known as DCT-1/NRAMP2)-mediated transport (Erikson et al., 2002; Garrick et al., 2003), ZIP8- and transferrin (Tf)-dependent transport (Aschner and Gannon, 1993) mechanisms are all involved in shuttling Mn across the BBB (Figure 1). Although non-protein-bound Mn enters the brain more rapidly than Tf-bound Mn (Murphy et al., 1991; Rabin et al., 1993), the question remains as to which form represents the predominant mechanism of transport in situ. Analyses of transport mechanisms which are based on tracer techniques employing bolus injections of Mn into the circulation cannot be easily interpreted. While tracer studies represent a sensitive technique for quantifying Mn transport, it must be noted that the information derived from such studies does not necessarily reflect the chemically active or functional forms in which Mn exists and is transported in vivo. This is due to the saturation of blood ligands for Mn and the likelihood that Mn in the free form exists at concentrations in excess to those expected under physiological conditions. Thus, transport kinetics under such conditions may not necessarily mimic physiological conditions. Leak-pathways for Mn also likely exist, especially in areas lacking intact BBB, namely the circumventricular organs (Figure 1).
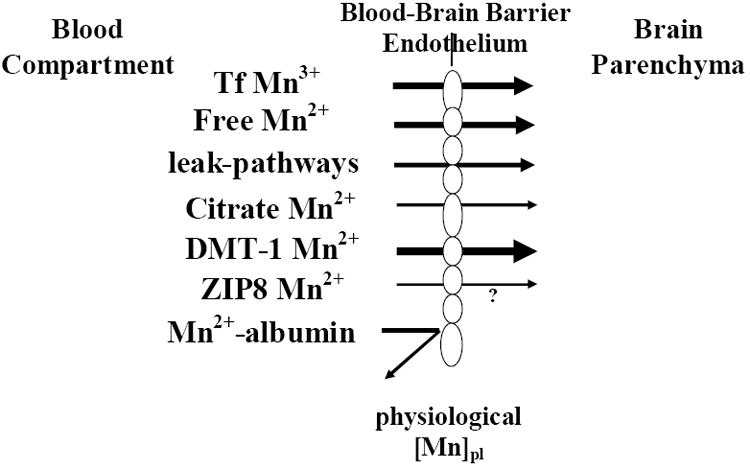
Mechanisms of Mn transport across the blood-brain barrier (BBB) under physiological exposure levels (physiological Mn plasma levels). Transporters associated with Mn transport (relevant to its oxidation state) are indicated. Mn bound to albumin is excluded from passing the BBB given its size. Arrow size depicts the relative importance of each of the transporters in this process, bolder arrows representing more prominent transport mechanisms. Please refer to the discussion for additional details. Since it has yet to be determined whether ZIP8 functions to transport Mn across the BBB, the process has been annotated with a question mark.
This brief review will address experimental evidence supporting of each of the above mentioned transporters in shuttling Mn across the BBB. No unique mammalian Mn transporters have been identified. The affinity of divalent Mn (2+) towards endogenous ligand is relatively low. Divalent Mn (2+) does not avidly complex with sulfhydryl (-SH) groups or amines, and it shows little variation in its stability constants for endogenous complexing ligands such as glycine, cysteine, riboflavin and guanosine. Within the plasma, approximately 80% of Mn is bound to globulin and albumin, and a small fraction of trivalent (3+) Mn is bound to Tf (Aisen et al., 1969). There is much theoretical and some experimental evidence suggesting that Tf, the principal Fe-carrying protein of the plasma, functions prominently in Mn transport across the BBB. In the absence of Fe, the binding sites of Tf can accommodate a number of other metals, raising the possibility that Tf functions in vivo as a transport agent for many of these metals. Mn binding to Tf is time-dependent (Keefer et al., 1970; Scheuhammer and Cherian, 1985; Aschner and Aschner, 1990). When complexed with Tf, Mn is exclusively present in the trivalent oxidation state, with 2 metal ions tightly bound to each Tf molecule (Aisen et al., 1969). At normal plasma Fe concentrations (0.9-2.8 μg/ml), normal iron binding capacity (2.5-4 μg/ml) and at normal Tf concentration in plasma — 3 mg/ml with 2 metal-ion-binding sites per molecule (Mr 77000) of which only 30% are occupied by Fe+3 — Tf has available 50 μ mole of unoccupied Mn3+ binding sites per liter.
Since Tf receptors are present on the surface of the cerebral capillaries (Fishman et al., 1985; Jeffries et al., 1984; Partridge et al., 1987) and endocytosis of Tf is known to occur in these capillaries (Partridge et al., 1987), it has been suggested that Mn (in the trivalent oxidation state) enters the endothelial cells complexed with Tf. Mn is then released from the complex in the endothelial cell interior by endosomal acidification, and the apo-Tf Tf complex is returned to the luminal surface (Morris et al., 1992a; Morris et al., 1992b). Mn released within the endothelial cells is subsequently transferred to the abluminal cell surface for release into the extracellular fluid (Figure 1). The endothelial Mn is delivered to brain-derived Tf for extracellular transport and subsequently taken up by neurons, oligodendrocytes and astrocytes for usage and storage. Support for receptor-mediated endocytosis of a Mn-Tf complex in cultured neuroblastoma cells (SHSY5Y) was recently demonstrated by Suarez and Eriksson (1993). Sloot and Gramsbergen (1994) have demonstrated anterograde axonal transport of 54Mn in both nigrostriatal and striatonigral pathways. Furthermore, in vivo intravenous administration of ferric-hydroxide dextran complex significantly inhibits Mn brain uptake, and high Fe intake reduces CNS Mn concentrations, corroborating a relationship between Fe and Mn transport (Aschner and Aschner, 1990; Diez-Ewald et al., 1968).
The distribution of Tf receptors in relationship to CNS Mn accumulation is noteworthy. The pallidum, thalamic nuclei and substantia nigra contain the highest concentrations of Mn (Barbeau et al., 1976). Interestingly, Fe concentrations in these structures are the highest as well (Hill and Switzer, 1984). Although the areas with dense Tf distribution (Hill et al., 1985) do not correspond to the distribution of Mn (or Fe), the fact that Mn-accumulating areas are efferent to areas of high Tf receptor density suggests that these sites may accumulate Mn through neuronal transport (Sloot and Gramsbergen, 1994). For example, the Mn-rich areas of the ventral-pallidum, globus pallidus and substantia nigra receive input from the nucleus accumbens and the caudate-putamen (Walaas and Fonnum, 1979; Nagy et al., 1978), two areas abundantly rich in Tf receptors.
A second prominent Mn transporter is the divalent metal transporter-1 (DMT-1; also known as NRAMP-2 and DCT-1; Figure 1). It is best known for its role as an intestinal, interluminal protein responsible for Fe regulation (Conrad and Umbreit, 2002; Mackenzie and Hediger, 2004). Indeed, gene transcription for this protein is actually regulated by Fe concentration via an iron-response element (IRE) located on the mRNA (Garrick et al., 2003). There is growing evidence that DMT-1 is involved in brain Mn delivery. It has been well established that DMT-1 has an affinity for Mn (Roth and Garrick, 2003). In the microcytic anemia (mk) mouse and the phenotypically similar Belgrade (b) rat (Fleming et al., 1997; Fleming et al., 1998), orthologous mutations (glycine 185 to arginine) in the DMT-1 gene result in significantly reduced dietary iron (Fe) absorption. The role of the defective DMT-1 allele in the transport of Mn across the BBB has been evaluated in homozygous Belgrade (b/b) rats that exhibit hypochromic anemia and also in heterozygous (+/b) Belgrade rats (Chua and Morgan, 1997). Plasma clearance and uptake by the CNS after intravenous injection of radioactive 54Mn bound to Tf or mixed with serum have demonstrated that plasma clearance of Mn-Tf was much slower than Mn-serum, but both were faster than the clearance of Fe-Tf. Uptake of 54Mn, and 59Fe by the brain was less in b/b rats than in +/b rats, suggesting that the defective DMT-1 allele affects the metabolism of both metals and that Mn and Fe might share DMT-1 transporters in the BBB (Chua and Morgan, 1997). Other in vitro work also strongly supports a role for this transporter in Mn movement across the BBB. Interestingly, DMT-1 activity is pH-dependent and probably Fe-dependent as well. Additionally, because metal transport via DMT-1 is an active process, it is likely to be temperature-dependent. This is interesting because data suggest that Mn transport across rat brain endothelial (RBE4) cells in culture is, among other things, temperature-, pH- and Fe-dependent (Fitsanakis et al. 2005). While the mammalian data are probably more germane to human Mn transport, it is also known that mutations in NRAMP-2, the DMT-1 homologue in bacteria and yeast, disrupt Mn transport (Kehres and Maguire, 2003; Portnoy et al., 2002; Rosakis and Koster, 2004; Rosakis and Koster, 2005). However, it is clear that DMT-1 is not the sole transporter of Mn across the BBB, since in the Belgrade rat, Mn is efficiently transported into the CNS (Crossgrove and Yokel, 2004). This is not altogether surprising since many metals and other molecules are dependent on numerous transporters and other proteins to facilitate their movement across various barrier systems. For an extensive review on the role of DMT-1 in mammalian transport of multiple metals, please refer to Garrick et al. (2003).
A small fraction of Mn is found in plasma as Mn-citrate (Crossgrove et al., 2003; Yokel and Crossgrove, 2004). In additional studies, these authors have assessed the regulation of Mn movement across the blood-brain barrier (BBB) with the in situ brain perfusion technique. The influx rates of Mn were compared to their predicted diffusion rates, which were determined from their octanol/aqueous partitioning coefficients and molecular weights. Results from these studies have suggested that a Mn citrate tridentate complex with a non-coordinated central carboxylate recognition moiety is likely a substrate for the organic anion transporter or a monocarboxylate transporter (MCT). Candidates for transport of Mn citrate may include MCT and/or members of the organic anion transporter polypeptide (OATP) or ATP-binding cassette (ABC) superfamilies. However, as pointed out by these authors, the most likely candidate for mediating transport of the Mn across the BBB is DMT-1.
Finally, recent evidence implies a role for the ZIP transporter proteins in Mn transport; although it has yet to be determined whether these proteins are functional at the BBB. The ZIP transporter proteins, members of the solute-carrier-39 (SLC39) metal-transporter family, have 14 members — highly conserved orthologs — between mouse and human (Eide, 2004). In plants, several ZIP proteins have been implicated in divalent metal transport, including zinc (Zn), Fe and Mn. A recent in vitro study (He et al., 2006) in mouse fetal fibroblast cultures established that ZIP8 has a high affinity for Mn. The Km of 2.2 μM for Mn2+ is close to physiological concentrations and within the same range determined in many cell lines or tissues. However, like many of the previously mentioned studies, one must be cautious in extrapolating into the intact animal, because the ability of this protein to transport a particular cation does not necessarily imply that this protein is physiologically relevant. Thus, whether Slc39 and organic transporters function in Mn transport remains to be tested under physiologically relevant conditions. To date, the strongest evidence-based studies on Mn imply a physiological role for the transport of Mn both by Tf receptors and DMT-1. Definitive studies to assess other protein functions (Slc39, MCT) in physiological roles have yet to be carried out.
3. Positron Emission Tomography (PET) Imaging in Mn Neurotoxicity: Old Questions, New Approaches
Mn is an essential element (see above), but exposure to excessive concentrations increases brain Mn levels resulting in a Parkinsonian-like syndrome. Parkinson's disease (PD) is the second most prevalent neurodegenerative disease and its hallmark neuropathology is the loss of nigrostriatal dopamine (DA) neurons in the substantia nigra pars compacta (SNpc). The progressive loss of DA neurons leads to the classical clinical symptoms of PD including resting tremor, hypoactivity, loss of balance and bradykinesia. Etiological and pathological data suggest that there are both genetic and environmental components that cause and contribute to dopaminergic degeneration (Jenner, 1998). Epidemiological studies also suggest that long-term Mn2+ exposure may be a risk factor for PD (see above).
Early work examining miners exposed to Mn described a constellation of extra-pyramidal symptoms with features similar to those exhibited by patients with idiopathic PD (Calne et al., 1994; Lee, 2000; Olanow, 2004; Pal et al., 1999). These features included bradykinesia, rigidity and gait abnormalities compounded by impaired dexterity and clumsiness (Calne et al., 1994; Olanow, 2004; Pal et al., 1999). Individuals with Mn-induced Parkinsonism also have more dystonia and fewer tremors than patients with idiopathic PD (Calne et al., 1994; Olanow, 2004; Pal et al., 1999). Furthermore, while L-dopa therapy is an effective means to ameliorate the clinical expression of PD, the benefits of L-dopa therapy have been described as having mixed results in Mn-exposed workers. Some reports describe the beneficial effects of L-dopa in the early treatment of Mn-induced Parkinsonism (Huang et al., 1989), while other reports describe no benefit (Lu et al., 1994). Therefore, both similarities and differences in the clinical expression of Mn-induced Parkinsonism and idiopathic PD exist.
From a neuropathological perspective, there are a very limited number of human studies in which autopsy samples of Mn-exposed brains have been examined (Bernheimer et al., 1973; Yamada et al., 1986 and references within). Furthermore, many of these brain autopsy examinations were performed in the early 1900's upon individuals working in heavily contaminated environments. Nevertheless, even from this limited number of human brain samples, there is clear evidence that basal ganglia nuclei are primary targets for Mn neurotoxicity. These early studies described significant neuropathological changes such as cell loss and gliosis in the globus pallidus, caudate, putamen and subthalamic nucleus (Bernheimer et al., 1973; Yamada et al., 1986 and references within). For the most part, the substantia nigra pars compacta has been described as being intact (Yamada et al., 1986), although one case indicated pathological involvement, loss of DA and the presence of Lewy bodies (Bernheimer et al., 1973). These neuropathological findings appear different from the consistent and marked degeneration of DAergic neurons of the substantia nigra pars compacta documented in idiopathic PD (Bernheimer et al., 1973).
The clinical expression and neuropathology of Mn-induced Parkinsonism has been duplicated to a large extent in studies using non-human primates (Chandra et al., 1979; Eriksson et al., 1987; Neff et al., 1969; Olanow et al., 1996; Pentschew et al., 1963). The early studies used extremely high doses of Mn in attempting to mimic human exposure (Pentschew et al., 1963; Neff et al., 1969). More recent studies have used lower dosing protocols for longer times of exposure (Chandra et al., 1979; Eriksson et al., 1987; Olanow et al., 1996). In general, the behavioral, neurochemical and neuropathological findings in primates agree strongly with findings documented in the human literature. Similar to human studies, non-human, primate studies have demonstrated motor function abnormalities following high doses of Mn exposure, including rigidity, hypoactivity, postural instability and action tremor (Neff et al., 1969; Eriksson et al., 1987, 1992; Shinotoh et al., 1995). From a neuropathological and neurochemical perspective, non-human primates exposed to high doses of Mn also exhibit alterations in dopamine levels as well as cell loss and gliosis in basal ganglia structures, especially in the globus pallidus (Pentschew et al., 1963; Chandra et al., 1979; Bird et al., 1984; Eriksson et al., 1987).
3a. Contribution of PET imaging to the understanding of Mn neurotoxicity
The advent of new technologies such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) in the early 1980s provided scientists the tools necessary to visualize and quantify the chemistry of the living brain. PET and SPECT are nuclear medicine imaging techniques that produce an image or map of functional processes in the body). These new technologies provided the advantage of being able to assess brain chemistry in human beings and in experimental animals in both healthy states and during active states of disease. Today, brain imaging is commonly used in many hospitals and research institutions throughout the United States and throughout the world. The first application of PET imaging to study Mn-induced neurological dysfunction was performed by a group led by Dr. Donald Calne (Wolters et al., 1989). In this study, the investigators performed PET imaging in 4 patients with Mn-induced Parkinsonism who had been working for more than 2 years in the Mn smelting industry in Taiwan. These individuals expressed bradykinesia and rigidity with masked facial expression, clumsiness, impaired dexterity and gait abnormalities. [18F]-fluorodopa PET studies were performed in these Mn-exposed individuals, and the results were compared to 21 normal, healthy controls and 13 patients with idiopathic PD. [18F]-fluorodopa PET provides an index of the ability of dopamine neurons to synthesize dopamine from L-dopa and represents an indirect measure of nigrostriatal dopaminergic neuronal integrity. [18F]-fluorodopa PET has been shown to be significantly decreased in patients with idiopathic PD (Brooks et al., 1990; Vingerhoets et al., 1994). As expected, in the study by Wolters et al., (1989), PD patients exhibited a dramatic decrease in the [18F]-fluorodopa activity in the striatum relative to controls. On the other hand, normal activity was measured in Mn-exposed workers that exhibited clinical signs of Parkinsonism and elevated levels of Mn in the blood and hair. Based on these findings, the authors concluded that Mn-exposed workers have an intact nigrostriatal dopaminergic system and that the Parkinsonian symptoms must be associated with the disturbance of post-synaptic striatal or pallidal neurons.
In a subsequent study by the same group of investigators published 8 years later (Shinotoh et al., 1997), they performed a follow up investigation in which they repeated [18F]-fluorodopa PET and also added [11C]-raclopride PET, a radioligand that binds to D2-dopamine receptors. They found that while Parkinsonian symptoms had progressed since their previous study, [18F]-fluorodopa PET was still normal in all of the patients, thereby corroborating their previous findings. [11C]-raclopride PET revealed a small (12%) but significant decrease in caudate/occipital ratios, for which the authors inferred that there was no major change in striatal D2 receptors.
More recently, SPECT studies with dopamine transporter radioligands have suggested that dopamine transporters in the striatum are decreased in Parkinsonian patients with a history of Mn exposure (Huang et al., 2003; Kim et al., 2002). Dopamine transporter levels in the striatum have been used as markers of dopamine terminal integrity in the diagnosis of PD (Pirker et al., 2002). These findings suggest that dopamine terminals in the striatum are degenerating in individuals with a history of Mn exposure. One of the confounding factors in this study is that it remains unknown whether the individuals with Mn exposure were already in the “pre-clinical” stages of PD and had merely experienced coincidental Mn exposure. Similarly, a case report by Racette et al., (2005a) of a female with a history of alcoholic cirrhosis with Parkinsonian symptoms showed MRI findings consistent with elevated Mn accumulation in the basal ganglia. The increase in brain Mn levels observed in this patient was due to the fact that, in liver disease Mn biliary excretion is impaired. The patient exhibited reductions in [18F]-fluorodopa in the striatum as compared to normal, age-matched controls, but not to the same degree as PD patients. This study is also confounded by the fact that this woman could have had PD with Mn accumulation in the basal ganglia due to her liver cirrhosis. However, the possibility that Mn exposure accelerates the progression of idiopathic PD cannot be ruled out. Therefore, the relationship between Mn exposure and PD remains a controversial topic in the human literature.
3b. PET studies in Mn-intoxicated monkeys
There are several PET imaging studies in non-human primates, providing additional insight into potential mechanisms of Mn-induced neurotoxicity. Shinotoh et al. (1995), were the first to use PET imaging in Mn-exposed primates. They exposed 3 male, adult rhesus monkeys to cumulative MnCl2 (single i.v. injections from 10 to 14 mg/kg) doses ranging from 71-87 mg Mn/kg of body weight and performed [18F]-fluorodopa PET to assess dopamine synthesis, [11C]-raclopride PET to examine D2-dopamine receptors and [18F]-fluoro-deoxyglucose PET to evaluate glucose metabolism. These PET studies were performed at baseline (prior to Mn administration) and at various time points after the initiation of Mn exposure. During Mn administration, two of the animals exhibited hypoactivity and weakness of the limbs; one animal did not express any degree of clinical abnormality. The latter result suggests that individual animals or human beings may have differential susceptibilities to Mn intoxication. At this point a cautionary note must be considered, namely that individual susceptibility to Mn in single animals may not be discriminated from experimental variation about a threshold for an effect. To ascertain this possibility, additional studies with larger animals will have to be carried out. The studies showed no significant changes from baseline for any of the PET studies during the course of Mn administration despite the fact that 2 out of the 3 animals exhibited clinical symptoms of Parkinsonism with significant accumulation of Mn in the basal ganglia, which was confirmed by MRI (Shinotoh et al., 1995).
Studies by Eriksson and colleagues using PET also showed that Mn-exposed monkeys expressed normal [11C]-L-Dopa turnover, but [11C]-nomifensin uptake (a measurement of dopamine transporter levels) declined progressively to reach a 60% reduction from baseline in the striatum, thereby suggesting dopaminergic terminal loss (Eriksson et al., 1992a). [11C]-raclopride was transiently decreased in the early stages of Mn intoxication, but levels normalized by the end of the studies. These animals were administered very high doses of Mn (400 mg MnO2 per injection on 12 different occasions for a cumulative dose of 4.8 grams of MnO2 or approximately 960 mg MnO2/kg body weight) and this very high dose paradigm may have caused the effect on dopamine transporter levels. They developed an unsteady gait with clumsiness of the hands and feet as well as hypoactivity. These studies suggested two possibilities: 1) the number of dopaminergic terminals in the striatum was constant with a reduced amount of dopamine transporter protein or 2) there was dopaminergic terminal loss with no change or with a compensatory increase in L-dopa activity. In either case, no conclusive statement could be made, and no post-mortem examination of the brain was performed.
Post-mortem studies of Mn-intoxicated primates have also provided conflicting information. For example, three reports have described decreased levels of dopamine in the striatum (Neff et al., 1969; Chandra et al., 1979; Eriksson et al., 1987) following relatively high doses of Mn exposure, while one report indicates no change in striatal dopamine levels and a normally appearing substantia nigra (Olanow et al., 1996). All primate studies noted prominent neuropathological changes (i.e., gliosis and cell loss) in the globus pallidus with some degree of involvement in the caudate, putamen and subthalamic nucleus (Neff et al., 1969; Chandra et al., 1979; Eriksson et al., 1987; Olanow et al., 1996; Pentschew et al., 1963). Finally, a report by Eriksson et al., (1992b) using receptor autoradiography indicated a significant decrease in striatal [3H]-SCH23390 binding to D1-dopamine receptors with no effect on D2-dopamine receptors and a significant decrease in [3H]-mazindol binding to dopamine transporters in the caudate and putamen. The latter study seems to confirm the PET findings by the same investigators (Eriksson et al., 1992a), namey that Mn-intoxicated monkeys exhibit decreased levels of dopamine transporters in the striatum.
3c. What can be surmised from the PET studies in Mn-exposed humans and primates?
As discussed above, Mn exposure likely alter different dopaminergic neuron markers or processes. Nevertheless, there is evidence to suggest that [18F]-fluorodopa PET is not affected by Mn exposure (Shinotoh et al., 1995; Wolters et al., 1989), consistent with the idea that Mn intoxication does not alter the integrity of nigrostriatal dopaminergic neurons. However, using radioligands that measure dopamine transporters, the data suggest that chronic exposure to high levels of Mn significantly decreases the level of this protein. Furthermore, there is evidence to suggest that dopamine transporters are targets for Mn, since acute Mn administration has been shown to transiently increase dopamine transporter levels in the striatum as measured by PET (Chen et al., 2006). The question that remains is whether, in the case of chronic Mn intoxication, the loss of dopamine transporters indicates the loss of dopaminergic terminals or the modulation of the protein. Taken together, the data suggest that the latter may be the case since PET studies assessing the conversion of L-dopa to dopamine are consistently found to be normal. Alternatively, the idea that Mn intoxication causes dopamine terminal loss without altering [18F]-fluorodopa PET cannot be dismissed. For example, in Wilson's disease, a genetic disorder of copper deposition in the basal ganglia, there are case reports of patients in which PET studies to measure dopamine transporter levels demonstrate significant loss in the striatum with no evidence of a change in [18F]-L-Dopa PET (Westermank et al., 1995). Importantly, similar to Mn intoxication, Parkinsonian symptoms and dystonia are prominent clinical features in Wilson's disease (Jeon et al., 1998). In both conditions, metal homeostasis in the caudate and putamen is disrupted. Interestingly, recent studies have documented an increase in the copper concentration in the globus pallidus, caudate and putamen of Mn-exposed primates (Guilarte et al., 2006).
3d. PET studies on Mn neurotoxicity
The molecular mechanisms by which Mn produces extra-pyramidal symptoms and other central nervous system effects are not well understood. To this end, Guilarte et al. (2006) have studied non-human primates chronically exposed to Mn. These animals received behavioral training for a variety of tests of cognitive function as well as monitoring of motor function and activity (See Schneider in this review). A comprehensive approach was undertaken, in which three important elements involved in dopaminergic neurotransmission — dopamine transporters, dopamine receptors and dopamine release — were measured using PET. Following baseline training of the animals for cognitive and motor assessment, PET studies were performed on the same day for D2-dopamine receptors ([11C]-raclopride), dopamine transporters ([11C]-methylphenidate) and in vivo dopamine release ([11C]-raclopride with amphetamine challenge). These PET studies were performed at baseline and during chronic Mn exposure (Guilarte et al., 2006). While no significant changes in [11C]-raclopride and [11C]-methylphenidate binding potential were present in any of the animals during Mn exposure, a significant and progressive decrease of in vivo dopamine release was measured in all of the animals (Figure 2) and (Guilarte et al., 2006). The lack of effect of Mn exposure on [11C]-methylphenidate binding despite observations of decreased transporter binding by other investigators (Huang et al., 2003; Kim et al., 2002) may be attributable to the magnitude and/or duration of Mn exposure, account for the differences between these studies.
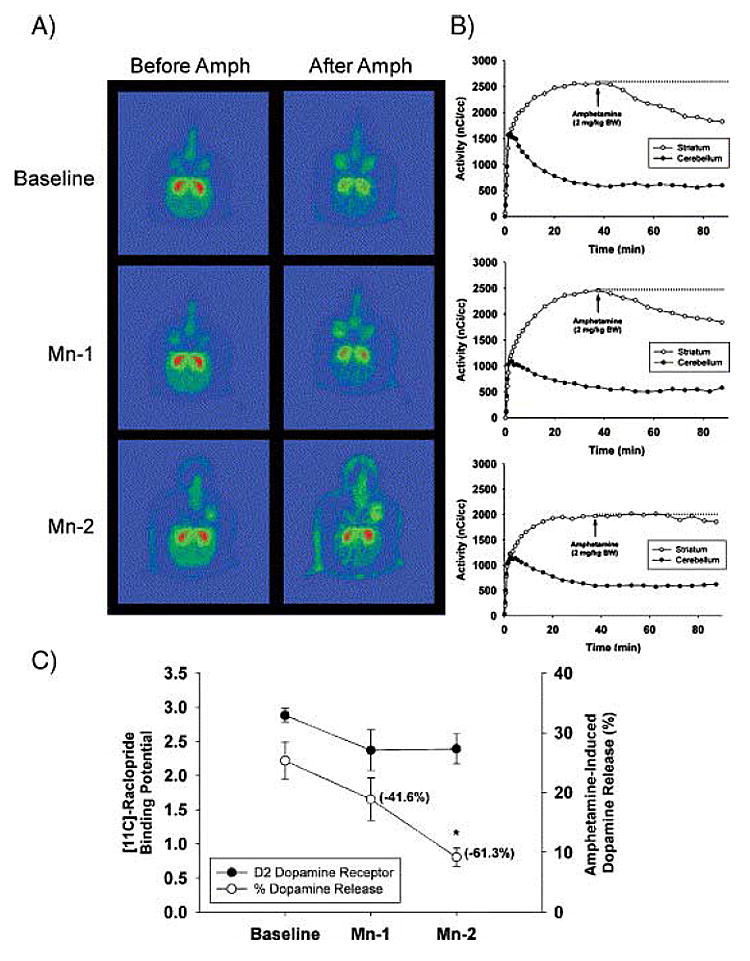
Fig. 2. Effect of chronic Mn exposure on [11C]-raclopride BP and AMPH-induced dopamine release. (A) Representative pseudocolor trans-axial images of [11C]-raclopride binding to D2-DAR in the striatum of one animal at baseline and at the Mn-1 and Mn-2 time points. Red areas represent high levels of binding and green areas represent low levels of [11C]-raclopride binding to D2-DAR. Note the progressive lack of a change in [11C]-raclopride levels in the striatum after AMPH administration from baseline to Mn-2. (B) [11C]-raclopride time–activity curves in the striatum and cerebellum before and after AMPH in the same animal as in panel A. Each graph corresponds to the adjacent images in panel A. At baseline (top graph in B), there is a dramatic decrease in [11C]-raclopride levels in the striatum after AMPH administration. Increasing Mn exposure reduces the effectiveness of AMPH-induced DA released to displace [11C]-raclopride from the striatum (see middle and lower graph in B). (C) Quantitative data on [11C]-raclopride BP and AMPH-induced in vivo DA release for all animals. For dopamine release, the numbers in parenthesis are the mean percent change from baseline. Each value is the mean ± SEM of 4 Mn-exposed animals. *p < 0.05. (Reprinted from: Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates Experimental Neurology 202:381-390, 2006 with permission from Elsevier).
The decrease of in vivo dopamine release was most pronounced at a time in which the Mn-exposed animals expressed deficits in fine motor dexterity and activity (Guilarte et al., 2006). The marked effect of Mn exposure on in vivo dopamine release measured by PET has not been described in earlier studies, and it represents a new mechanism by which Mn exposure may affect basal ganglia function. These results show that nigrostriatal dopamine system dysfunction is possible in the absence of a change in a marker of dopamine terminal integrity. These findings may help explain previous observations that L-dopa therapy is not an effective means of ameliorating the clinical symptoms in Mn-exposed humans or primates since the problem may not be one of dopamine synthesis or loss of dopamine terminals but one of dopamine release. Furthermore, these findings provide a new avenue for investigating the effects of Mn on the nigrostriatal dopaminergic system.
Notably, in a previous study in which acute high dose Mn was given to 2 non-human primates, a transient but reproducible increase in striatal DAT levels was ascertained by PET (Chen et al., 2006). In this study by Chen et al., (2006) it was also shown that Mn inhibits the binding of the cocaine analog [3H]-WIN 35,428 to rat striatal membranes and the uptake of [3H]-dopamine to rat striatal synaptosomes. The acute effect of Mn on dopamine transporter levels measured by PET is different from the lack of a significant effect of chronic Mn exposure on striatal dopamine transporter levels described by Guilarte et al., (2006). It is likely that this variation in effect is based on whether Mn is given acutely or chronically. The consistent finding with both studies is that dopamine transporters are direct targets for Mn-induced effects. As demonstrated in the work by Chen et al., (2006), acute Mn increased DAT levels. On the other hand, in the chronic study (Guilarte et al., 2006); Mn abrogates the stimulatory effect of amphetamines on dopamine release, which is presumed to be due to a Mn-induced inhibition of dopamine transporters. In summary, these studies clearly point out that molecular imaging with PET, SPECT and other modalities will play an increasingly important role in defining the complex neurotoxic effects of Mn on the living brain.
4. Behavioral Effects of Mn Intoxication on Non-Human Primates
As discussed above, much has been learned about the basic toxicology of Mn from studies in rodents, and especially from the relatively few studies on the effects of Mn exposure in non-human primates, a species whose behavioral repertoire more closely resembles that generated by the human neurobehavioral system. Because Mn affects dopaminergic systems and its neuropathology closely resembles PD (see above), studies on the behavioral consequences of Mn exposure in non-human primates are extremely valuable.
To date, most studies have examined outcomes following relatively high level acute or chronic exposure to Mn. Less is known about the effects of chronic exposure to lower levels of Mn or about the threshold exposures sufficient for altering cognitive and motor function. This is of primary importance as concerns about possible adverse effects from long-term exposure to increasing ambient levels of Mn in the environment continue to mount (Aschner et al., 2005). To begin to address this issue, this section of the review will also briefly discuss data being collected as part of an on-going, multi-disciplinary study assessing the behavioral, neuroimaging and neuropathological consequences of chronic exposure to different levels of Mn in non-human primates.
4a. Effects of Mn on Motor Functioning
A number of studies have described the effects of Mn exposure on motor functioning in non-human primates. Although these studies used different levels of cumulative exposure and different forms of Mn, the types of motor dysfunctions noted (mostly associated with increasing cumulative exposure) include rigidity and bradykinesia (Olanow et al., 1996), intention tremor (Newland and Weiss, 1992; Suzuki et al., 1975), uncoordination (Neff et al., 1969; Suzuki et al., 1975), hyperactivity (Eriksson et al., 1992), unsteady gait and clumsiness (Eriksson et al., 1992).
In a study of 3 rhesus monkeys, Olanow et al. (1996) described the development of marked generalized bradykinesia, rigidity and abnormal extensor posturing of the hindlimbs after only 2 intravenous injections of 10 mg/kg each of Mn chloride. Movements suggestive of facial dystonia were also noted. A second animal also developed moderately severe bradykinesia, rigidity, extensor posturing and grimacing suggestive of facial dystonia after 2 injections of Mn chloride (total 23 mg/kg). Interestingly, a third animal did not develop any neurological signs after receiving 87 mg/kg of Mn over a 43 day period (Olanow et al., 1996). The motor dysfunction in the 2 symptomatic animals did not respond to L-dopa therapy.
In contrast to the rapid development of motor symptoms described above, other investigators have reported much more slowly evolving motor function deficits following chronic exposure to Mn. Mella (1923) injected monkeys intramuscularly with 5 mg of Mn chloride every other day for 18 months and reported that the animals developed choreoathetoid movements, tremor and rigidity. Gupta et al. (1980) described the development of muscular weakness and rigidity in the lower limbs of rhesus monkeys after 18 months of daily exposure to Mn (25 mg/kg Mn chloride, p.o.). Eriksson et al. (1987) exposed each of 4 monkeys to a cumulative dose of 8 g of Mn oxide by repeated subcutaneous injections of 0.2 g of Mn per injection over a 5 months period. All animals developed hyperactive behaviors after about 2 months of Mn administration and then became hypoactive with an unsteady gait and some action tremors by approximately 5 months of Mn exposure. The animals developed increasing weakness and clumsiness throughout the latter part of the study period. In another study, Eriksson et al. (1992) administered Mn oxide to 2 monkeys on 11 occasions over a 4 months period, with 0.4 g of Mn oxide injected subcutaneously on each occasion. Both animals developed an unsteady gait, minor clumsiness of the hands and feet and hypoactivity after 4-5 months. Eriksson et al. (1992a) also administered Mn oxide by subcutaneous injection to 3 monkeys on 13 occasions (0.2 g Mn per injection) over a 26 months period. The authors reported that the monkeys in this study did not show any obvious symptoms (although one animal, however, did show a time-dependent decrease in locomotor activity) from this “low-dose Mn intoxication” as compared to the animals in the previous study which received larger amounts of Mn during a shorter period of time. One report on the behavioral outcome of rhesus monkeys exposed to Mn by inhalation (exposure to Mn dust particles, less than 5 μm in diameter at a concentration in air of 30 mg/m3 with a total of 4 g of Mn dust blown through the inhalation chamber in a six hour exposure period each day) (Bird et al., 1984) claimed that the animals showed neither abnormal movements nor abnormal neurological signs during the 2 years of exposure. It is noteworthy that generalizing on the effect of exposure duration on neurotoxicity is somewhat difficult, given the different species and Mn salts used in various experimental paradigms.
One study has recently examined the neurobehavioral development of rhesus monkeys raised on cow's milk formula (containing 50 μg Mn/L), soy formula (containing 300 μg Mn/L) or soy formula supplemented with Mn chloride (containing 1,000 μg Mn/L) through 4 months of age (Golub et al., 2005). Motor endpoints thought to be potentially reflective of Mn-induced developmental neurotoxicity were more consistently present during observation sessions (compared to animals that did not receive Mn), and an increase in overall spontaneous activity was witnessed (Golub et al., 2005).
As part of an on-going multi-disciplinary study (Schneider and Guilarte labs) assessing the behavioral, neuroimaging and neuropathological consequences of chronic exposure to different levels of Mn sulfate in non-human primates, recent studies have examined several aspects of motor functioning in monkeys in “low,” “intermediate” and “high” exposure groups. Motor assessments included automated activity measurements, motor ratings and assessment of fine motor skills (Schneider and Pope-Coleman, 1995). Animals were randomly assigned to treatment groups and received intravenous injections of Mn sulfate (MnSO4, doses calculated as the salt) at either 10 mg/kg/week for 5 weeks and then 15 mg/kg/week for the remainder of the study period (“low” dose group), 15 mg/kg/week for 5 weeks and then 20 mg/kg/week for the remainder of the study period (“intermediate” dose group), or 20 mg/kg/week for 5 weeks and then 25 mg/kg/week for the remainder of the study period (“high” dose group). The cumulative amount of Mn administered to the “low” dose group was 156.7 ± 9.5 mg/kg of body weight over 272 ± 17 days. Animals in the “intermediate” dose group received 210.4 ± 1.3 mg Mn/kg of body weight over 271 ± 3 days, and animals in the “high” dose group received 97.5 ± 21.9 mg Mn/kg of body weight over 68.3 ± 18.2 days.
In the “low” dose group, motor rating scores, although significantly different from baseline by the end of the study period, did not indicate the presence of Parkinson-like or other clinically significant gross motor disorders. No abnormal movements (i.e., dystonia or dyskinesia) were observed in any of these animals during the study. However, the animals did develop considerable difficulty with fine motor skills by the end of the study period. Fine motor skills were assessed using 2 boards made of clear Plexiglas that contained 16 wells of small (14 mm) or large diameter (22 mm), all of which were 17 mm deep (Schneider and Pope-Coleman, 1995). An “easy” version of the board consisted of 12 large and 4 small wells while a “difficult” version of the board consisted of 12 small and 4 large wells. Animals were required to retrieve a standard reward pellet from each well as quickly as possible. The primary measure obtained was the number of errors made per well (i.e., the number of attempts to remove a pellet from a well and/or the number of times a pellet was dropped). On this test of fine motor skills, animals made 0.98 ± 0.80 errors/well on the “easy” board and 2.45 ± 0.91 errors per well on the “difficult” board during pre-Mn baseline testing. Over the course of the manganese exposure period, performance on the “easy” board did not significantly change whereas animals made significantly more errors on the “difficult” version of the task at the end of the Mn exposure period (6.25 ± 0.35 errors) (Schneider et al., 2006).
Overall activity during 2 – 3 consecutive 24 hr. periods was recorded prior to manganese exposure (1 -3 separate sessions) and following manganese exposure (1 session approximately every 2 - 4 weeks) using a personal activity monitor (Actitract; IM Systems, Inc.) placed into a pocket on the back of a jacket worn by the animals during the evaluation period. Overall activity levels in the “low” dose group were significantly decreased from baseline by the end of the study period (normalized activity levels were 100 ± 4.4; in the pre-Mn baseline period at 41.2 ± 3.9 at the end of the Mn exposure period) (Guilarte et al., 2006). Studies with the “intermediate” and “high” dose groups are ongoing. Although there is not yet enough data to comment on the results of the studies on “intermediate” dose animals, total activity initially decreased in all “high” dose animals and then increased significantly above baseline levels, coinciding with the development of hyperkinesis (a medical condition resulting in uncontrolled muscle movement, akin to spasms). All “high dose” animals developed dystonia as well as spontaneous choreiform dyskinesias (involuntary movement with jerky displacements of short duration affecting the limbs and the face) that appeared at approximately 7 – 12 weeks of Mn exposure. Some animals in this group also developed significant ataxia. Due to the significant motor disturbances exhibited by these animals, they were euthanized much sooner after initiation of Mn exposure than animals that received lower amounts of Mn per injection (“low” and “intermediate” exposure groups) and thus had shorter durations of exposure and received lower cumulative amounts of Mn. However, that these “high” dose animals developed significant motor problems with shorter durations of exposure and lower cumulative amounts of Mn than the other Mn-exposed animals suggests that it is not just the cumulative exposure that is important but the amount per exposure that plays a significant role in determining outcome from Mn exposure.
4b. Effects of Mn on Cognitive and Behavioral Functioning
Compared to the number of studies examining the motor effects of Mn exposure, there have been considerably fewer studies exploring the cognitive and behavioral outcomes of Mn exposure in non-human primates. Newland and Weiss (1992) investigated the effects of Mn on effortful responding and schedule-controlled behavior in 3 Cebus monkeys in an effort to evaluate the effects of Mn on motor properties and response patterns on operant tasks requiring different levels of physical exertion. Monkeys were required to push against a spring-loaded device that resisted extension with a force that approximated their body weight and had to move the device through an angle of about 45 degrees (an arc length of 10 cm) after which it returned to the starting position. This behavior was maintained using a multiple fixed-ratio, fixed-interval schedule of performance. During the fixed ratio schedule, the reinforcement cycle was triggered after 20 responses were completed. During the fixed-interval 90-sec schedule, the reinforcement cycle was initiated by the first response to occur after 90 sec had elapsed. Lights situated in front of the animals signaled which reinforcement schedule was in effect. Mn chloride (5 or 10 mg/kg, calculated as the salt) was administered to the animals by intravenous injections separated by at least 1 week. After only 2 administrations of 5 mg Mn/kg, a disruption of effortful responding (an increase in the number of incomplete responses) was observed, although only under the fixed-ratio schedule of reinforcement that maintained a high rate of response. Only acute administration of the 10 mg Mn/kg dose caused a transient increase in incomplete responding under the fixed-interval schedule. Overall response rates and patterns were not affected by Mn exposure despite an increase in response failures (Newland and Weiss, 1992; Newland, 1999). Action tremor appeared in all monkeys after a cumulative dose of 40 mg/kg. The apparent lack of effect of Mn toxicity on motivation (i.e., no effects on rate of responding) and the disruption of only a behavior that required considerable exertion on the part of the animal suggests a subtle effect of relatively low Mn exposure on motor performance, but not upon cognitive/behavioral performance (Newland and Weiss, 1992).
Golub et al. (2005), in their study of the neurobehavioral development of rhesus monkeys raised on cow's milk formula, soy formula or soy formula supplemented with Mn chloride, examined performance with a number of behavioral parameters. Group differences were not apparent on the relative performances of specific cognitive tasks (i.e., delayed nonmatch to sample, object discrimination, position discrimination and reversal and continuous performance test) that assessed learning, memory and attention. Overall, infant animals (up to 18 months of age) were noted to be poorly engaged in the cognitive function tests regardless of the treatment group, suggesting that additional testing at more mature ages is necessary. However, the soy group infants were described as having poorer participation across tasks and slower attainment of performance criteria on some tasks (Golub et al., 2005). In addition, significant differences between cow's milk and soy groups were found on the duration of the daily sleep period (longer in soy groups), frequency of cling behaviors (increased in soy groups), play behaviors (decreased in soy groups), premature response during a reward delay test, failure to improve performance with experience and greater response rates during early continuous performance testing in the soy + Mn group. These results may indicate an increase in impulsivity or a lack of normal wariness (Golub et al., 2005). Despite limitations in interpreting behavioral data obtained from infant monkeys, these results suggest that developmental Mn exposure may negatively influence certain aspects of neurobehavioral development.
In an on-going study of the consequences of chronic exposure to different levels of Mn sulfate in non-human primates, several aspects of cognitive and behavioral functioning in monkeys in “low,” “intermediate” and “high” exposure groups were examined. In the “low” dose group, the effect of Mn exposure on spatial working memory performance (variable delayed response task) varied among the animals and thus, group analyses of these behavioral data showed inconsistent changes in each group's performance across task delays during Mn exposure. However, there was a trend toward decreased performance at short and intermediate delays (i.e., 2 – 10 second delays), and chronic Mn exposure interfered with the expression of learning effects such as those observed in normal animals during long-term task performance. Visual discrimination performance (reference memory) was not affected in these animals, and no consistent changes were observed in the cognitive component of an object retrieval task (executive functioning) (Schneider and Pope-Coleman, 1995). In contrast, certain stereotypical behaviors such as grooming and the licking/biting of fingers significantly increased in frequency during the Mn exposure period.
As with the “low” dose exposure group, variable effects of “intermediate” dose Mn exposure on spatial working memory performance have been observed to date, with one animal showing a consistent and reliable decrease in performance at short and intermediate delays. Neither visual discrimination nor object retrieval performance has been affected by the “intermediate” dose of Mn exposure. Preliminary analyses of behavioral data also suggest an increased frequency of certain stereotypical behaviors as seen with the “low” dose exposure group.
To date, animals in the “high” dose Mn group have shown no changes in performance of the spatial working memory task, but all animals have developed early-appearing deficits in a non-spatial working memory task (delayed matching to sample). There was no deterioration in performance of the reference memory task or on the object retrieval task.
In summary, there have been a number of studies examining Mn toxicity in non-human primates. However, most have focused on imaging parameters, post-mortem pathological changes and effects on gross motor behavior (Olanow et al., 1996; Eriksson et al., 1987; Eriksson, et al., 1992, Eriksson et al., 1992a). Most studies have used either Mn chloride or Mn oxide and a variety of exposure routes including oral administration, inhalation and subcutaneous or intravenous injection. In ongoing studies in our lab (Guilarte et al., 2006), Mn sulfate was chosen as the experimental choice for treatment, since it is one of the main combustion products of methyl-cyclopentadienyl Mn tricarbonyl (MMT) (Ressler et al., 1999; Zayed et al 1999), an antiknock additive to unleaded fuel that contributes to environmental deposition of Mn (Aschner et al., 2005). Although the neurobehavioral toxicity of Mn in rodents has been shown to differ by the form of Mn administered (Gianutsos and Murray, 1982; Komura and Sakamoto, 1992; Zheng et al., 2000) as well as by the route of administration, these parameters have not been systematically manipulated in non-human primate studies. Thus, the effects of different exposure types and forms of Mn may influence outcomes, but this cannot be stated with any certainty at this time.
While it is relatively easy to detect motor dysfunction in monkeys resulting from Mn exposure, it is much more difficult to detect and quantify subtle cognitive and behavioral abnormalities that might arise, particularly when these abnormalities may precede other overt manifestations of toxicity. Variation in the appearance of these more subtle signs, as we and others have observed in monkeys, reflects a variation in individual susceptibility to the behavioral effects of Mn exposure. These variations have also been described in Mn-exposed humans (Iregren, 1990). It is possible that more consistent cognitive deficits might be observed with more prolonged Mn exposure, more frequent dosing or examination of performance on tasks that tap into different cognitive domains than we have studied or on tasks with greater demands on the processes already explored. Additional work is clearly necessary to better understand the long-term effects of different forms, doses and dosing regimens of Mn on cognitive and motor functioning in both adult and developing Mn-exposed non-human primates.
5. Human Studies of Mn Intoxication
While information exists on Mn transport and mechanisms of Mn-induced neurotoxicity in a variety of species, significant challenges remain in extrapolating the information to humans. Several crucial issues in human Mn toxicity remain unresolved, three of which will be detailed below: 1) the lack of a clearly defined clinical standard to distinguish manganism from idiopathic Parkinson's disease (IPD), 2) the lack of reliable biological markers to assess the internal dose of Mn and its relationship to environmental exposure and 3) the lack of a successful clinical therapeutic strategy for treatment. This section will briefly discuss each of these issues, providing a critical review and directions for future research.
5a. Diagnosis of Manganism in Humans
As mentioned above, typical signs and symptoms shown by manganism patients resemble those of IPD, including tremor, rigidity, bradykinesia and posture instability (Barbeau et al., 1976; Calne et al., 1994; Inoue and Makita, 1996; Mena et al., 1967). The patients may initially exhibit palpitations, headache, memory loss, hand tremor, lower limb myalgia and hyper-myotonia. Without treatment, symptoms usually progress. Giddiness, myasthenia, frequent tetany and numbness in the arms and legs may occur. In severe cases, the patient may display tremors at the angle of the lips and at the tip of the tongue, the tongue bitten while speaking, profound trembling of the upper extremities while writing or carrying objects and abnormal nose-pointing function. More typically, the patient's handwriting becomes characteristically irregular, with particular difficulty drawing circles, and letters become progressively smaller and smaller. Additionally, a distinct, festinating gait may develop (Jiang et al., 2006a). Patients may also display neuropsychological difficulties that include apathy and even psychosis. After withdrawal from the occupational exposure, Mn concentrations in the patient's blood, urine or hair may return to normal after several months. Some symptoms may stabilize or improve to a certain extent; however, many symptoms that are associated with extrapyramidal damage persist. Severely intoxicated patients have difficulty simply coping with daily life.
Basic research suggests that the sites of Mn-induced neurological lesions are different from those observed in idiopathic Parkinson's disease (IPD). The brain regions primary targeted in manganism are the globus pallidus and the striatum of the basal ganglia, whereas the neurodegeneration in IPD occurs mainly in the substantia nigra (Calne et al., 1994; Cersosimo and Koller, 2006; Inoue and Makita, 1996; Olanow et al., 1996; Yamada et al., 1986; Walter et al. 2003). As such, it is argued that the differences in the sites of pathologic lesion would determine the clinical manifestation of manganism or IPD. Consequently, the lack of the response of manganism patients to L-dopa therapy, a criterion to differentiate manganism from IPD that is commonly acknowledged among clinicians, may mirror the specific lesion sites in the brain for manganism or IPD, leading to different responses to L-dopa.
Recent studies on human subjects, however, indicate that chronic exposure to Mn in certain occupations may increase the risk of acquiring or accelerating PD (Gorell et al., 1997; Racette et al., 2001, 2005a, b). Racette and colleagues (2005a) screened 1,423 welders in Alabama who were referred for medical evaluation for PD. By using the Unified Parkinson's Disease Rating Scale (UPDRS), these investigators reported that the estimated prevalence of Parkinsonism among active male welders ages 40 to 69 was about 0.98% to 1.3%, significantly higher than that of the age-standardized general population (et al., 2005a). In addition, the clinical symptoms and the response of welders to L-dopa treatment are not strikingly different from those of IPD patients (Racette et al., 2001). An earlier community-based study has suggested that Mn neurotoxicity may be a continuum of dysfunction with early, subtle changes at lower levels of exposure (Mergler et al., 1999). However, a more recent study using a nationwide cohort of Swedish welders has suggested that there is no relationship between welding and PD or any other movement disorders (Fored et al., 2006). Thus, no conclusive judgment can be made at present regarding the association between career welding practice and the etiology of idiopathic PD.
Despite these arguments, the critical point on which the entire debate rests is the credible diagnosis of manganism. Since there is no reliable biomarker available for clinical assessment of the degree of Mn neurotoxicity, the diagnosis must then take a patient's occupational history into consideration. Based on experience gained from Chinese workers (Crossgrove and Zheng, 2004; Jiang et al., 2006a), the diagnosis of manganism is usually less ambiguous for active working patients than for patients who have long ago left the job, and more consistent for smelters than for welders, likely due to welders' day-to-day job variation and inconsistent environmental exposure. Clearly, establishing standard criteria for diagnosing manganism is of paramount importance in research on Mn neurotoxicity in humans.
5b. Search for biomarker of Mn exposure
It should be emphasized that the symptoms of Mn intoxication, once established, usually become progressive and irreversible, reflecting to some extent the permanent damage of neuronal structures. Thus, searching for a reliable biological indicator or biomarker for early Mn exposure has become a daunting task in the clinical investigation of Mn neurotoxicity.
Because of the intracellular distribution and relatively short half life of Mn in the blood compartment (Zheng et al., 2000), blood Mn in general does not serve as a reliable indictor of the total body burden of Mn or of the overall disease status. A study on 97 career welders revealed that serum Mn concentrations do not correlate with welders' professional years. However, these welders indeed showed significantly higher serum levels of both Mn and iron (Fe) than those of control subjects (Lu et al., 2005). Thus, on a group comparison basis, serum Mn levels may serve reasonably well as an indicator of recent Mn exposure (e.g., welders vs. normal controls). However a relatively large variation in blood Mn among individuals, either due to diet or other environmental influences, disqualifies blood Mn for clinical uses. Additionally, blood Mn is not suitable for the estimation of the historical accumulation of Mn in the body following long-term, low-level Mn exposure. Nevertheless, a consistently high level of blood Mn in any individual above the local surveillance range should unequivocally suggest recent exposure to Mn.
Compared to blood Mn, urine Mn is even less likely to be a clinical indicator for Mn toxicity, because the primary route of Mn excretion (>95%) is via the bile to feces (Klaassen, 1974). Yet, the limited Mn urinary excretion as a background may prove to be valuable for the EDTA challenge test. Of eight reported Chinese patients who received EDTA treatment (Crossgrove and Zheng, 2004, Jiang et al., 2006a), five had urinary Mn levels nearly 2 fold higher than before EDTA treatment. Thus, some clinicians have recommended EDTA as a challenge agent to test Mn exposure, since a significant increase in urinary Mn levels after EDTA treatment may suggest the accumulation of Mn in the body. However, caution must be taken, for it remains unclear whether EDTA mobilizes physiological Mn in normal subjects, to what extent the elevated urinary Mn level is considered a confident reflection of Mn body overload and how the EDTA dose regimen should be designed to facilitate a reasonable assessment. Therefore, it is necessary to build the database on Mn excretion by EDTA among normal subjects in order to evaluate the potential uses of EDTA for Mn diagnosis more comprehensively.
A body of evidence suggests that Mn-induced neurotoxicities appear to be associated with altered Fe metabolism at both systemic and cellular levels (Chua and Morgan, 1996; Li et al., 2004; Malecki et al., 1999; Zheng and Zhao, 2001; Zheng et al., 1999). Among career welders, serum concentrations of ferritin and Tf are higher, while serum Tf receptor levels are lower than those of controls. Interestingly, serum Tf receptor levels decline as serum Mn concentration increases. These findings suggest that exposure to welding fumes among welders disturbs the serum homeostasis of Fe and the proteins associated with Fe metabolism (Li., et al., 2004; Lu et al., 2005). In vivo Mn exposure in animals appears to facilitate the influx of Fe from the blood to the cerebral spinal fluid (CSF) (Zheng et al., 1999). Moreover, in vitro treatment of cultured cells with Mn promotes cellular Fe overload (Li et al., 2005, 2006; Malecki et al., 1999; Zheng and Zhao, 2001). In humans, a dysfunction in Fe metabolism has been seen in IPD patients. High levels of total Fe, decreased ferritin, Fe-associated oxidative stress and abnormal mitochondrial complex-I have been repeatedly reported in the postmortem substantia nigra of IPD patients (Dexter et al., 1991; Griffiths and Crossman, 1993; Loeffler et al., 1995; Sofic et al., 1991). Thus, accumulation of Fe in selected brain areas may induce the generation of Fe-mediated reactive oxygen species; the ensuing oxidative stress may then lead to neuronal cell death (Connor, 1997; Youdim et al., 1993). Since Fe and related metabolizing proteins are readily assayed in serum, this theory must be tested.
The life brain scan by MRI is another promising technique with potential uses for diagnosing Mn neurotoxicity (see also above). An increased T1-weighted (the time associated with the return of longitudinal magnetization to its equilibrium condition is termed the spin lattice relaxation time, T1, and reflects the strength and nature of magnetic interactions between the spins and their atomic neighborhood). Mn signal in the globus pallidus area has been found among workers occupationally exposed to Mn (Dietz et al. 2001; Jiang et al., 2006a; Kim et al., 1999a; Lucchini et al., 2000; Nelson et al., 1993), in patients receiving long-term total parenteral nutrition (Alves et al., 1997; Ejima et al., 1992; Fell et al., 1996; Fredstrom et al., 1995; Iwase et al.,2000; Nagatomo et al., 1999; Quaghebeur et al., 1996) and in clinical cases of hepatic failure (Butterworth et al., 1995; Chetri et al., 2003; Hauser et al., 1994; Hazell et al. 1999; McKinney et al., 2004; Spahr et al., 2002). Kim and his colleagues have reported that a welder with more than 10 years of Mn exposure whose clinical manifestations included a masked face, asymmetric resting tremor and bradykinesia had symmetrical high signal intensities in the globus pallidus on the T1-weighted image. The intensity, however, nearly completely disappeared six months after he discontinued the welding practice (Kim et al., 1999b). This has led the authors to conclude that MRI, similar to blood Mn measurements, may be useful for detecting recent Mn exposure.
5c. Therapy
Another challenging issue in the clinical aspect of the study of Mn toxicity is the lack of an effective therapeutic strategy for Mn-induced neurological impairment. Without treatment, the severity of symptoms usually increases, and the chance of recovery decreases. Treatment with L-dopa has been the first choice in most clinics for two reasons. First, a neurologist tends to control or alleviate the devastating symptoms associated with extrapyramidal injury. Clinical cases have shown that replacement of the lost dopamine could initially improve extrapyramidal symptoms (Lee, 2000; Mena et al., 1970; Rosenstock et al., 1971). Second, a neurologist tends to use L-dopa as a diagnostic tool to distinguish manganism from IPD. There are bodies of evidence supporting the view that L-dopa therapy generally has limited benefits in improving clinical symptoms among manganism patients, which contrasts to the response from IPD patients. A five-year follow-up study indicates that the response to L-dopa treatment decreases after two or three years (Huang et al., 1993). The same group of investigators further indicates that, ten years after the cessation of Mn exposure, the same patients continued to show progression in the severity of symptoms (Huang et al., 1993, 1998).
In severe cases of Mn poisoning, chelation therapy has been recommended in order to reduce the body burden of Mn. EDTA (ethylene diamine tetra acetic acid) is a commonly used chelating agent for treatment of a variety of metal-induced toxicities (e.g. lead), and it has been shown to increase toxin elimination, reducing the body burden of Mn and therefore proving useful in treating manganism patients at early stages of disease development (Hernandez et al., 2006). Among seven Chinese welders with manganism, EDTA treatment increased Mn excretion in urine and decreased Mn concentrations in blood (Crossgrove and Zheng, 2004). Although the clinical symptoms were not alleviated significantly among these patients, EDTA chelation therapy appears to be helpful in reducing blood Mn levels in acutely poisoned patients. A similar conclusion using EDTA therapy has also been reported by other investigators, although they questioned the efficacy of this treatment for reducing neurological symptoms (Calne et al., 1994; Discalzi et al., 2000; Ono et al., 2002). This limited therapeutic benefit of EDTA is expected. The four carboxyl groups in the EDTA structure, while essential to its chelating property, render the molecule poorly lipophilic and thus prevent it from effectively crossing the blood-brain barrier (Fenstermacher et al., 1988; Schlageter et al., 1987; Von Holst et al., 1989). EDTA likely chelates mainly the extracellular Mn ions.
Recently, an antibacterial drug, sodium para-aminosalicylic acid (PAS), has emerged as a potentially beneficial drug for treating severe manganism. PAS is an FDA-approved drug used to treat tuberculosis (TB). Clinically, the drug has never been considered as the chelating drug for metal poisoning, although the carboxyl group, along with the hydroxyl and amine groups, in PAS's structure do appear to provide an ideal chelating moiety for metals. Recent evidence has pointed out that chelation of Fe in individuals with an excessive Fe burden may reduce TB viability and replication (Cronje et al., 2005), which may partly contribute to PAS's effectiveness in treating TB.
In an animal study published in 1975, Tandon et al. (1975) showed that PAS was capable of removing Mn from the liver and testes of Mn-exposed rats. The human cases in which PAS was used successfully in the treatment of manganism were first introduced to English language literature by Drs. Ky and Hu of Guangxi Medical University (Ky et al., 1992). In 2004, this group of investigators conducted a follow-up study in one of the two cases reported in 1992 (Jiang et al., 2006b). Combined with 85 other cases receiving PAS treatment, most of which are in Chinese literature, the clinical data appear to support the effectiveness of PAS for treating manganism (Jiang et al., 2006b). Following intravenous PAS infusion, most — if not all — signs and symptoms are reportedly significantly alleviated. Remarkably, not only does PAS reverse extrapyramidal syndromes immediately after therapy, but it also been shown to ensure a stable prognosis long after therapy (Jiang et al., 2006b), suggesting the possible repair of damaged neurons. The exact mechanism(s) of PAS therapy remain unknown. Based on the characteristics of its chemical structure and better lipophilicity, PAS may penetrate the blood-brain barrier more readily than EDTA. Whether PAS chelates Mn from its brain deposit site or combats Mn-induced inflammatory processes remains unknown and warrants further investigation.
6. Conclusions
Mn is an essential mineral that is found at low levels in virtually all diets. Mn ingestion represents the principal route of human exposure, although inhalation also occurs, predominantly in occupational cohorts. Regardless of intake, animals generally maintain stable tissue Mn levels as a result of homeostatic mechanisms that tightly regulate the absorption and excretion of this metal. However, high-dose exposures are associated with increased tissue Mn levels, resulting in adverse neurological, reproductive, and respiratory effects. In humans, Mn-induced neurotoxicity, commonly referred to as manganism or Parkinson's disease-like syndrome, is of paramount concern and is considered to be one of the most sensitive endpoints. Mn neurotoxicity is associated with motor dysfunction syndrome that is recognized as a form of Parkinsonism. While much has been learned about transport mechanisms of Mn into the CNS, as well as its neurological effects, many challenges remain. More rigorous quantitative risk assessments that would extrapolate data obtained in rodents and nonhuman primates to humans will require biologically-based dosimetry models with better appreciation for neuronal transfer rates within the brain, mechanisms of manganese-induced neurotoxicity, and the relevance of olfactory transport to brain manganese delivery in primates. This review, and recent studies described herein ((Guilarte et al., 2006; Schneider et al., 2006) also establish that individuals in the general population are exposed to Mn in their living environment and exhibit blood Mn levels within the range of those achieved in experimental animals. This suggests that the behavioral and neuroimaging changes observed in the in non-human primates are likely to occur in individuals under specific living conditions. Finally, it is clear that additional studies are necessary in order to identify potential efficacious treatments for Mn intoxication along with reliable biomarkers of exposure, representing fruitful areas for future research.
Acknowledgments
This work was supported in part by NIEHS 10563 and DoD W81XWH-05-1-0239 (MA), NIEHS 010975 (TRG), NIEHS10975 (JSS), and NIEHS 008146 (WZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisen P, Aasa R, Redfield AG. The chromium, manganese, and cobalt complexes of transferrin. J Biol Chem. 1969;244:4628–4633. [Abstract] [Google Scholar]
- Alves G, Thiebot J, Tracqui A, Delangre T, Guedon C, Lerebours E. Neurologic disorders due to brain manganese deposition in a jaundiced patient receiving long-term parenteral nutrition. J Parenter Enteral Nutr. 1997;21:41–45. [Abstract] [Google Scholar]
- Archibald FS, Tyree C. Manganese poisoning and the attack of trivalent manganese upon catecholamines. Arch Biochem Biophys. 1987;256:638–650. [Abstract] [Google Scholar]
- Aschner M, Aschner JL. Manganese transport across the blood-brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24:857–860. [Abstract] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: Species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. [Abstract] [Google Scholar]
- Aschner M, Gannon M. Manganese (Mn) transport across the blood-brain barrier: Saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33:345–349. [Abstract] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Manganese. Sep, 2000. Public Health Service, http://www.atsdr.cdc.gov/toxprofiles/tp151.html. [Abstract] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders. Neurotoxicol. 1984;5:13–35. [Abstract] [Google Scholar]
- Barbeau A, Inoué N, Cloutier T. Role of manganese in dystonia. Adv Neurol. 1976;14:339–352. [Abstract] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. [Abstract] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. J Neurol Sci. 1973;20:415–455. [Abstract] [Google Scholar]
- Bird ED, Anton AH, Bullock B. The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkey. Neurotoxicology. 1984;5:59–66. [Abstract] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int J of Hyg Environ Health. 2003;206:517–529. [Abstract] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. [Abstract] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS. Differing patterns of striatal 18F-dopa uptake in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–555. [Abstract] [Google Scholar]
- Butterworth RF, Spahr L, Fontaine S, Layrargues GP. Manganese toxicity, dopaminergic dysfunction and hepatic encephalopathy. Metab Brain Dis. 1995;10:259–267. [Abstract] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurol. 1994;44:1583–1586. [Abstract] [Google Scholar]
- Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006;27:340–346. [Abstract] [Google Scholar]
- Chandra SV, Shukla GS, Srivastawa RS, Singh H, Gupta VP. An exploratory study of manganese exposure to welders. Clin Toxicol. 1981;18:407–416. [Abstract] [Google Scholar]
- Chandra SV, Srivastava RS, Shukla GS. Regional distribution of metals and biogenic amines in the brain of monkeys exposed to manganese. Toxicol Lett. 1979;4:189–192. [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–236. [Abstract] [Google Scholar]
- Chetri K, Choudhuri G. Role of trace elements in hepatic encephalopathy: zinc and manganese. Indian J Gastroenterol. 2003;22 Suppl 2:S28–30. [Abstract] [Google Scholar]
- Chia SE, Foo SC, Gan SL, Jeyaratnam J, Tian CS. Neurobehavioral functions among workers exposed to manganese ore. Scand J Work Environ Health. 1993;19:264–720. [Abstract] [Google Scholar]
- Chua AC, Morgan EH. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol Trace Elem Res. 1996;55:39–54. [Abstract] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol. 1997;167:361–369. [Abstract] [Google Scholar]
- Connor JR. Metals and Oxidative Damage in Neurological Disorders. Plenum Press; New York: 1997. pp. 23–39. [Google Scholar]
- Conrad ME, Umbreit JN. Pathways of iron absorption. Blood Cells Mol Dis. 2002;29:336–55. [Abstract] [Google Scholar]
- Cotzias GC, Horiuchi K, Fuenzalida S, Mena I. Chronic manganese poisoning: Clearance of tissue manganese concentrations with persistence of the neurological picture. Neurology. 1968;18:376–382. [Abstract] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharm Vital Stat Gen Sci. 1837;1:41–42. [Google Scholar]
- Cronje L, Edmondson N, Eisenach KD, Bornman L. Iron and iron chelating agents modulate Mycobacterium tuberculosis growth and monocyte-macrophage viability and effector functions. FEMS Immunol Med Microbiol. 2005;45:103–112. [Abstract] [Google Scholar]
- Cronje L, Bornman L. Iron overload and tuberculosis: a case for iron chelation therapy. Int J Tuberc Lung Dis. 2005;9:942. [Abstract] [Google Scholar]
- Crossgrove J, Allen D, Bukaveckas B, Rhineheimer S, Yokel R. Manganese distribution across the blood-brain barrier I Evidence for carrier-mediated influx of manganese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24:3–13. [Abstract] [Google Scholar]
- Crossgrove J, Yokel R. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25:451–460. [Abstract] [Google Scholar]
- Crossgrove JS, Zheng W. Review of manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–553. [Europe PMC free article] [Abstract] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–1975. [Abstract] [Google Scholar]
- Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. [Abstract] [Google Scholar]
- Diez-Ewald M, Weintraub LR, Crosby WH. Inter relationship of iron and manganese metabolism. Proc Soc Exp Biol Med. 1968;129:448–151. [Abstract] [Google Scholar]
- Dickerson RN. Manganese intoxication and parenteral nutrition. Nutrition. 2001;17:689–693. [Abstract] [Google Scholar]
- Discalzi G, Pira E, Hernandez EH, Valentini C, Turbiglio M, Meliga F. Occupational Mn Parkinsonism: Magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation. Neurotoxicology. 2000;21:863–866. [Abstract] [Google Scholar]
- Eide DJ. The SLC39 family of metal ion transporters. Pflueg Arch Eur J Physiol. 2004;447:796–800. [Abstract] [Google Scholar]
- Ejima A, Imamura T, Nakamura S, Saito H, Matsumoto K, Momono S. Manganese intoxication during total parenteral nutrition. Lancet. 1992;339:426. [Abstract] [Google Scholar]
- Erikson K, Shihabi ZK, Aschner J, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–156. [Abstract] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–480. [Abstract] [Google Scholar]
- Erikson KM, Syversen T, Aschner JL, Aschner M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol and Pharmacol. 2005;19:415–421. [Abstract] [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, Bjurling P, Langstrom B, Hedstrom KG, Heilbron E. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992a;66:403–407. [Abstract] [Google Scholar]
- Eriksson H, Gillberg PG, Aquilonius SM, Hedstrom KG, Heilbron E. Receptor alterations in manganese intoxicated monkeys. Arch Toxicol. 1992;66:359–364. [Abstract] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrom KG, Theodorsson-Norheim E, Kristensson K, Stalberg E, Heilbron E. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch Toxicol. 1987;61:46–52. [Abstract] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrom KG, Theodorsson-Norheim E, Kristensson K, Stalberg E, Heilbronn E. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch Toxicol. 1987;61:46–52. [Abstract] [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, Bjurling P, Langstrom B, Hedstrum KG, Heilbronn E. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992a;66:403–407. [Abstract] [Google Scholar]
- Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet. 1996;347:1218–1221. [Abstract] [Google Scholar]
- Fenstermacher J, Kaye T. Drug “diffusion” within the brain. Ann N Y Acad Sci. 1988;531:29–39. [Abstract] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. [Abstract] [Google Scholar]
- Fishman JB, Handrahan JB, Rubir JB, Connor JR, Fine RE. Receptor-mediated trancytosis of transferrin across the blood-brain barrier. J Cell Biol. 1985;101:423A. [Abstract] [Google Scholar]
- Fitsanakis VA, Piccola G, Aschner JL, Aschner M. Manganese (Mn) transport in rat brain endothelial cell (RBE4) monolayers cultured in the presence of astrocyte conditioned media (ACM) is an active process. J Neurosci Res. 2005;81:235–243. [Abstract] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp-2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp-2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. [Europe PMC free article] [Abstract] [Google Scholar]
- Fleming MD, Trenor CC, III, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nature Genet. 1997;16:383–386. [Abstract] [Google Scholar]
- Fored CM, Fryzek JP, Brandt L, Nise G, Sjogren B, McLaughlin JK, Blot WJ, Ekbom A. Parkinson's disease and other basal ganglia or movement disorders in a large nationwide cohort of Swedish welders. Occup Environ Med. 2006;63:135–140. [Europe PMC free article] [Abstract] [Google Scholar]
- Fredstrom S, Rogosheske J, Gupta P, Burns LJ. Extrapyramidal symptoms in a BMT recipient with hyperintense basal ganglia and elevated manganese. Bone Marrow Transp. 1995;15:989–992. [Abstract] [Google Scholar]
- Garrick M, Dolan K, Horbinski C, Ghio A, Higgins D, Porubcin M, Moore E, Hainsworth L, Umbreit J, Conrad M, Feng L, Lis A, Roth J, Singleton S, Garrick L. DMT1: A mammalian transporter for multiple metals. Biometals. 2003;16:41–54. [Abstract] [Google Scholar]
- Gianutsos G, Murray MT. Alterations in brain dopamine and GABA following inorganic or organic manganese administration. Neurotoxicology. 1982;3:75–82. [Abstract] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, sow formula or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. [Abstract] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposures to metals as risk factors for Parkinson's disease. Neurology. 1997;48:650–658. [Abstract] [Google Scholar]
- Griffiths PD, Crossman AR. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson's disease and Alzheimer's disease. Dementia. 1993;4:61–65. [Abstract] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006 In press. [Abstract] [Google Scholar]
- Gupta SK, Murthy RC, Chandra SV. Neuromelanin in manganese-exposed primates. Toxicol Lett. 1980;6:17–20. [Abstract] [Google Scholar]
- Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–875. [Abstract] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. [Abstract] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, Member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–80. [Abstract] [Google Scholar]
- Hernandez EH, Discalzi G, Valentini C, Venturi F, Chio A, Carmellino C, Rossi L, Sacchetti A, Pira E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology. 2006;27:333–339. [Abstract] [Google Scholar]
- Hill JM, Ruff MR, Weber RJ. Transferrin receptors in rat brain: Neuropeptide-like pattern and relationship to iron distribution. Proc Natl Acad Sci USA. 1985;82:4553–4557. [Europe PMC free article] [Abstract] [Google Scholar]
- Hill JM, Switzer RC., III The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11:595–603. [Abstract] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism: ten years of follow-up. Neurology. 1998;50:698–700. [Abstract] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB. Chronic manganese intoxication. Arch Neurol. 1989;46:1104–1106. [Abstract] [Google Scholar]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W, Calne DB. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. [Abstract] [Google Scholar]
- Huang CC, Weng YH, Lu CS, Chu NS, Yen TC. Dopamine transporter binding in chronic manganese intoxication. J Neurol. 2003;250:1335–1339. [Abstract] [Google Scholar]
- Human Health and Animal Nutrition. Academic Press; New York: pp. 185–225. [Google Scholar]
- Hurley LS, Keen CL. Manganese. In: Underwood E, Mertz W, editors. Trace Elements in Human Health and Animal Nutrition. Academic Press; New York: 1987. pp. 185–225. [Google Scholar]
- Inoue N, Makita Y. Neurological aspects in human exposures to manganese. In: Chang LW, editor. Toxicology of Metals. CRC Press; Boca Raton: 1996. pp. 415–421. [Google Scholar]
- Iregren A. Psychological test performance in foundry workers exposed to low levels of manganese. Neurotoxicol Teratol. 1990;12:673–675. [Abstract] [Google Scholar]
- Iwase K, Kondoh H, Higaki J, Tanaka Y, Yoshikawa M, Hori S. Hyperintense basal ganglia on T1-weighted magnetic resonance images following postoperative parenteral nutrition in a pancreatoduodenectomized patient. Dig Surg. 2000;17:190–193. [Abstract] [Google Scholar]
- Jeffries WA, Brandon MR, Hunt SV, Williams AF, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;132:162–163. [Abstract] [Google Scholar]
- Jenner P. Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov Disord. 1998;13:24–34. [Abstract] [Google Scholar]
- Jeon H, Kim JM, Jeong JM, Kim KM, Chang YS, Lee DS, Lee MC. Dopamine transporter imaging with [123I]-β-CIT demonstrate presynaptic nigrostriatal dopaminergic damage in Wilson's disease. J Neurol Neurosurg Psychiat. 1998;65:60–64. [Europe PMC free article] [Abstract] [Google Scholar]
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W. Effective Treatment of Manganese-Induced Occupational Parkinsonism with PAS-Na: A Case of 17-Year Follow-Up Study. J Occup Env Med. 2006a;48:644–649. [Europe PMC free article] [Abstract] [Google Scholar]
- Jiang YM, Zheng W, Long L, Zhao W, Li X, Mo XA. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology. 2006b in press. [Europe PMC free article] [Abstract] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–2039. [Abstract] [Google Scholar]
- Keefer RC, Barak AJ, Boyett JD. Binding of manganese and transferrin in rat serum. Biochim Biophys Acta. 1970;221:390–393. [Abstract] [Google Scholar]
- Keen CL. Overview of manganese toxicity. In: Velazquez S, EPA Liaison, editor. Proceedings of the Workshop on the Bioavailability and Oral Toxicity of Manganese. US EPA, Environmental Criteria and Assessment Office; 1995. pp. 3–11. [Google Scholar]
- Kehres D, Maguire M. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–90. [Abstract] [Google Scholar]
- Kim Y, Kim J, Ito K, Lim HS, Cheong HK, Kim JY. Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology. 1999a;20:249–252. [Abstract] [Google Scholar]
- Kim Y, Kim KS, Yang JS, Shin YC, Park IJ, Kim E. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999b;20:901–907. [Abstract] [Google Scholar]
- Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Dis. 2002;17:568–575. [Abstract] [Google Scholar]
- Klaassen CD. Biliary excretion of manganese in rats, rabbits, and dogs. Toxicol Appl Pharmacol. 1974;29:458–468. [Abstract] [Google Scholar]
- Komaki H, Maisawa S, Sugai K, Kobayashi Y, Hashimoto T. Tremor and seizures associated with chronic manganese intoxication. Brain Dev. 1999;21:122–124. [Abstract] [Google Scholar]
- Komura J, Sakamoto M. Effects of manganese forms on biogenic amines in the brain and behavioral alterations in the mouse: Long-term oral administration of several manganese compounds. Environ Res. 1992;57:34–44. [Abstract] [Google Scholar]
- Ky SQ, Deng HS, Xie PY, Hu WD. A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Brit J Ind Med. 1992;49:66–69. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee JW. Manganese Intoxication. Arch Neurol. 2000;57:597–599. [Abstract] [Google Scholar]
- Li GJ, Liu J, Waalkes MP, Zheng W. Molecular mechanism of distorted iron regulation in the choroids plexus and selected brain regional capillaries following in vivo manganese exposure. Neurotoxicology. 2006 in press. [Europe PMC free article] [Abstract] [Google Scholar]
- Li GJ, Zhang LL, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004;46:241–248. [Europe PMC free article] [Abstract] [Google Scholar]
- Li GJ, Zhao Q, Zheng W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol Appl Pharmacol. 2005;205:188–200. [Europe PMC free article] [Abstract] [Google Scholar]
- Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, Nguyen H, Brickman CM, LeWitt PA. Transferrin and iron in normal, Alzheimer's disease, and Parkinson's disease brain regions. J Neurochem. 1995;65:710–724. [Abstract] [Google Scholar]
- London RE, Toney G, Gabel SA, Funk A. Magnetic resonance imaging studies of the brains of anesthetized rats treated with manganese chloride. Brain Res Bull. 1989;23:229–235. [Abstract] [Google Scholar]
- Lu CS, Huang CC, Chu NS, Calne DM. Levodopa failure in chronic manganism. Neurol. 1994;44:1600–1602. [Abstract] [Google Scholar]
- Lu L, Zhang LL, Li GJ, Guo W, Liang WN, Zheng W. Serum concentrations of manganese and iron as the potential biomarkers for manganese exposure in welders. Neurotoxicology. 2005;26:257–265. [Europe PMC free article] [Abstract] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren A, Alessio L. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Environ Health. 1995;21:143–149. [Abstract] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [Abstract] [Google Scholar]
- Lucchini R, Albini E, Placidi D, Gasparotti R, Pigozzi MG, Montani G. Brain magnetic resonance imaging and manganese exposure. Neurotoxicology. 2000;21:769–775. [Abstract] [Google Scholar]
- Mackenzie B, Hediger M. Slc11 family of H+-coupled metal-ion transporters NRAMP1 and DMT. Pflug Arch. 2004;447:571–579. [Abstract] [Google Scholar]
- Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11:171–176. [Abstract] [Google Scholar]
- Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res. 1999;56:113–122. [Abstract] [Google Scholar]
- McKinney AM, Filice RW, Teksam M, Casey S, Truwit C, Clark HB. Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology. 2004;46:291–295. [Abstract] [Google Scholar]
- Mella H. The experimental production of basal ganglion symptomatology in macacus rhesus. Trans Am Neurol Assoc. 1923;49:131–143. [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods in Cell Biol. 1995;48:451–482. [Abstract] [Google Scholar]
- Mena I, Court J, Fuenzalida S, Papavasiliou PS, Cotzias GC. Modification of chronic manganese poisoning. Treatment with L-dopa or 5-OH tryptophane. N Engl J Med. 1970;282:5–10. [Abstract] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology. 1967;17:128–136. [Abstract] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine MP, Hudnell K. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–342. [Abstract] [Google Scholar]
- Morris CM, Candy JM, Keith AB, Oakley A, Taylor G, Pullen RGL, Bloxham CA, Gocht A, Edwardson JA. Brain iron homeostasis. J Inorganic Biochem. 1992b;47:257–265. [Abstract] [Google Scholar]
- Morris CM, Keith AB, Edwardson JA, Pullen RGL. Uptake and distribution of iron and transferrin in the adult brain. J Neurochem. 1992a;59:300–306. [Abstract] [Google Scholar]
- Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. [Abstract] [Google Scholar]
- Murphy VA, Wadhwani KC, Smith QR, Rapoport SI. Saturable transport of manganese (II) across the rat blood-brain barrier. J Neurochem. 1991;57:948–954. [Abstract] [Google Scholar]
- Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, Esswein E, Renton K, Spies A, Boulle A, Naik I, Iregren A, Rees DJ. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24:885–894. [Abstract] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, Osame M. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999;162:102–105. [Abstract] [Google Scholar]
- Nagy JI, Carter DA, Fibiger HC. Evidence for a GABA-containing projection from the enopenduncular nucleus to the lateral habenula in the rat. Brain Res. 1978;145:360–364. [Abstract] [Google Scholar]
- Neff NH, Barrett RE, Costa E. Selective depletion of caudate nucleus dopamine and serotonin during chronic manganese dioxide administration to squirrel monkeys. Experientia. 1969;25:1140–1141. [Abstract] [Google Scholar]
- Nelson K, Golnick J, Korn T, Angle C. Manganese encephalopathy: utility of early magnetic resonance imaging. Br J Ind Med. 1993;50:510–513. [Europe PMC free article] [Abstract] [Google Scholar]
- Newland MC, Weiss B. Persistent effects of manganese on effortful responding and their relationship to manganese accumulation in the primate globus pallidus. Toxicol Appl Pharmacol. 1992;113:87–97. [Abstract] [Google Scholar]
- Newland MC. Animal models of manganese's neurotoxicity. Neurotoxicology. 1999;20:415–432. [Abstract] [Google Scholar]
- Nonet ML. Studying mutants that affect neurotransmitter release in C elegans. In: Bellen HJ, editor. Neurotransmitter Release. New York: Oxford Univ Press; 1999. [Google Scholar]
- Olanow CW. Manganese-induced Parkinsonism and Parkinson's disease. Ann NY Acad Sci. 2004;1012:209–223. [Abstract] [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, Beal MF, Calne DB, Perl DP. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–498. [Abstract] [Google Scholar]
- Ono K, Komai K, Yamada M. Myoclonic involuntary movement associated with chronic manganese poisoning. J Neurol Sci. 2002;199:93–96. [Abstract] [Google Scholar]
- Pal PKr, Samil A, Calne DB. Manganese neurotoxicity: A review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [Abstract] [Google Scholar]
- Partridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987;36:892–895. [Abstract] [Google Scholar]
- Pentschew A, Ebner FF, Kovatch RM. Experimental manganese encephalopathy in monkeys. J Neuropathol Exp Neurol. 1963;22:488–499. [Abstract] [Google Scholar]
- Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffmann M, Brucke T. Progression of dopaminergic degeneration in Parkinson's disease and atypical parkinsonism: A longitudinal ß-CIT SPECT study. Mov Dis. 2002;17:45–53. [Abstract] [Google Scholar]
- Portnoy M, Jenesn L, Culotta V. The distinct methods by which manganese and iron regulate the NRAMP transporters in yeast. Biochem J. 2002;362:110–124. [Europe PMC free article] [Abstract] [Google Scholar]
- Quaghebeur G, Taylor WJ, Kingsley DP, Fell JM, Reynolds AP, Milla PJ. MRI in children receiving total parenteral nutrition. Neuroradiology. 1996;38:680–683. [Abstract] [Google Scholar]
- Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61:509–517. [Abstract] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: Clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. [Abstract] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005a;64:230–235. [Abstract] [Google Scholar]
- Racette BA, Antenor JA, McGee-Minnich L, Moerlein SM, Videen TO, Kotagal V, Perlmutter JS. [18F]-FDOPA PET and clinical features in parkinsonism due to manganism. Mov Dis. 2005b;20:492–496. [Abstract] [Google Scholar]
- Ressler T, Wong J, Roos JW. Manganese speciation in exhaust particulates of automobiles using MMT containing gasoline. J Synchrtron Radiat. 1999;6:656–658. [Abstract] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR. C elegans II. New York: Cold Spring Harbor Laboratory Press; 1997. [Abstract] [Google Scholar]
- Roels H, Ghyselen P, Buchet J, Ceulemans E, Lauwerys R. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med. 1992;49:25–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, de Fays M, Bernard A, Stanescu D. Epidemiological Survey Among Workers Exposed to Manganese: Effects on Lung, Central Nervous System, and some Biological Indices. Am J Indus Med. 1987;11:307–327. [Abstract] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Brit J Ind Med. 1992;49:25–34. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosakis A, Koster W. Transition metal transport in the green microalga Chlamydomonas reinhardtii-genomic sequence analysis. Res Microbiol. 2004;155:201–210. [Abstract] [Google Scholar]
- Rosakis A, Koster W. Divalent metal transport in the green microalga chlamydomonas reinhardtii is mediated by a protein similar to prokaryotic nramp homologues. Biometals. 2005;18:107–120. [Abstract] [Google Scholar]
- Rosenstock HA, Simons DG, Meyer JS. Chronic manganism. Neurologic and laboratory studies during treatment with levodopa. JAMA. 1971;217:1354–1358. [Abstract] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. [Abstract] [Google Scholar]
- Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, Perl DP, Olanow W, Calne DB. MRI and PET studies of manganese-intoxicated monkeys. Neurology. 1995;45:1199–1204. [Abstract] [Google Scholar]
- Shinotoh H, Snow BJ, Chu NS, Huang CC, Lu CS, Lee C, Takahashi H, Calne DB. Presynaptic and postsynaptic striatal dopaminergic function in patients with manganese intoxication. Neurology. 1997;48:1053–1056. [Abstract] [Google Scholar]
- Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985;840:163–169. [Abstract] [Google Scholar]
- Schlageter NL, Carson RE, Rapoport SI. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:1–8. [Abstract] [Google Scholar]
- Schneider JS, Pope-Coleman A. Cognitive deficits precede motor deficits in a slowly progressing model of parkinsonism in the monkey. Neurodegeneration. 1995;4:245–255. [Abstract] [Google Scholar]
- Schneider JS, Tinker JP, Van Velson M, Menzaghi F, Lloyd GK. The nicotinic acetylcholine receptor agonist SIB-1508Y improves cognitive functioning in chronic low dose MPTP-treated monkeys. J Pharmacol Exp Ther. 1999;290:731–739. [Abstract] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Res. 2006 In Press. [Europe PMC free article] [Abstract] [Google Scholar]
- Sloot WN, Gramsbergen JB. Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res. 1994;657:124–132. [Abstract] [Google Scholar]
- Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem. 1991;56:978–982. [Abstract] [Google Scholar]
- Spahr L, Burkhard PR, Grotzsch H, Hadengue A. Clinical significance of basal ganglia alterations at brain MRI and 1H MRS in cirrhosis and role in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2002;17:399–413. [Abstract] [Google Scholar]
- Stredrick DL, Stokes AH, Worst TH, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology. 2004;25:543–553. [Abstract] [Google Scholar]
- Suarez N, Eriksson H. Receptor-mediated endocytosis of a manganese complex of transferrin into neuroblastoma (SHSY5Y) cells in culture. J Neurochem. 1993;61:127–131. [Abstract] [Google Scholar]
- Suzuki Y, Mouri T, Suzuki Y, Nishiyama K, Fujii N, Yano H. Study of subacute toxicity of manganese dioxide in monkeys. Tokushima J Exp Med. 1975;22:5–10. [Abstract] [Google Scholar]
- Takeda A, Akiyama T, Sawashita J, Okada S. Brain uptake of trace metals, zinc and manganese, in rats. Brain Res. 1994;640:341–344. [Abstract] [Google Scholar]
- Tandon SK, Chandra SV, Singh J, Husain R, Seth PK. Chelation in metal intoxication. I. In vivo effect of chelating agents on liver and testis of manganese administered rats. Environ Res. 1975;9:18–25. [Abstract] [Google Scholar]
- Thomas JH, Lockery SH. Neurobiology. In: Hope IA, editor. C elegans: A practical approach. New York: Oxford Univ Press; 1999. pp. 143–179. [Google Scholar]
- USEPA. Health Assessment Document for Manganese. United States Environmental Protection Agency; 1984. EPA 600/8-83-013F. [Google Scholar]
- Vingerhoets FJG, Snow BJ, Lee CS, Schulzer M, Mak E, Calne DB. Longitudinal fluorodopa positron emission tomography studies of the evolution of idiopathic parkinsonism. Ann Neurol. 1994;36:759–764. [Abstract] [Google Scholar]
- Von Holst H, Ericson K, Edner G. Positron emission tomography with 68-Ga-EDTA and computed tomography in patients with subarachnoid hemorrhage. Acta Neurochir. 1989;97:146–149. [Abstract] [Google Scholar]
- Walaas I, Fonnum F. The distribution and origin of glutamate decarboxylase and choline acetyltransferase in ventral pallidum and other basal forebrain regions. Brain Res. 1979;177:325–336. [Abstract] [Google Scholar]
- Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D. Brain parenchyma sonography discriminates Parkinson's disease and atypical parkinsonian syndromes. Neurology. 2003;60:74–77. [Abstract] [Google Scholar]
- Westermark K, Tedroff J, Thuomas KA, Hartvig P, Langstrom B, Andersson Y, Hornfeldt K, Aquilonius SM. Neurological Wilson's disease studied with magnetic resonance imaging and with positron emission tomography using dopaminergic markers. Mov Dis. 1995;10:596–603. [Abstract] [Google Scholar]
- Wicks SR, Yeh RT, Gish WR, Waterston RH, Plasterk RH. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nature Gen. 2001;28:160–164. [Abstract] [Google Scholar]
- Wolters ECh, Huang CC, Clark C, Peppard RF, Okada J, Chu NS, Adam MJ, Ruth TJ, Li D, Calne DB. Positron Emission Tomography in manganese intoxication. Ann Neurol. 1989;26:647–651. [Abstract] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: A neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. [Abstract] [Google Scholar]
- Yokel RA, Crossgrove JS. Manganese toxicokinetics at the blood-brain barrier. Res Rep Health Eff Inst. 2004;119:7–58. [Abstract] [Google Scholar]
- Youdim MBH, Ben-Shachar D, Riederer P. The possible role of iron in the etipathology of Parkinson's disease. Mov Disord. 1993;8:1–12. [Abstract] [Google Scholar]
- Zayed J, Hong B, L'Esperance G. Characterization of manganese-containing particles collected from the exhaust emissions of automobiles running with MMT additive. Environ Sci Technol. 1999;33:3341–3346. [Google Scholar]
- Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats. Toxicol Sci. 2000;54:295–301. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–342. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng W, Zhao Q. Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–179. [Europe PMC free article] [Abstract] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.taap.2007.03.001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1950780?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.taap.2007.03.001
Article citations
Hereditary haemorrhagic telangiectasias with recurrent ischemic stroke hinted by manganese deposition in the basal ganglia: a case report and literature review.
BMC Neurol, 24(1):375, 07 Oct 2024
Cited by: 0 articles | PMID: 39375614 | PMCID: PMC11457332
Review Free full text in Europe PMC
Dopaminergic REST/NRSF is protective against manganese-induced neurotoxicity in mice.
J Biol Chem, 300(9):107707, 22 Aug 2024
Cited by: 0 articles | PMID: 39178947 | PMCID: PMC11421342
Genetic and epigenetic modulations in toxicity: The two-sided roles of heavy metals and polycyclic aromatic hydrocarbons from the environment.
Toxicol Rep, 12:502-519, 04 May 2024
Cited by: 1 article | PMID: 38774476 | PMCID: PMC11106787
Review Free full text in Europe PMC
Manganese in autism spectrum disorder and attention deficit hyperactivity disorder: The state of the art.
Curr Res Toxicol, 6:100170, 25 Apr 2024
Cited by: 2 articles | PMID: 38737010 | PMCID: PMC11088232
Review Free full text in Europe PMC
In Vitro Assessment of the Neuro-Compatibility of Fe-20Mn as a Potential Bioresorbable Material for Craniofacial Surgery.
Medicina (Kaunas), 60(3):440, 07 Mar 2024
Cited by: 0 articles | PMID: 38541166 | PMCID: PMC10972363
Go to all (300) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates.
Environ Health Perspect, 117(3):325-332, 03 Oct 2008
Cited by: 88 articles | PMID: 19337503 | PMCID: PMC2661898
Review Free full text in Europe PMC
Pathophysiology of manganese-associated neurotoxicity.
Neurotoxicology, 33(4):881-886, 21 Dec 2011
Cited by: 66 articles | PMID: 22202748 | PMCID: PMC3350837
Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water.
Arch Toxicol, 88(1):47-64, 06 Jul 2013
Cited by: 35 articles | PMID: 23832297 | PMCID: PMC3859803
"Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies".
BMC Pharmacol Toxicol, 17(1):57, 04 Nov 2016
Cited by: 129 articles | PMID: 27814772 | PMCID: PMC5097420
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIEHS NIH HHS (8)
Grant ID: R01 ES008146
Grant ID: R01 ES010563-06
Grant ID: ES10975
Grant ID: ES010975
Grant ID: ES10563
Grant ID: R01 ES010563
Grant ID: R01 ES010563-07
Grant ID: ES008146





